Abstract
Background
Ovarian Cancer (OC) constitute the eighth most common cancers among women worldwide. Surgery remains the cornerstone in the management of OC. Intraoperative frozen section (FS) diagnosis is widely used to decide the surgery course. We aimed to assess the reliability of intraoperative FS diagnosis for treatment planning of patients with suspected OC from a multidisciplinary perspective. The clinical consequences of reclassification and the multidisciplinary management of the therapy plan, is the secondary aim of this study. To our knowledge, this information is sparely investigated.
Methods
A single-center, retrospective population-based study of patients who underwent surgery for suspected OC between 2018 and 2020. Histopathological outcomes were classified as benign, borderline, or malignant. The FS diagnosis was the diagnostic test, and the final histopathology report was the gold standard. Diagnostic capability for treatment planning was assessed, and modifications made possible by overall clinical knowledge were discussed.
Results
A total of 358 patients were identified, of whom 187 were included in the FS group. Overall accuracy was 89.8%, and 19 patients were reclassified; the malignancy grade of 15 tumors was underestimated. Prevalence, sensitivity, specificity, positive predictive value, and negative predictive value for invasive malignancies on FS were 54.0% (CI 46.6–61.3%), 88.1% (CI 80.2–93.7%), 98.8% (CI 93.7–99.9%), 98.9% (CI 92.7–99.8%), and 87.6% (CI 80.6–92.4%), respectively. Tumors incorrectly graded by FS tended to be of borderline-related.
Conclusions
The reliability of the FS methodology was an accurate test to help perform appropriate surgery and plan swift oncological treatment. FS is a reliable method to diagnose invasive malignancies and benign pathology. The communication between the pathologist, surgeon, and medical oncologist is highly important for both intraoperative decision-making and postoperative patient care.
Introduction
Ovarian cancer is the eighth most common cancer among females worldwide and the most lethal of gynecological malignancies, causing 184,799 deaths in 2018 [Citation1]. The relative survival in Sweden differs largely depending on the stage [Citation2]. The 5-year survival rate in the early stages (I–II) is around 90% and is less than 45% in advanced stages (III–IV). However, most tumors are detected in the late stages, contributing to the poor prognosis [Citation3]. Symptoms are usually sparse, vague, and similar to other illnesses, further contributing to late discovery [Citation4–6]. Ovarian tumors are multifaceted diseases, exhibiting different features of origin, risk factors, treatment susceptibility, and survival [Citation7–10]. Staging according to the International Federation of Gynecology and Obstetrics (FIGO) and histological classification moderated by the World Health Organization (WHO) are keys to prognostication [Citation11,Citation12]. Globally, ovarian tumors are typically carcinomas predominated by the high-grade serous subtype [Citation13,Citation14]. The primary treatment regime is commonly surgery, with the objective to correctly diagnose and stage the tumor, as well as remove all visible cancer [Citation15,Citation16].
It is important to adapt the surgery depending on tumor malignancy and spread; for example, by performing lymph node dissection for staging and upfront debulking surgery in advanced stages to achieve macroscopic radicality [Citation16,Citation17]. The frozen section (FS) is performed to avoid additional major abdominal surgery and prolonged general anesthesia, as well as to avoid surgical overtreatment, including potential postoperative complications mainly associated with more extensive surgery. An important situation to employ FS is when fertility preservation is pursued [Citation18,Citation19]. Consequently, the frozen section technique is relevant when it guides the surgeon in the intraoperative situation to optimize the surgical procedure while the patient is still under anesthesia [Citation20]. FS diagnosis often shows a high level of accuracy, particularly when performed by a pathologist specializing in gynecologic pathology [Citation21]. Nevertheless, reclassification is not entirely uncommon in the final pathology report, especially when interpreting tumors associated with borderline histology [Citation22].
In Sweden, the processing time of the final histopathological diagnosis may be prolonged due to organizational factors, posing an additional need for a high reliance on FS diagnosis for rapid postoperative treatment planning. This is of utmost importance, based on observations of disease progression and reduced overall survival in patients with delayed start of chemotherapy [Citation23]. A systematic Cochrane review from 2016 analyzed 38 studies and showed that frozen sections with a benign diagnosis were accurate in 94%, a borderline diagnosis in 73%, and a malignant diagnosis in 99%. Overall, for malignant tumors, the sensitivity and specificity for FS were 90% (95% CI, 87.6–92.0) and 99.5% (95% CI, 99.2–99.7), respectively [Citation24].
This study aimed to compare the histopathological diagnosis of the intraoperative FS and the final histopathological diagnosis in patients who underwent surgery for suspected ovarian cancer to assess the reliability of frozen section diagnosis in relation to the postoperative management of the patient. The data was collected in the context of preoperative assessment and postoperative treatment to assure transferability and enable a multidisciplinary discussion of the results. Another aim with this study was to investigate the clinical consequences of reclassification and the multidisciplinary management of the therapy plan. To our knowledge, this information is sparely investigated.
Materials and methods
Study population
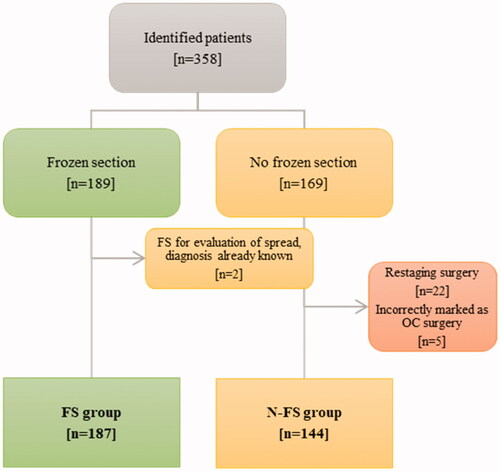
The research was conducted as a retrospective population-based review after receiving approval from the Swedish Ethical Review Authority (2020-02818). The primary data source was patients who underwent elective surgery for suspected ovarian cancer between the 1 July 2018 and the 30 June 2020 at the tertiary center for gynecologic surgery in Lund, Skåne University Hospital. Patient data were retrieved from the surgical management IT-system Orbit 5.10.7 (EVRY Healthcare System AB, Kristianstad, Sweden) by filtering for the following cause of surgery: malignant tumor in the ovary or tumor of uncertain or unknown nature in the ovary. All patients were then identified in the laboratory information management system used by pathologists and divided into two groups. A frozen section group (FS-group), including patients for whom FS diagnostics was performed for intraoperative tumor diagnosis, and a non-frozen section group (N-FS group), for whom no frozen section was assessed. Exclusion criteria were restaging surgeries or surgery incorrectly registered as ovarian tumor operation. Among the diagnoses of primary ovarian malignancy, tubo-ovarian epithelial cancer and epithelial peritoneal cancer were included.
Statistical analyses
Descriptive and analytical statistics were performed using the statistical software IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp Data included both preoperative findings such as laboratory and imaging reports and intraoperative information comprising the type of surgery and postoperative staging and treatment. Nominal and ordinal data were coded to enable subsequent statistics. Qualitative data were described in counts (n) and percentages (%) and quantitative nonparametric data in medians and interquartile ranges (IQR).
Gynecological pathologists performed the histopathological diagnosis. Formalin-fixed and paraffin-embedded material was used for the final histopathological diagnosis and constituted the diagnostic reference standard (gold standard), while the frozen section diagnosis was the diagnostic test. Histopathological tumor diagnoses were classified as benign, borderline, or malignant. The presence of a preoperative diagnosis was documented.
Computation of 95% confidence intervals for prevalence, sensitivity, specificity, and predictive values were performed according to Newcomb and calculated separately for each outcome: benign, borderline, and malignant. Since these statistical measures require a binary classification system, the three outcomes were compared 1:2. For example, the values computed for the benign outcome were performed by setting benign tumors as the positive outcome and grouping borderline and malignant tumors as negative outcomes. Patients with an inconclusive diagnosis on FS were also included in these computations.
Finally, the actual impact of differences between diagnoses at the per-operative FS and final diagnoses, on post-operative treatment planning, were discussed.
Results
Study population
In total, 331 patients fit the inclusion and exclusion criteria, comprising the total study population, and were divided into the FS-group and the N-FS group, as shown in .
Patient characteristics of the total study population are shown in the supplementary material (Table S1). The distribution of histopathology diagnoses of the whole study group is detailed in .
Table 1. Distribution of histopathology (n).
The frozen section group characteristics
Clinicopathological characteristics of the FS-group are shown in .
Table 2. Clinicopathological characteristics of frozen section group (FS).
Frozen section regardless of preoperative diagnosis
In 19 patients (10.2%), the FS was performed regardless of their preoperative diagnosis. Motivation for FS was identified in 13 patients, namely, to distinguish between a borderline and a malignant tumor (n = 5), to determine diagnosis if the preoperative diagnosis was not fully established (n = 5) or suspicion of another coinciding tumor, separate from the preoperatively tested tumor (n = 1), or uncertainties between the preoperative diagnosis and the intraoperative findings (n = 2). FS were likely unnecessarily performed in six patients because of incomplete communication between the surgeon and the pathologist.
The non-frozen section group
FS for diagnostic purposes was not performed in 144 patients, as presented in supplementary material Table S2. Out of the 98 patients primarily operated due to ‘suspicion of cancer’, the final histopathologic diagnosis was benign in 64, borderline in 11, and malignant in 23 patients.
Forty-six patients were preoperatively identified as malignant due to preoperatively histopathologic diagnoses or high clinical suspicion of malignancy, resulting in a ‘treat and confirm’-approach. Twenty-six of these patients had interval-debulking surgery, and 20 patients with peritoneal carcinomatosis underwent upfront surgery. All patients from this group had high-grade serous carcinoma (HGSC) in the final diagnosis.
Collection of tissue samples
In the total study population, the surgeon asked for an FS diagnosis in 62 (49.2%) out of 126 benign cases, 24 (68.6%) out of the 35 borderline cases, and 101 (59.4%) out of 170 malignant cases.
Material sampled for FS by the surgeon almost exclusively contained parts of the uterus adnexa for benign (98.4%) and borderline (100%) diagnoses. In cases of malignancy, the surgeon sampled extra-adnexal tissue in 33.6% of cases.
Reliability of frozen section diagnosis
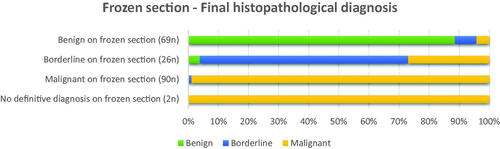
One hundred sixty-eight patients were correctly diagnosed by the FS technique, providing an overall accuracy of 89.8%. Seventeen (9.1%) were subjected to reclassification on final histopathological diagnosis, with 15 cases underestimated and two cases overestimated by FS. Two patients (1.1%) received an inconclusive diagnosis on FS and were later diagnosed with malignant tumors: one with adult granulosa cell tumor and the other with gastrointestinal stromal tumor (GIST). and supplementary material Table S3 illustrate the concordance between the FS and final histopathological diagnosis.
Figure 2. Frozen section diagnosis and corresponding final histopathological diagnosis in percentages, illustrating positive predictive value (PPV) for the three diagnoses; 88.4, 69.2, and 98.9% for benign, borderline, and malignant tumors, respectively.
Twelve of the 17 reclassified tumors (70.6%) were borderline related: borderline serous tumor (n = 1), low-grade serous carcinoma (n = 3), borderline tumor (n = 3), mucinous carcinoma (n = 4), and mucinous cystadenoma (n = 1). Five of the reclassified tumors were related to endometriosis: borderline endometrioid (n = 1), endometrioid carcinoma (n = 3), and clear cell carcinoma (n = 1) ().
Table 3. Tumor categories based on histopathological characteristics.
The Supplementary Table S4 illustrates FS diagnosis as a diagnostic test with statistics of individual outcomes. For the benign and malignant outcomes, sensitivity was 98.4% (95% CI, 91.3–99.9%) and 88.1% (95% CI, 80.1–93.7%), and specificity was 93.6% (95% CI, 87.8–97.2%) and 98.8% (95% CI, 93.7–99.9%). The sensitivity and specificity for the borderline outcome was 75.0% (95% CI, 53.2–90.2%) and 95.1% (95% CI, 90.6–97.7%).
The clinical consequence of reclassification
Of the 19 patients subjected to reclassification on FS, 15 were underdiagnosed, 2 were over-diagnosed, and 2 received an inconclusive diagnosis. In all, 6 out of 19 patients had their treatment changed due to the misclassification during FS. For detailed information on each restaged patient, see supplementary material Table S5.
Discussion
We evaluated the reliability of intraoperative FS diagnosis for treatment planning of patients with suspected ovarian cancer. The FS as a diagnostic method showed a high specificity (98.8%) and a lower sensitivity (88.1%) for malignant diagnosis. Since only 12% of the women were premenopausal, few fertility-preserving requirements were needed. Laparotomy instead of a minimally invasive technique was used in most cases to remove larger tumors without spillage.
In a Cochrane review on FS including 38 studies, 11,181 participants had a median of 29% malignant tumors, while in our study, 54% malignant tumors were recorded [Citation24]. The differences in malignancy representation can be explained by differences in the study populations. In our study, we included both early and advanced stages treated in a tertiary center, while the Cochrane review included only early stages treated in both secondary and tertiary centers. Ratnavelu et al. found the sensitivity and specificity to correctly distinguishing malignant tumors from benign and borderline tumors on FS to be 90.0 and 99.5%, respectively [Citation24], which is in line with the 88.1 and 98.8% recorded in our study.
Our results show that FS diagnosis is a reliable method to diagnose malignant tumors and rule out a benign pathology, which is in concordance with other studies [Citation25–27]. Since the sensitivity was lower than its specificity in both studies, FS diagnosis might be criticized for under-diagnosis, possibly resulting in a secondary surgery, but rarely for extensive surgical over-treatment.
Among the malignant tumors, some are more difficult to diagnose on FS. In the present study, two patients with an adult granulosa cell tumor and a GIST, did not receive a conclusive frozen section diagnosis. The lack of diagnosis is possibly a consequence of the rarity of the tumors and, in addition, a less specific histologic picture requiring ancillary diagnostic methods for diagnosis. In both cases, the malignancy concern was discussed during the intraoperative communication about the FS.
A systematic review by Heatley et al. found that clear cell carcinoma and all types of mucinous tumors were particularly difficult to diagnose [Citation22]. Furthermore, mucinous cancer being frequently reclassified has been observed in a literature review by Hiroshi Yoshida et al. [Citation28], with a discordant rate of 40.5%. This result is in line with our results, where one of two clear cell carcinomas and four of thirteen mucinous lesions were correctly diagnosed.
In accordance with earlier research, the borderline tumor diagnosis was the least reliable on FS [Citation22]. Tumors of the borderline type were also most frequently reclassified, and 27% of borderline tumors in our study were eventually identified with invasive components. Tumor heterogeneity in borderline tumors causes varying histopathologic morphology within the same tumor; thus, prolonged time for FS can be considered to perform a more thorough sampling in the per-operative situation. The adnexa was usually sent in its entirety to the pathology department to minimize the sampling error, allowing the pathologist to make the macroscopic sampling. The accuracy, i.e. the agreement between FS for borderline tumors and final diagnosis in our study, was 66.6%, which was comparable with a study by Taejong et al. that showed a 64.4% agreement rate [Citation29].
For those with benign diagnoses, the sensitivity and specificity were 98.4% and 93.6%, respectively. Since sensitivity was higher than specificity, an overdiagnosis of benign tumors might occur, rsesulting in under-treatment rather than over-treatment, as previously discussed.
The FS is a time-consuming assignment for the pathology department. Thus, a reasonable indication for FS is required. Good knowledge about clinical data and macroscopic tumor characteristics is relevant for the surgeon to decide if FS is appropriate or not. To optimize the interpretation, a multidisciplinary perioperative collaboration, including communication between the surgeon, pathologist, and in some selected cases, the medical oncologist is highly important.
Finally, in our study, the treatment plan needed an adjustment after the reclassification in 6 out of 19 patients. One patient needed a second surgery, and five were treated with adjuvant chemotherapy. Because of the preoperative concern of borderline pathology, all those five patients, who received adjuvant chemotherapy, had a complete surgical staging, and no delayed start in adjuvant chemotherapy.
Due to our clinical routines to offer prophylactic surgery to all patients of non-fertile age, no patients in the over-diagnosed group were considered over-treated.
With respect to postoperative chemotherapy treatment, this study has established a high reliance on the FS diagnosis regarding the malignancy diagnosis, supporting the thought that chemotherapy planning based on FS diagnosis can be initiated when treatment would otherwise be delayed.
Observations show that patients prefer to receive individualized and forthright information in the diagnostic phase, even when information is limited and preliminary [Citation30]. In light of the results of this study, it would be beneficial for patients to receive a probable diagnosis based on FS and the probability of requiring chemotherapy soon after surgery.
The main strength of this study is the population-based approach by including consecutive cases from our region, including the study of the clinical aftermath. The healthcare system in Sweden provides inhabitants uniformity of access to governmental financed healthcare, irrespective of socioeconomic status, and has easily accessible documentation of patient information owing to national personal identification numbers. Due to the centralized cancer care in Sweden, the patients have access to high-qualified care, including gynecologic oncologists, gynecology pathologists, medical oncologists, specialist nurses, and clinical trial units.
Further, a novelty with the present study, is a careful survey of the reclassified patients. Despite diagnose difficulties in some of the cases, the intraoperative communication between specialists resulted in adequate treatment decision, which minimalize the risk for reoperation and secondary delay in chemotherapy.
Another advantage of our study is the relatively short time interval from the start to the end of recruitment, which minimizes the influence of changes in clinical routines, terminology, diagnostic criteria, and issues involving specimen handling, especially for borderline tumors, over the years [Citation31,Citation32]. A retrospective study by Stefanie Avril et al. showed that over-diagnosis of cystadenoma/fibroma as serous borderline tumors had been a clinical problem over the years. Out of 81 consecutive cases diagnosed as borderline ovarian tumors over 10 years at a single tertiary center, the diagnosis of serous borderline tumors was rejected due to a diagnosis of serous cystadenoma/fibroma in seven (9%) patients [Citation33].
Additional strengths of this study were the concordance of results with earlier research and the relatively large study group.
The main weakness of this study is its retrospective nature, which limits the study to preexisting information and may have affected the results and its applicability. The pathologists performing the final histopathological diagnosis were aware of the FS diagnosis, thereby entailing the risk of bias and potential overestimation of the frozen section’s reliability. The final histopathological result is based on more conclusive material, with better technical quality, including access to ancillary methods (mostly immunohistochemistry), and often examined by more than one pathologist; therefore, it is unlikely that this bias had any noticeable influence.
Similar studies have previously been performed, but to our knowledge, never in Sweden and rarely in consideration of clinicopathological variables including the impact on further treatment. Therefore, the information that this study provides can most likely contribute to the field of research and provide practical guidance in managing ovarian cancer.
Conclusion
The reliability of frozen section diagnosis at surgery of suspected ovarian cancer was observed to be high in this study. Both measures of diagnostic capability and agreement with the gold standard proved this, with an overall accuracy of 89.8%. Underestimation of malignancy was observed, but overestimation was rare. The multidisciplinary collaboration could triage the care of the patients based on the frozen sections, which is highly important for both intraoperative decision-making and postoperative patient care. These results contribute to improving cancer care among patients undergoing surgery for suspected ovarian cancer, with guidance on how to handle information for optimal surgical procedures and early oncological treatment. Knowledge concerning frozen sections in relation to different histopathologic subtypes is needed, and chemotherapy treatment can preliminary be planned accordingly.
| Abbreviations | ||
| OC | = | ovarian cancer |
| FS | = | frozen section |
| N-FS | = | non-frozen section |
| FIGO | = | International Federation of Gynecology and Obstetrics |
| WHO | = | World Health Organization |
| CI | = | Confidence interval |
| IQR | = | interquartile range |
| PPV | = | positive predictive value |
| NPV | = | negative predictive value |
| LGSOG | = | low-grade serous ovarian carcinoma |
| BOT | = | borderline ovarian tumor |
| GIST | = | gastrointestinal stromal tumor |
| BSO | = | bilateral salpingo-oophorectomy |
| MLAC | = | mesonephric-like adenocarcinoma of Mullerian origin |
| FATWO | = | female adnexal tumor of probable Wolffian origin |
| MANEC | = | mixed adenoneuroendocrine carcinoma of the ovary |
| SCTAT | = | sex cord tumor with annular tubules |
Supplemental Material
Download MS Word (29.4 KB)Acknowledgements
Susann Ullén for statistical support. No funding has been received for this study.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The datasets used and/or analyzed in the present study are available from the corresponding author upon reasonable request.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Berek JS. Lymph node-positive stage IIIC ovarian cancer: a separate entity? Int J Gynecol Cancer. 2009;19(Suppl 2):S18–S20.
- Lheureux S, Gourley C, Vergote I, et al. Epithelial ovarian cancer. Lancet. 2019;393(10177):1240–1253.
- Bankhead CR, Kehoe ST, Austoker J. Symptoms associated with diagnosis of ovarian cancer: a systematic review. BJOG. 2005;112(7):857–865.
- Goff BA, Mandel L, Muntz HG, et al. Ovarian carcinoma diagnosis. Cancer. 2000;89(10):2068–2075.
- Bankhead CR, Collins C, Stokes-Lampard H, et al. Identifying symptoms of ovarian cancer: a qualitative and quantitative study. BJOG. 2008;115(8):1008–1014.
- Peres LC, Cushing-Haugen KL, Köbel M, et al. Invasive epithelial ovarian cancer survival by histotype and disease stage. J Natl Cancer Inst. 2019;111(1):60–68.
- Prat J. Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012;460(3):237–249.
- Kurman RJ, Shih IM. The dualistic model of ovarian carcinogenesis: revisited, revised, and expanded. Am J Pathol. 2016;186(4):733–747.
- Wentzensen N, Poole EM, Trabert B, et al. Ovarian cancer risk factors by histologic subtype: an analysis from the ovarian cancer cohort consortium. J Clin Oncol. 2016;34(24):2888–2898.
- Prat J. Ovarian, fallopian tube and peritoneal cancer staging: rationale and explanation of new FIGO staging 2013. Best Pract Res Clin Obstet Gynaecol. 2015;29(6):858–869.
- Mutch DG, Prat J. 2014 FIGO staging for ovarian, fallopian tube and peritoneal cancer. Gynecol Oncol. 2014;133(3):401–404.
- Kurman RJ, Carcangiu ML, Herrington CS, et al. editors. World Health Organisation classification of tumours of the female reproductive organs. Lyon: International Agency for Research on Cancer; 2014. (World Health Organization classification of tumours; vol. 6)
- Coburn SB, Bray F, Sherman ME, et al. International patterns and trends in ovarian cancer incidence, overall and by histologic subtype. Int J Cancer. 2017;140(11):2451–2460.
- Colombo N, Sessa C, du Bois A, ESMO-ESGO Ovarian Cancer Consensus Conference Working Group, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease†. Ann Oncol. 2019;30(5):672–705.
- Trimbos B, Timmers P, Pecorelli S, et al. Surgical staging and treatment of early ovarian cancer: long-term analysis from a randomized trial. J Natl Cancer Inst. 2010;102(13):982–987.
- du Bois A, Reuss A, Pujade-Lauraine E, et al. Role of surgical outcome as prognostic factor in advanced epithelial ovarian cancer: a combined exploratory analysis of 3 prospectively randomized phase 3 multicenter trials: by the arbeitsgemeinschaft gynaekologische onkologie studiengruppe ovarialkarzinom (AGO-OVAR) and the groupe d’Investigateurs nationaux pour les etudes des cancers de lOvaire (GINECO). Cancer. 2009;115(6):1234–1244.
- Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36(19):1994–2001.
- Xu Z, Becerra AZ, Justiniano CF, et al. Complications and survivorship trends after primary debulking surgery for ovarian cancer. J Surg Res. 2020;246:34–41.
- Gal AA. The centennial anniversary of the frozen section technique at the Mayo Clinic. Arch Pathol Lab Med. 2005;129(12):1532–1535.
- Bige O, Demir A, Saygili U, et al. Frozen section diagnoses of 578 ovarian tumors made by pathologists with and without expertise on gynecologic pathology. Gynecol Oncol. 2011;123(1):43–46.
- Heatley MK. A systematic review of papers examining the use of intraoperative frozen section in predicting the final diagnosis of ovarian lesions. Int J Gynecol Pathol. 2012;31(2):111–115.
- Mahner S, Eulenburg C, Staehle A, et al. Prognostic impact of the time interval between surgery and chemotherapy in advanced ovarian cancer: analysis of prospective randomised phase III trials. Eur J Cancer. 2013;49(1):142–149.
- Ratnavelu ND, Brown AP, Mallett S, et al. Intraoperative frozen section analysis for the diagnosis of early stage ovarian cancer in suspicious pelvic masses. Cochrane Database Syst Rev. 2016;3(3):CD010360.
- Morton R, Anderson L, Carter J, et al. Intraoperative frozen section of ovarian tumors: a 6-year review of performance and potential pitfalls in an Australian Tertiary Referral Center. Int J Gynecol Cancer. 2017;27(1):17–21.
- Bajracharya Shakya A, Jain V, Sekhon R, et al. Diagnostic accuracy of intraoperative frozen section in ovarian neoplasms: experience in a tertiary oncology Centre. Kathmandu Univ Med J. 2018;16(63):259–262.
- Cross PA, Naik R, Patel A, et al. Intra-operative frozen section analysis for suspected early-stage ovarian cancer: 11 years of Gateshead Cancer Centre experience. BJOG. 2012;119(2):194–201.
- Yoshida H, Tanaka H, Tsukada T, et al. Diagnostic discordance in intraoperative frozen section diagnosis of ovarian Tumors: a literature review and analysis of 871 cases treated at a Japanese Cancer Center. Int J Surg Pathol. 2021;29(1):30–38.
- Song T, Choi CH, Kim HJ, et al. Accuracy of frozen section diagnosis of borderline ovarian tumors. Gynecol Oncol. 2011;122(1):127–131.
- Jelicic L, Brooker J, Shand L, et al. Experiences and health care preferences of women with ovarian cancer during the diagnosis phase. Psychooncology. 2019;28(2):379–385.
- Hauptmann S, Friedrich K, Redline R, et al. Ovarian borderline tumors in the 2014 WHO classification: evolving concepts and diagnostic criteria. Virchows Arch. 2017;470(2):125–142.
- Seidman JD, Soslow RA, Vang R, et al. Borderline ovarian tumors: diverse contemporary viewpoints on terminology and diagnostic criteria with illustrative images. Hum Pathol. 2004;35(8):918–933.
- Avril S, Hahn E, Specht K, et al. Histopathologic features of ovarian borderline tumors are not predictive of clinical outcome. Gynecol Oncol. 2012;127(3):516–524.