Abstract
Background
Organ-sparing treatment for muscle-invasive bladder cancer by maximal transurethral removal of the tumor (TURB) followed by chemoradiation (CRT) has shown promising results in recent studies, and is therefore considered to be an acceptable alternative for the standard of radical cystectomy (RC) in selected patients. We report on outcomes in a single-center, retrospective CRT cohort in comparison to a RC and radiotherapy only (RT) cohort.
Patients and methods
The patient population included n = 84 CRT patients, n = 93 RC patients, and n = 95 RT patients. Primary endpoints were local control (LC) up to 2 years and overall survival (OS) up to 5 years. Cox regression was performed to determine risk factors for LC and OS in the CRT group. Acute genito-urinary (GU) and gastro-intestinal (GI) toxicity were scored with CTCAE version 4 for the RT and CRT cohort. Logistic regression was used to determine risk factors for toxicity. We followed the EQUATOR guidelines for reporting, using the STROBE checklist for observational research.
Results
Baseline characteristics were different between the treatment groups with in particular worse comorbidity scores and higher age in the RT cohort. The CRT schedule was completed by 96% of the patients. LC at 2 years was 83.4% (90% CI 76.0–90.8) for CRT vs. 70.9% (62.2–79.6) for RC and 67.0% (56.8–77.2) for RT. OS at 5 years was 48.9% (38.4–59.4) for CRT vs. 46.6% (36.4–56.8) for RC, and 27.6% (19.4–35.8) for RT. High T stage was significantly associated with worse LC and OS in the CRT group. GU/GI toxicity grade ≥2 occurred in 43 (48.3%) RT patients and 38 (45.2%) CRT patients.
Conclusions
The organ-preserving strategy with CRT was feasible and tolerable in this patient population, and the achieved LC and OS were satisfactory in comparison to the RC cohort and literature.
Background
For years, radical cystectomy (RC) with pelvic lymph node dissection is considered the cornerstone of treatment of N0M0 muscle-invasive bladder cancer. Less invasive and organ-sparing treatments, among which transurethral removal of the bladder tumor (TURBT), external beam radiotherapy (EBRT), and partial cystectomy, provided inferior results. However, multimodality treatment, consisting of maximal TURBT, followed by EBRT and radiosensitizing chemotherapy, resulted in comparable outcomes and is now included in guidelines for properly selected patients [Citation1]. Since no randomized controlled trial is performed to compare RC to multimodality treatment, debate remains about what is best. Recent non-randomized studies suggest comparable local control (LC) and cancer-specific survival rates for multimodality treatment [Citation2–8], however, the results of these studies have to be interpreted with caution because of differences between patient populations receiving the treatment of interest. In the elderly and medically unfit subpopulation, survival after RC is worse with increasing age and comorbidity [Citation9–12], suggesting that bladder sparing treatment could be a safer and non-inferior treatment for these patients. Nevertheless, comorbidities, like vascular disease, can be a contra-indication for the addition of chemotherapy to the bladder-sparing treatment. In the medically unfit subgroup not suitable for chemotherapy, EBRT without chemotherapy could however still be a feasible alternative treatment to maintain quality of life and achieve acceptable LC rates [Citation13–17].
Although the proportion of MIBC patients receiving bladder-sparing treatment has increased over time, radiation is still mainly applied in elderly MIBC patients who are considered unfit for surgery. In this study, we aimed to assess real-world data on the long-term clinical outcome of bladder-sparing treatment (CRT) for non-metastatic MIBC patients (elderly and unfit-for-surgery population) for the primary endpoints LC and survival, and to compare this with outcomes of RC and radiotherapy (RT) only. We hypothesized that with CRT acceptable outcomes can be achieved compared to RC when taking into consideration the differences with respect to age and comorbidity. The results of this study might help physicians to guide treatment decisions on MIBC in the context of the aging population.
Patients and methods
We followed the EQUATOR guidelines for reporting, using the STROBE checklist for observational research, which is a checklist of 22 items that are considered essential for good reporting of observational studies (checklist included in the Suppl. file) [Citation18].
Patients and data collection
This single-center, retrospective cohort study was conducted at the Erasmus Medical Center Cancer Institute, Rotterdam, The Netherlands. Three treatment patient groups were established: a consecutive patient cohort with muscle-invasive, non-metastatic bladder cancer (clinical stage T2-4N0M0) who received EBRT (total dose of 66 Gy) with concomitant radiosensitizing chemotherapy (CRT group) or with RT only (RT group) between January of 2010 and December of 2017. The third treatment group consisted of consecutive patients aged ≥ 60 years with pT2-4N0M0 urothelial cell carcinoma who were treated with RC between January 2008 and December 2018, and who did not receive prior treatment with chemotherapy (induction or neo-adjuvant) or salvage cystectomy. Data were retrospectively collected from hospital records. Survival data were checked with the civil register. Charlson comorbidity index (CCI, not age-adjusted) was calculated as an indicator for comorbidity [Citation19]. This cohort study was reviewed and approved by the local medical ethics committee or the Erasmus Medical Center, Rotterdam, The Netherlands (nr MEC 2017-233).
Sample size
The patient population included N = 84 CRT patients, N = 93 RC patients, and N = 95 RT patients, as a result of the patient numbers treated at our hospital in the specified period. We performed a priori no sample size calculations since the main goal of the study is to report cumulative incidences with confidence intervals; we do not primarily aim at testing differences or non-inferiority between treatment groups.
Treatment
RC was the treatment of first choice, when feasible, and consisted of cystoprostatectomy in men and anterior exenteration in women with pelvic lymph node dissection and urinary diversion. Patients who were considered unfit or were unwilling to undergo RC received bladder-sparing treatment. Maximum feasible TURBT was performed in all patients. Presence of distant metastatic disease was excluded by CT of thorax and abdomen, not older than 8 weeks at the start of treatment. Patients were treated with curative intent. The CRT schedule consisted of EBRT up to a total dose of 66 Gy in 33 fractions 5 times a week, combined with 2 cycles of Fluorouracil 500 mg/m2 + 12 mg/m2 Mitomycin C in week 1 and Fluorouracil 500 mg/m2 in week 4 [Citation19]. Exclusion criteria for chemotherapy were ischemic heart disease, a severe worsened liver- or kidney function, or deficiency of the dihydropyrimidine dehydrogenase (DPYD)-gene (associated with development of severe toxicity from fluorouracil [Citation20]). If chemotherapy was not a valid option, either due to a patient’s general condition or comorbidities, patient received the same EBRT schedule, but without chemotherapy (RT group). Target volume for radiation was the empty bladder volume including macroscopic tumor extension (clinical target volume, CTV) with a 1.5 cm circumferential margin to account for variation in patient positioning, empty bladder volume and rectal filling. Conformal 3D RT (3–4-field technique) was used for the majority of patients; a limited number was treated with intensity-modulated RT (IMRT).
Evaluation and follow-up
Patients were evaluated during treatment once per 1–2 weeks by the treating physician to assess acute toxicity as well as three months later at follow-up. Follow-up consisted of a visit to the referring urologist every 3 months during the first 2 years including cystoscopy and urinary cytology on indication. After 2 years, follow-up including cystoscopy was performed twice a year up to 5 years follow-up post-treatment [Citation21]. However, the follow-up schedule was often personalized in elderly and frail patients.
Endpoints and outcome measures
Primary endpoints of this study were 2-year LC rate, and 5-year overall survival (OS). Secondary endpoint was acute toxicity Grade ≥2. Toxicity scores were retrospectively assigned based on information from medical records using the CTCAE scale version 4.0 (https://evs.nci.nih.gov/ftp1/CTCAE/). Genito-urinary (GU) Items scored were cystitis, hematuria, bladder spasms, urine retention, incontinence and urgency. Gastrointestinal (GI) items scored were diarrhea and urgency. Patients with a urine catheter or colostomy at baseline were excluded from toxicity analyses.
Local recurrence was defined as a histologically proven urothelial carcinoma of the bladder or a papillary bladder tumor on cystoscopy or urine cytology that showed a suspicion of high-grade urothelial carcinoma. If cause of death was not recorded, we defined a probable cause of death for all deaths within 2 years. For this purpose, additional information was obtained from general practitioner. If there were signs of recurrence or metastasis in the last patient records, we defined the cause of death as probably bladder cancer-related. If there were no signs of recurrence in the past 6 months, we defined the cause of death as other.
Statistical analyses
Follow-up time was calculated from start of therapy to date of last contact or event. Baseline characteristics were compared using Kruskal–Wallis test (age) or Chi-square (categorical/ordinal variables). Logistic regression was used to evaluate potential predictive factors for acute toxicity. Uni- and multi-variable analysis for 2-year LC and 5-year OS was performed using Cox regression for the CRT and RC group (separately and combined). Known factors from literature were tested. Because of limited statistical power, we dichotomized variables around median values. Factors with a p value of <0.2 at univariable analysis were included in the multivariable model using the backward selection method. Robustness of the model was tested by manually entering/removing variables with a p value between 0.2 and 0.3. Kaplan–Meier curves were calculated to estimate LC and OS rates as a function of time. All analyses were performed with IBM SPSS Statistics for Windows version 25 (IBM Corp., Armonk, NY, USA) and R version 4.0 (RStudio, PBC, Boston, MA, USA). Significance level for all tests was set at p ≤ 0.05.
Results
Study patients
A total of N = 84 CRT patients, N = 95 RT patients, and N = 93 RC patients were included. Reasons for exclusion were: receiving an alternative shorter RT schedule on request for fragile patients (63 Gy in 28 fractions, n = 67).
Baseline characteristics
Median age in the CRT group (N = 84) was 75.5 years vs. 79.0 years in the RT group (N = 95) and 69.0 in the RC group (N = 93). Median follow-up was 28.5 months (range 3–60). As expected, because of patient selection, patient and tumor characteristics were significantly different between the treatment groups (). RC patients were younger and had a lower CCI. Also, gender ratio, WHO performance status (WHO-PS), T stage distribution and proportion of patients with additional carcinoma in situ (CIS) were different between treatment groups. Nine patients received induction or neo-adjuvant chemotherapy and then switched from planned RC to CRT. Thirty-five patients had no strict contra-indications for RC, but opted for bladder sparing treatment instead. Five patients (CRT n = 1, RT n = 4) did not complete RT (one patient with severe comorbidity died after 3 fractions, 4 patients stopped after 44–60 Gy). Three CRT patients did not complete chemotherapy but did finish their RT schedule.
Table 1. Baseline characteristics.
Local control (LC)
Evaluation of LC was according schedule for the majority of the patients up to 2 years of follow-up; after this time point, part of the patients was not in follow-up anymore and data therefore less reliable. A total of 11 patients had local recurrence within 2 years in the CRT group. For the RC and RT group, these numbers were 21 and 22, respectively. Corresponding LC at 2 years was 83.4% (90% CI 76.0–90.8) for CRT vs. 70.9% (62.2–79.6) for RC and 67.0% (56.8–77.2) for RT (). Furthermore, we observed that another 8 patients in the CRT group developed a local failure between 2 and 5 years follow-up vs. only 2 cases in the RC group. Within the RT group there were another eight cases of local failure within 2–5 years of follow-up (while follow-up beyond 2 years was quite limited in this treatment group with fragile patients). Corresponding Kaplan–Meier estimates up to 5 years are shown in Suppl. Figure A.
Figure 1. Local control (Kaplan–Meier estimates) in the radiotherapy only (RT), chemoradiation (CRT), and radical cystectomy (RC) cohorts.
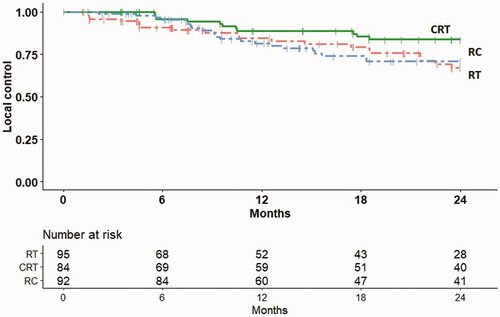
In the CRT group, T-stage was a significant factor at multivariable analysis (). In the RC group no prognostic factors were found significant, and the estimated HRs for T stage and WHO PS score were much lower compared to the results for the CRT group (, Suppl. Table 1(a)).
Table 2. Results of the Cox regression analysis for the CRT group for the endpoint of local recurrence within 2 years after start treatment.
Survival
Five-year OS was 48.9% (38.4–59.4) for CRT, 46.6% (36.4–56.8) for RC, and 27.6% (19.4–35.8) for RT (). Within the CRT group, T stage was the only significant prognostic factor (). None of the tested factors were prognostic for OS in the RC group (Suppl. Table 2(a)). In the combined group, T stage was a prognostic factor (Suppl. Table 2(b)).
Figure 2. Overall survival (Kaplan–Meier estimates) in the radiotherapy only (RT), chemoradiation (CRT), and radical cystectomy (RC) cohorts.
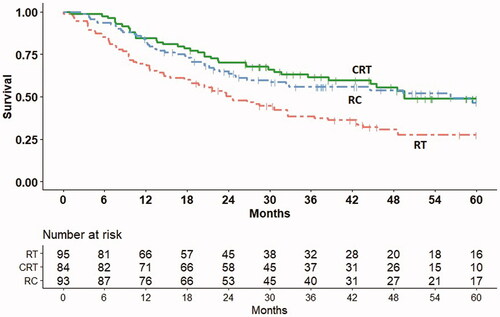
Table 3. Results of the Cox regression analysis for the CRT group for the endpoint of overall survival within 5 years after start treatment.
For all deaths within 2 years, we evaluated whether it was probably or likely related to bladder cancer. In the CRT group, 64.0% (16 out of 25) were scored as ‘bladder cancer related’, for RC and RT these numbers were 93.9% (31/33) and 53.2% (25/47), respectively.
Acute toxicity
Acute toxicity was evaluable in 89 RT patients (94%) and 84 CRT patients (100%). Bladder sparing treatments were tolerated well. GU- and/or GI-toxicity grade ≥2 occurred in 43 (48.3%) RT-patients and 38 (45.2%) CRT-patients. Notably, GU-symptoms grade ≥2 were already present before treatment in 12 and 5%, respectively, probably partly caused by the tumor and partly as comorbidity. Grade 3 symptoms, requiring hospitalization, occurred in 4 patients. No grade 4 or 5 toxicity occurred. In 94.5% of patients, symptoms did return to baseline at the end of the acute toxicity period (3 months post-treatment). Mean time to maximum toxicity was 30 d for both GU (range 5–70, IQR 24) and GI toxicity (range 7–62, IQR 24). There was no significant difference in toxicity proportions between the RT and CRT groups. Additionally, we performed logistic regression to define baseline variables that increased the probability of grade ≥2 toxicity. None of the evaluated variables reached significance (age above or below 75, gender, WHO-PS, treatment schedule, smoking status, history of non-MIBC, year of treatment, and T-stage), except for the presence of hydronephrosis, OR of 6.1 (2.5–14.9, 95% CI; p < 0.05). Acute toxicity in RC patients was assessed through Clavien–Dindo scoring, 70% of patients had a score of 2 or lower, i.e., no radiological or surgical intervention of any kind was necessary; 4 patients experienced a life-threatening complication and 1 patient died within 30 d. A comparison with the CRT and RT group was not possible because of the differences in scoring methods.
Discussion
To our knowledge, this is one of the largest cohort studies reporting on outcomes of bladder-sparing multimodality treatment (CRT) in unfit-for-surgery MBIC patients. With CRT, acceptable LC and OS rates were achieved and treatment was well tolerated with a treatment compliance of 96% and acceptable acute toxicity rates.
Comparing outcomes to RC and RT only treatment groups from our hospital, we observed trends as can be expected based on literature: worse outcomes with RT only and similar outcomes with RC. A general problem in comparing outcomes in this study (as well as in other studies) is that baseline characteristics of the treatment groups are significantly different and therefore comparisons should be interpreted with caution. Of note is that we observed a significant number of local failures in the CRT group beyond 2 years of follow-up whereas in the RC group local failure beyond 2 years was rare.
In terms of OS, we showed comparable results between CRT and RC. Boustani et al., who described a large cohort (n = 164) of patients > 80 years treated with either RC or CRT, also found no difference in progression-free and OS between treatment strategies [Citation22]. Disease progression was the only significant factor influencing OS in their series. Williams et al. however showed decreased survival for bladder-sparing therapy in the elderly (HR 1.49 for OS), as compared to RC by propensity matching [Citation23].
The RT group showed poor outcomes in terms of OS. This can partly be explained by the observed high numbers of death due to other causes. At baseline, these patients were generally older and had worse performance and more comorbidity. This is in line with the population-based study of Korpics et al., showing in a propensity-matched study that CRT is better than RT alone in terms of survival in the elderly [Citation24].
Grade 2–3 acute GU and GI toxicity rates ranged from 29 to 70% in elderly patient cohorts treated with either RT or CRT [Citation25–34]. Fonteyne et al. performed a systematic review, evaluating curative treatment strategies in elderly patients and found that RC was associated with higher short-term mortality rates and more early and late complications in this patient category [Citation9]. Our data supports that in the elderly and unfit patient group, CRT is associated with acceptable toxicity, with a very low rate of grade ≥3 acute toxicity. However, in this elderly subgroup grade 1–2 toxicity can already be a reason for deterioration with respect to quality of life and performance. Unfortunately, we could not identify predictive factors for toxicity of (C)RT, except for the presence of hydronephrosis, but considering the large confidence interval, its significance is hard to interpret. Hydronephrosis is not mentioned as predictive factor in other studies performed in selected (frail, medically unfit) patients. In the study of Lutkenhaus et al. [Citation28], tumor size was found predictive. Bamias et al. [Citation25] reported a correlation between comorbidity index and toxicity.
Although several studies argue that radical or partial cystectomy should be the treatment of choice and that other treatments are inferior [Citation10,Citation35], our data suggest that, with good patient selection, less invasive therapy with chemoradiation (CRT) is a good alternative. The population-based study of Gray et al. showed that the elderly subgroup is denied aggressive treatment, under which chemoradiotherapy, regularly [Citation36]. However, performance status, not chronologic age, was a prognostic factor in other studies [Citation25,Citation32,Citation34], underlining the importance of appropriate patient selection. Comprehensive geriatric assessment (CGA), or abbreviated versions, like G8 which is recommended in the current guideline [Citation1], could help guide patient selection and prevent undertreatment, although this is still to be prospectively validated in MIBC patients [Citation37]. After all, left untreated, MIBC is an aggressive disease with high rates of progression to metastasis (38% after 6 months) and cancer-specific mortality (5Y rate of 86%) [Citation38].
There are several limitations to our study. First, we were not able in this retrospective setting to retrieve full information on the results of the TURB, like the histology, whereas this is relevant information with prognostic value [Citation39,Citation40]. Second, especially in the RT group which included the most fragile patients, follow-up was not always performed according to schedule. Frail patients were often only seen in case of complaints and did not always undergo follow-up cystoscopy. This may have impacted the reported LC rates in this group. Third, the acute toxicity rates may be underestimated because of the retrospective nature of the scoring. Lastly, the available patient numbers per treatment group (around 90 per group) limits the statistical power of this study.
Conclusions
Our real-world data support that a bladder preserving strategy, i.e., TURB followed by CRT, is a feasible alternative for RC in selected patients, with good tolerability and acceptable LC and survival outcomes. Future research will have to confirm these observations. Important results for the clinic are expected from the currently ongoing nationwide prospective Dutch cohort study ‘BlaZIB’ [Citation41].
Supplemental Material
Download MS Word (93.8 KB)Acknowledgments
The authors thank Yvette van Norden for statistical advice.
Disclosure statement
The authors report there are no competing interests to declare.
Data availability statement
The data of this study are available upon request; the corresponding author can be contacted for this purpose.
References
- Witjes JA, Bruins HM, Cathomas R, et al. European association of urology guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2020 guidelines. Eur Urol. 2021;79(1):82–104.
- Booth CM, Siemens DR, Li G, et al. Curative therapy for bladder cancer in routine clinical practice: a population-based outcomes study. Clin Oncol (R Coll Radiol). 2014;26(8):506–514.
- Seisen T, Sun M, Lipsitz SR, et al. Comparative effectiveness of trimodal therapy versus radical cystectomy for localized muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2017;72(4):483–487.
- Bekelman JE, Handorf EA, Guzzo T, et al. Radical cystectomy versus bladder-preserving therapy for muscle-invasive urothelial carcinoma: examining confounding and misclassification biasin cancer observational comparative effectiveness research. Value Health. 2013;16(4):610–618.
- Kaushik D, Wang H, Michalek J, et al. Chemoradiation Vs radical cystectomy for muscle-invasive bladder cancer: a propensity score-weighted comparative analysis using the national cancer database. Urology. 2019;133:164–174.
- Vashistha V, Wang H, Mazzone A, et al. Radical cystectomy compared to combined modality treatment for Muscle-Invasive bladder cancer: a systematic review and Meta-Analysis. Int J Radiat Oncol Biol Phys. 2017;97(5):1002–1020.
- Arcangeli G, Arcangeli S, Strigari L. A systematic review and Meta-analysis of clinical trials of bladder-sparing trimodality treatment for muscle-invasive bladder cancer (MIBC). Crit Rev Oncol Hematol. 2015;94(1):105–115.
- Kulkarni GS, Hermanns T, Wei Y, et al. Propensity score analysis of radical cystectomy versus Bladder-Sparing trimodal therapy in the setting of a multidisciplinary bladder cancer clinic. J Clin Oncol. 2017;35(20):2299–2305.
- Fonteyne V, Ost P, Bellmunt J, et al. Curative treatment for muscle invasive bladder cancer in elderly patients: a systematic review. Eur Urol. 2018;73(1):40–50.
- Goossens-Laan CA, Leliveld AM, Verhoeven RH, et al. Effects of age and comorbidity on treatment and survival of patients with muscle-invasive bladder cancer. Int J Cancer. 2014;135(4):905–912.
- Megwalu II, Vlahiotis A, Radwan M, et al. Prognostic impact of comorbidity in patients with bladder cancer. Eur Urol. 2008;53(3):581–589.
- Turgeon GA, Souhami L. Trimodality therapy for bladder preservation in the elderly population with invasive bladder cancer. Front Oncol. 2014;4:206.
- Witjes J, Lebret T, Comperat EM, et al. Updated 2016 EAU guidelines on muscle-invasive and metastatic bladder cancer. Eur Urol. 2017;71(3):462–475.
- Ploussard G, Daneshmand S, Efstathiou JA, et al. Critical analysis of bladder sparing with trimodal therapy in muscle-invasive bladder cancer: a systematic review. Eur Urol. 2014;66(1):120–137.
- Gomez-Millan J. Radiation therapy in the elderly: more side effects and complications? Crit Rev Oncol Hematol. 2009;71(1):70–78.
- Smaaland R, Akslen LA, Tonder B, et al. Radical radiation treatment of invasive and locally advanced bladder carcinoma in elderly patients. Br J Urol. 1991;67(1):61–69.
- Moonen L, Voet H, de Nijs R, et al. Muscle-invasive bladder cancer treated with external beam radiation: influence of total dose, overall treatment time, and treatment interruption on local control. Int J Radiat Oncol Biol Phys. 1998;42(3):525–530.
- Simera I, Moher D, Hoey J, et al. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40(1):35–53.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- van Kuilenburg AB. Dihydropyrimidine dehydrogenase and the efficacy and toxicity of 5-fluorouracil. Eur J Cancer. 2004;40(7):939–950.
- IKNL. Urotheelcelcarcinoom van de blaas, landelijke richtlijn versie 1.0. 2009. Available from: www.oncoline.nl
- Boustani J, Bertaut A, Galsky MD, et al. Radical cystectomy or bladder preservation with radiochemotherapy in elderly patients with muscle-invasive bladder cancer: retrospective international study of cancers of the urothelial tract (RISC) investigators. Acta Oncol. 2018;57(4):491–497.
- Williams SB, Shan Y, Jazzar U, et al. Comparing survival outcomes and costs associated with radical cystectomy and trimodal therapy for older adults with Muscle-Invasive bladder cancer. JAMA Surg. 2018;153(10):881–889.
- Korpics MC, Block AM, Martin B, et al. Concurrent chemotherapy is associated with improved survival in elderly patients with bladder cancer undergoing radiotherapy. Cancer. 2017;123(18):3524–3531.
- Bamias A, Tsantoulis P, Zilli TP, et al. Outcome of patients with nonmetastatic muscle-invasive bladder cancer not undergoing cystectomy after treatment with noncisplatin-based chemotherapy and/or radiotherapy: a retrospective analysis. Cancer Med. 2016;5(6):1098–1107.
- Bonet M, Bonfill T, Nunez M, et al. Curative radiation therapy for very elderly bladder cancer patients with localized disease. Clin Transl Oncol. 2018;20(7):899–905.
- Hammer L, Laufer M, Dotan Z, et al. Accelerated hypofractionated radiation therapy for elderly frail bladder cancer patients unfit for surgery or chemotherapy. Am J Clin Oncol. 2019;42(2):179–183.
- Lutkenhaus LJ, van Os RM, Bel A, et al. Clinical results of conformal versus intensity-modulated radiotherapy using a focal simultaneous boost for muscle-invasive bladder cancer in elderly or medically unfit patients. Radiat Oncol. 2016;11:45.
- McPherson VA, Rodrigues G, Bauman G, et al. Chemoradiotherapy in octogenarians as primary treatment for muscle-invasive bladder cancer. Can Urol Assoc J. 2017;11(1–2):24–30.
- Sakaguchi M, Maebayashi T, Aizawa T, et al. Clinical results for bladder cancer treated by radiotherapy without concurrent standard chemotherapy. Anticancer Res. 2016;36(10):5519–5525.
- Santacaterina A, Platania A, Palazzolo C, et al. Very elderly (>80 years), frail patients with muscle-invasive bladder cancer and comorbidities: is curative irradiation feasible? Tumori. 2015;101(6):609–613.
- Tran E, Souhami L, Tanguay S, et al. Bladder conservation treatment in the elderly population: results and prognostic factors of muscle-invasive bladder cancer. Am J Clin Oncol. 2009;32(4):333–337.
- Turgeon GA, Souhami L, Cury FL, et al. Hypofractionated intensity modulated radiation therapy in combined modality treatment for bladder preservation in elderly patients with invasive bladder cancer. Int J Radiat Oncol Biol Phys. 2014;88(2):326–331.
- Wujanto C, Tey J, Chia D, et al. Radical radiotherapy in older patients with muscle invasive bladder cancer. J Geriatr Oncol. 2019;10(2):292–297.
- Hollenbeck BK, Miller DC, Taub D, et al. Aggressive treatment for bladder cancer is associated with improved overall survival among patients 80 years old or older. Urology. 2004;64(2):292–297.
- Gray PJ, Fedewa SA, Shipley WU, et al. Use of potentially curative therapies for muscle-invasive bladder cancer in the United States: results from the national cancer data base. Eur Urol. 2013;63(5):823–829.
- VanderWalde NA, Chi MT, Hurria A, et al. Treatment of muscle invasive bladder cancer in the elderly: navigating the trade-offs of risk and benefit. World J Urol. 2016;34(1):3–11.
- Martini A, Sfakianos JP, Renström-Koskela L, et al. The natural history of untreated muscle-invasive bladder cancer. BJU Int. 2020;125(2):270–275.
- Kimura S, Mari A, Foerster B, et al. Prognostic value of concomitant carcinoma in situ in the radical cystectomy specimen: a systematic review and Meta-Analysis. J Urol. 2019;201(1):46–53.
- Chung PW, Bristow RG, Milosevic MF, et al. Long-term outcome of radiation-based conservation therapy for invasive bladder cancer. Urol Oncol. 2007;25(4):303–309.
- Ripping TM, Kiemeney LA, van Hoogstraten LMC, et al. Insight into bladder cancer care: study protocol of a large nationwide prospective cohort study (BlaZIB). BMC Cancer. 2020;20(1):455.
