Abstract
Purpose
This prospective study aims to assess the diagnostic test characteristics of Na[18F]F PET/CT for the skeletal staging of cancer in morbidly obese patients compared with 99mTc-methylene diphosphonate (MDP), whole-body planar (WBS), SPECT, and SPECT/CT acquisitions.
Material and methods
One hundred seventeen obese patients (BMI 46.5 ± 6.1 kg/m2 and mean age, 59.0 years; range 32–89 years) with BMI > 40 kg/m2 were prospectively enrolled and underwent [99mTc]Tc-MDP WBS, SPECT, SPECT/CT, and Na[18F]F PET/CT within two weeks for the osseous staging of a malignancy. Images were assessed qualitatively using a 3-point scale. Patient and lesion-based diagnostic test characteristics were estimated using an optimistic and pessimistic dichotomization method.
Results
Bone metastases were confirmed in 44 patients. Patient-based optimistic diagnostic test characteristics were (sensitivity, specificity, overall accuracy): Na[18F]F PET/CT (95.5%, 95.9%, 95.7%), [99mTc]Tc-MDP WBS (52.3%, 71.2%, 64.1%), SPECT (61.4%, 80.8%, 73.5%) and SPECT/CT (65.9%, 91.8%, 82.1%). Lesion-based optimistic diagnostic test characteristics were: Na[18F]F PET/CT (97.7%, 97.9%, 97.7%), [99mTc]Tc-MDP WBS (39%, 67%, 48.9%), SPECT (52.9%, 93.6%, 67.3%) and SPECT/CT (65.9%, 91.8%, 82.1%). There was no significant difference in the specificity of Na[18F]F and SPECT/CT. All other pairwise comparisons were significant (p<.001). ROC curve analysis showed a high overall accuracy of Na[18F]F with significantly higher AUCs for Na[18F]F PET/CT compared to [99mTc]Tc-MDP WBS, SPECT, and SPECT/CT on both patient and lesion-based analysis (p<.001). Moreover, Na[18F]F PET/CT changed patient management in 38% of patients.
Conclusions
Na[18F]F PET/CT may be the preferred imaging modality for skeletal staging in morbidly obese patients. The technique provides excellent diagnostic test characteristics superior to [99mTc]Tc-MDP bone scan (including SPECT/CT), impacts patient management, has an acceptable radiation exposure profile, and is well-tolerated. Further cost-effectiveness evaluations are warranted.
Introduction
Obesity is a substantial public health crisis [Citation1]. Its prevalence has been increasing rapidly, nearly tripling since 1975. In 2016, more than 1.9 billion adults aged 18 years and older were overweight. Of these, over 650 million adults were obese [Citation2]. It is estimated that 2.7 billion adults will be overweight, over 1 billion affected by obesity, and 177 million adults severely affected by obesity by 2025 [Citation3]. Obesity is responsible for approximately 20% of all malignancies, and it is an independent prognostic factor for developing distant metastases and death [Citation4]. Moreover, several biologic mechanisms contribute to a worse prognosis in obese patients with breast and other cancer [Citation5].
Traditionally, skeletal scintigraphy with 99mTc-labeled bisphosphonates is the mainstay imaging technique for screening for bone metastases [Citation6]. However, conventional bone scan has certain limitations due to the poor quality of images in obese patients secondary to high background soft tissue activity and attenuation [Citation7]. The use of Na[18F]F PET/CT has been demonstrated to be superior to conventional planar imaging due to its favorable pharmacokinetics. This technique is less susceptible to artifacts induced by body habitus and therefore retains its image quality and may offer superior diagnostic confidence. We have previously reported the high diagnostic accuracy of Na[18F]F PET/CT in obese patients to detect bone metastases in a retrospective analysis in morbidly obese patients [Citation8].
In this study, we prospectively evaluated and performed a head-to-head comparison of the diagnostic performance of Na[18F]F PET/CT with [99mTc]Tc-methylene diphosphonate (MDP) using planar whole-body (WBS), SPECT, and SPECT/CTacquisitions to detect bone metastases in morbidly obese patients.
Material and methods
Patient population
We prospectively recruited morbidly obese patients with a BMI ≥40 kg/m2 in whom skeletal staging was performed for solid tumors between January 2019 and February 2021: routine primary staging/restaging or when clinical suspicion of bone metastases prompted a new imaging workup (e.g., bone pain, elevated tumor marker, suspicious lesion(s) by a different imaging modality). All enrolled patients underwent [99mTc]Tc-MDP WBS, SPECT and SPECT/CT, and Na[18F]F PET/CT within two weeks. The local ethics committee approved the study (2018/1130), and informed consent was obtained from each patient. Patients with incomplete follow-up or missing scan-specific clinical data were excluded from the study. The study’s primary endpoint was to evaluate the sensitivity of Na[18F]F PET/CT to detect bone metastases in obese patients compared with conventional [99mTc]Tc-MDP bone scan. Other diagnostic test characteristics (specificity, positive predictive value [PPV], negative predictive value [NPV], positive likelihood ratio [PLR], negative likelihood ratio [NLR], and area under the curve [AUC]) served as supporting endpoints. Clinical follow-up of six months or longer was planned and used to determine the reference standard.
Body mass index (BMI)
Patient height and weight were measured and recorded at the time of the scan, in underclothes and without shoes. Based on this information, BMI was calculated as the body weight in kilograms divided by the height in square meters (kg/m2). The WHO criteria for appropriate BMI classification for an Asian population specifies class III or extreme obesity as a BMI ≥ 40 kg/m2 [Citation9].
Na[18F]F PET/CT
All patients were imaged on integrated PET/CT systems (Discovery 690 and 710, GE Healthcare). Images were acquired after intravenous (IV) injection of 2.2 MBq/kg (0.06 mCi/kg) Na[18F]F and a 60 to 90 min uptake period [Citation10,Citation11]. PET emission images were obtained in a three-dimensional mode at 3 min per bed position, from vertex to toes, and reconstructed with a standard iterative algorithm (ordered-subset expectation maximization, three iterative steps, and 32 subsets) and a filter cutoff of 6.4 mm as recommended by the manufacturer. A non-contrast-enhanced CT was performed using an auto tube current of 50–120 mA determined by an automated algorithm based on the planar view to achieve a noise index of 20, 140 kVp, and pitch 1.3. The CT axial images were reconstructed in a 512 × 512 matrix, with a thickness of 2.5 mm (Supplementary Table 1). The PET, CT, and fusion images were reviewed with Hermes Hybrid viewer version 2.2 on a PACS integrated workstation.
[99mTc]Tc-MDP scintigraphy
Whole-body scintigraphy, SPECT, and SPECT/CT imaging were performed 2–3 h after administration of 925–1110 MBq (25–30 mCi) [99mTc]Tc-MDP using a low-energy high resolution (LEHR) collimator with a matrix size of 256 × 1024 and a scan speed of 15 cm/minute using a dual-head gamma camera SPECT/CT system (Siemens Symbia T16) [Citation12]. If possible, SPECT images of the trunk were obtained (cervical region to the pelvis) using a two-bed position acquisition in the supine position with arms above the head. A 128 × 128 matrix size, non-circular orbit, 32 projections, 25 s per view were used, resulting in an imaging time of 26 min. SPECT data were reconstructed with an iterative technique (Flash3D, 8 iterations over 4 subsets). A low dose non-contrast-enhanced CT was performed using auto mAs (20−40 mAs), 120 kV, rotation time 0.6 sec, slice thickness 5 mm, pitch 1.5 (Supplementary Table 1) [Citation13]. SPECT/CT fusion images were reviewed with Hermes Hybrid viewer version 2.2 on a PACS integrated workstation.
Reference standard
Since a bone biopsy of all lesions for histology was not considered appropriate for practical and ethical reasons, the radiological and clinical follow-up of at least six months served as the reference standard comparator. Follow-up information included physical examination, laboratory tests, tumor markers, and other independent imaging studies (CT, MRI, and [18F]FDG PET/CT). For CT, the M.D. Anderson criteria were used for follow-up: an increase in the number of lesions and changes in CT characteristics (e.g., a lytic lesion changing to a blastic/sclerotic lesion or an increase in Hounsfield unit [HU] of the sclerotic lesion) were considered strong evidence of bone metastases [Citation14].
Image interpretation and data analysis
The [99mTc]Tc-MDP bone scan and Na[18F]F PET/CT studies were independently reviewed by two nuclear medicine specialists, who were blinded to all clinical details and results of other imaging methods. Any inter-observer disagreements were resolved by consensus reading. Each site of abnormal radiotracer uptake was graded using a three-point scale: 1 benign; 2 equivocal; 3 malignant based on the intensity of uptake, its anatomical location, and morphological features on CT using a previously published standardized reporting Scheme [Citation8]. Based on the CT-specific characteristics, the following lesions were considered benign: bone cysts, degenerative lesions (e.g., around joints), and uncomplicated fractures (without associated soft-tissue mass or cortical lysis). When the tracer uptake was localized to osteoblastic, osteolytic, or mixed lesions, the lesion was marked as metastatic or probably metastatic. Lesions with tracer uptake which were not typically benign or malignant on CT were considered as equivocal. Two thresholds were used: an “optimistic analysis” in which equivocal lesions were considered negative for bone metastasis and a “pessimistic analysis” in which equivocal lesions were considered positive for bone metastasis on a patient level. Images were analyzed using a patient-based and a lesion-based approach. Patients with widespread bone metastases (>15 lesions) on any modality were excluded from the lesion-based analysis for practical reasons, as identifying additional lesions would not have had clinical relevance. Because the skull and the appendicular skeleton are not included in the trunk SPECT/CT, it was not included in the lesion-based comparison.
Patient radiation dosimetry
The effective dose imparted by Na[18F]F (internal exposure) was calculated using the coefficient 0.089 mrem/mCi (0.024 mSv/MBq) according to ICRP publication 106 [Citation15]. The effective dose for [99mTc]Tc-MDP was calculated according to ICRP no. 80 [Citation16] using the coefficient 0.0057 (mSv/MBq). The volume CT Dose Index (CTDIvol) (mGy]) and Dose Length Product (mGy.cm) were directly obtained from the display screen of the CT workstation to estimate the effective dose from the CT scan (external exposure). The estimated effective dose was calculated by multiplying DLP (mGy.cm) with the ICRP conversion coefficient for the abdomen and pelvis in adults: k = 0.015 [mSv/(mGy.cm)] [Citation17].
Cost-effectiveness
The method described by Tateishi et al. was used to provide a crude estimate of the cost-effectiveness of Na[18F]F PET/CT [Citation18]. Briefly, effectiveness was defined as the proportion of correctly staged patients (i.e., accuracy). Costs were defined as the direct costs of diagnostic procedures from the payer’s perspective and based on the 2021 national average Medicare professional and technical fees extracted using the specific Current Procedural Terminology (CPT) codes for each modality. The average costs were $321 for [99mTc]Tc-MDP WBS, $728 for SPECT, $948 for SPECT/CT, and $1,443 for Na[18F]F PET/CT. Average cost-effectiveness ratios were calculated by dividing the expected costs by the expected effectiveness. Incremental cost-effectiveness ratios (ICER) were calculated by dividing the additional (marginal) expected costs by the additional (marginal) expected effectiveness.
Operator and Patient-Reported outcomes (PRO)
Using self-administered questionnaires, we evaluated patient comfort and experience for either modality. Performing technologists were also requested to complete a questionnaire to assess the convenience and difficulty of performing the scans in this patient population. Both questionnaires were administered electronically (Supplementary Table 2).
Statistical analysis
Using Monte-Carlo simulation, the required sample size was estimated to be 105 patients to have 80% power to detect a significant difference in the sensitivity between [99mTc]Tc-MDP WBS and Na[18F]F using a two-sided McNemar test and an alpha level of 5%. The sensitivity and specificity of [99mTc]Tc-MDP WBS were assumed to be 65% and 80%, and for Na[18F]F both 95%. The prevalence of metastatic disease was expected to be 30%.
Results are presented as mean ± standard deviation (SD). The sensitivity, specificity, PPV, NPV, PLR, NLR, and accuracy were calculated based on the results of the reference standard, together with exact 95% confidence intervals (CI). A weighted generalized score method and an omnibus Wald test were used to compare the overall test characteristics between imaging modalities [Citation19]. A pairwise comparison of individual test characteristics was performed using the McNemar test when significant differences were identified. Receiver operating characteristics (ROC) curve analysis, with an “optimistic” and “pessimistic” analysis, was performed separately for the imaging modalities and the values compared using DeLong’s test [Citation20]. Analyses were performed using the "compbdt", "mada", "meta", and "ROCit" commands in R (version 4.1.0) programming language [Citation21]. A Benjamini–Hochberg adjustment for false discovery rate was applied to the p-values for all pairwise comparisons of Na[18F]F with [99mTc]Tc-MDP using a threshold of 5% to account for multiple testing [Citation22]. Other comparisons are reported as nominal p-values. All tests were two-sided with significance at p ≤ .05.
Results
Study population
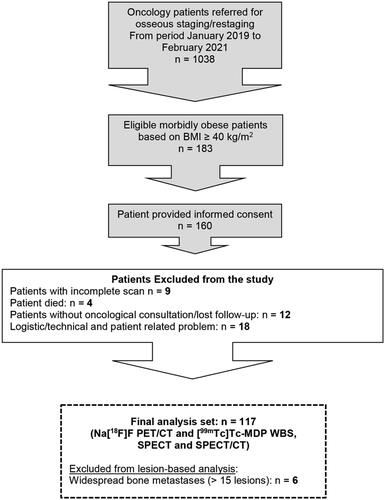
A total of 183 morbidly obese patients with a BMI ≥40 kg/m2 fulfilled the inclusion criteria, 160 provided informed consent, and 117 were evaluable (mean age, 59 years; range 32−89 years). For the lesion-based analysis, 6 patients with widespread disease were excluded, yielding 111 patients in this cohort (). A total of 266 scored lesions on any modality were evaluated (mean 2.4 lesions per patient; maximum of 12 lesions). The main reasons for drop-out were technical and patient-related: 18 subjects (e.g., production failure, suboptimal image quality due to radiotracer extravasation, SPECT/CT was not done due to patient high body habitus and if there is more than 2 week time difference between 2 tests). A summary of the main patient characteristics is presented in . The average follow-up period was 445 ± 119 days (median 210 days; range 182−820 days).
Table 1. Summary of main patient characteristics.
Patient-Based analysis
The reference standard classified 44 patients (38%) as positive for bone metastasis and 73 (62%) as negative (disease prevalence 37.6%; 95% CI 28.8−47.0%). In the patient-based analysis Na[18F]F PET/CT was negative in 69 (59%), equivocal in 3 (3%), and positive for bone metastasis in 45 (38%) patients (Supplementary Table 3). The individual patient test results and reference standard classification are shown in Supplementary Figure 1.
Using an optimistic cutoff, the sensitivity of Na[18F]F PET/CT, [99mTc]Tc-MDP WBS, SPECT, and SPECT/CT were 95.5%, 52.3%, 61.4 and 65.9%, and the specificity 95.9%, 71.2%, 80.8% and 91.8%, respectively. The PPV of Na[18F]F PET/CT, [99mTc]Tc-MDP WBS, SPECT, and SPECT/CT were 93.3%, 52.3%, 65.9%, 82.9%, the NPV 97.2%, 71.2%, 77.6, 81.7%, and the accuracy 95.7%, 64.1%, 73.5 and 82.1% respectively ().
Figure 2. Diagnostic test characteristics of Na[18F]F PET/CT, [99mTc]Tc-MDP WBS, SPECT, and SPECT/CT using a patient and lesion-based optimistic and pessimistic analysis (with 95% confidence intervals).
![Figure 2. Diagnostic test characteristics of Na[18F]F PET/CT, [99mTc]Tc-MDP WBS, SPECT, and SPECT/CT using a patient and lesion-based optimistic and pessimistic analysis (with 95% confidence intervals).](/cms/asset/5c9a374e-082c-48a0-9cf5-a14aef31d64f/ionc_a_2101899_f0002_b.jpg)
Using a pessimistic threshold, the sensitivity of Na[18F]F PET/CT, [99mTc]Tc-MDP WBS, SPECT, and SPECT/CT were 95.5%, 52.3%, 60.5 and 65.9%, and the specificity 91.8%, 57.5%, 60.3% and 84.9%, respectively. The PPV of Na[18F]F PET/CT, [99mTc]Tc-MDP WBS, SPECT, and SPECT/CT were 87.5%, 42.6%, 45.6%, 72.5%, the NPV 97.1, 66.7%, 73.4%, 80.5% and the accuracy were 93.2%, 55.6%, 62.4% and 77.8% respectively ().
The omnibus Wald test p-values were significant for all diagnostic test characteristics in both scenarios (Supplementary Table 4), justifying a follow-up pairwise comparison (). This demonstrated a statistically significant difference favoring the sensitivity, PPV, NPV, and accuracy of Na[18F]F PET/CT over [99mTc]Tc-MDP WBS, SPECT and SPECT/CT for both optimistic and pessimistic classification scenarios (all McNemar p < .001) (Supplementary Table 4). In contrast, there were no statistically significant differences between the specificity of Na[18F]F PET/CT and [99mTc]Tc-MDP SPECT/CT in the optimistic analysis.
Table 2. Pairwise comparison of the diagnostic test characteristics of Na[18F]F-PET/CT with [99mTc]Tc-MDP WBS, SPECT and SPECT/CT: p-values for the McNemar test (proportions) and DeLong test (AUC).
Lesion-Based analysis
Out of 266 lesions, 172 (65%) were bone metastases and 94 (35%) were benign according to the reference standard (disease prevalence 64.7%; 95% CI 58.6−70.0%). On Na[18F]F PET/CT 93 (35%) were classified as benign, 3 (1%) as equivocal and 170 (64%) as malignant. A significant reduction in the proportion of equivocal lesions was observed with Na[18F]F PET/CT (1.1%; 95% CI 0.2−3.3%) compared with [99mTc]Tc-MDP WBS (10.2%; 95% CI 6.8−14.4%; nominal p < .0001) and SPECT (16.9%; 95% CI 0.13−22.0%; nominal p < .001), but not with SPECT/CT (3.8%; 95% CI 1.9−6.8%; nominal p = .092).
Using the optimistic and pessimistic classification scenarios, the per-lesion sensitivity, specificity, PPV, NPV, and accuracy of Na[18F]F PET/CT and [99mTc]Tc-MDP acquisitions were in concordance with the patient-based analysis (), showing significant differences between acquisitions for all test characteristics (Supplementary Table 5). Follow-up pairwise comparisons are shown in and show a significant difference between sensitivity, PPV, NPV, and accuracy of Na[18F]F and WBS, SPECT and SPECT/CT on lesion-based analysis for both “optimistic” and “pessimistic” analyses (p < .001). There was no statistically significant difference between the specificity of Na[18F]F and SPECT/CT in the optimistic analysis.
ROC analysis
The patient-based ROC curve analysis showed a high area under the curve (AUC) for Na[18F]F in both classification scenarios (). For the optimistic analysis (), the AUC of Na[18F]F was 0.957 (95% CI 0.912−0.999), compared to [99mTc]Tc-MDP WBS 0.618 (95% CI 0.511−0.724), SPECT 0.711 (95% CI 0.610−0.812) and SPECT/CT 0.788 (95% CI 0.695−0.882). Similarly, for the pessimistic analysis (), the AUC was higher for Na[18F]F with 0.936 (95% CI 0.885−0.987), compared to [99mTc]Tc-MDP WBS 0.549 (95% CI 0.441−0.657), SPECT 0.629 (95% CI 0.524−0.734) and SPECT/CT 0.754 (95% CI 0.658−0.851). Concordant findings were observed for the lesion-based analysis (). Importantly, the AUC of Na[18F]F PET/CT was statistically superior to all other techniques using both analysis scenarios.
Figure 3. ROC curve of Na[18F]F PET/CT, [99mTc]Tc-MDP WBS, SPECT, and SPECT/CT using a patient and lesion-based optimistic and pessimistic analysis.
![Figure 3. ROC curve of Na[18F]F PET/CT, [99mTc]Tc-MDP WBS, SPECT, and SPECT/CT using a patient and lesion-based optimistic and pessimistic analysis.](/cms/asset/b79bd21e-1c91-496f-9ff1-358226d57bdd/ionc_a_2101899_f0003_c.jpg)
Table 3. Patient and lesion-based ROC curve analysis of Na[18F]F-PET/CT, [99mTc]Tc-MDP WBS, SPECT and SPECT/CT.
Impact on patient management
Na[18F]F PET/CT compared to WBS correctly up-staged 19 (16%; 95% CI 10−24%) patients and down-staged 26 (22%; 95% CI 15−31%) patients with an overall change in staging in 38% (95% CI 30−48%). For [99mTc]Tc-MDP SPECT/CT, these were 13 (11%) and 5 (4%) patients, respectively.
Patient radiation dosimetry
Patients were administered a mean activity of 248 ± 37 MBq (6.7 ± 1.0 mCi) of Na[18F]F, resulting in an estimated mean effective absorbed dose of 4.7 ± 0.7 mSv for PET imaging and 7.9 ± 1.7 mSv for the CT component. Patients were injected a mean activity of 1109 ± 28.1 MBq (30.0 ± 0.8 mCi) [99mTc]Tc-MDP. The effective dose of 1110 MBq (30 mCi) [99mTc]Tc-MDP bone scintigraphy in an obese adult is around 6.32 mSv and an additional 4–5 mSv for low dose CT incorporated in the SPECT/CT.
Cost-effectiveness
The cost-effectiveness ratio (i.e., the average cost per correctly staged patient) for [99mTc]Tc-MDP WBS was $501, for SPECT $990, for SPECT/CT $1,155, and for Na[18F]F PET/CT $1,508. When comparing to a scenario using [99mTc]Tc-MDP WBS, switching to [99mTc]Tc-MDP SPECT/CT or Na[18F]F PET/CT would be associated with an additional cost per additional correctly staged patient (ICER) of $3,483 and $3,551, respectively, and dominant over [99mTc]Tc-MDP SPECT $4,330.
Operator and Patient-Reported outcomes (PRO)
A total of 53 patients (47.7%) and 19 nuclear medicine technologists participated in the survey. The overall mean satisfaction score was 4.7/5.0 for Na[18F]F PET/CT and higher compared to 3.2/5.0 for [99mTc]Tc-MDP bone scan (Supplementary Table 6). The survey showed that the vast majority of respondents found Na[18F]F PET/CT more comfortable (94%; 95% CI 84−99%) and would prefer this modality for future studies (89%; 95% CI 78−96%), compared to [99mTc]Tc-MDP bone scan with 6% (95% CI 1−15%) and 11% (95% CI 4−22%) (all p < .001), respectively. For Na[18F]F PET/CT, 80% (95% CI 66−89%) were extremely satisfied, and 15% (95% CI 7 − 27%) were somewhat satisfied, while for [99mTc]Tc-MDP bone scan only 11% (95% CI 4 − 23%) were extremely satisfied. Among technologists, 84% (95% CI 60−97%) reported that Na[18F]F PET/CT was more convenient to perform and less prone to technical difficulties when imaging this patient population compared to [99mTc]Tc-MDP bone scan (89%; 95% CI 67−99%).
Discussion
Traditionally, imaging for evaluating osseous metastases is performed using [99mTc]Tc-MDP bone scan with a sensitivity ranging between 85% and 96% for detecting osseous metastasis in general patient cohorts [Citation23]. Subsequent technological advances, including SPECT and SPECT/CT, have improved the diagnostic test characteristics of whole-body planar imaging and are now considered best-practice in an oncological setting [Citation12,Citation13]. Nevertheless, it is well known in clinical practice that [99mTc]Tc-MDP bone scan in obese patients has poor quality due to high background soft tissue activity, scatter, and attenuation, even when using the latest hybrid acquisition protocols. Also, limitations in spatial resolution contribute to a lower sensitivity for detecting small osseous metastases [Citation24]. However, the diagnostic accuracy of [99mTc]Tc-MDP bone scan in the growing group of obese patients has not been appropriately studied.
The superior test characteristics for detecting osteoblastic metastases of Na[18F]F PET/CT compared to planar [99mTc]Tc-MDP imaging were initially demonstrated by Even-Sapir et al., showing a 30% benefit in sensitivity and 43% in specificity in an otherwise unselected high-risk population of prostate cancer patients [Citation25]. In the same study, the benefits of Na[18F]F PET/CT over SPECT were smaller, with improvements of 8% and 18%, respectively. When comparing with SPECT/CT, Chakraborty et al. [Citation26] studied Na[18F]F PET/CT in a cohort of patients with urinary bladder carcinoma, reporting a benefit of 12% and 13% in sensitivity and specificity with Na[18F]F PET/CT over [99mTc]Tc-MDP SPECT/CT, respectively. A recent meta-analysis of available studies by Liu et al. confirmed the consistently superior diagnostic performance of Na[18F]F PET/CT in detecting bone metastases on a patient and lesion basis [Citation27]. The pooled sensitivity and specificity were 93% (95% CI 91−96%) and 95% (95% CI 93−96%). Similar results were obtained by Sheikhbahaei et al. [Citation28] for Na[18F]F PET/CT in high-risk prostate cancer patients with a pooled sensitivity of 97% (95% CI 95−98%), but a somewhat lower pooled specificity of 84% (95% CI 81−87%). This analysis included a comparison with whole-body MRI (with diffusion-weighted imaging) and found a higher sensitivity (95% vs 83%) for Na[18F]F PET/CT, and comparable specificity (90% vs 90%) and AUC values (0.97 vs 0.94; p = .18).
In this prospective comparative study, we show that Na[18F]F PET/CT maintains its high sensitivity in morbidly obese patients and consistently outperforms the sensitivity of [99mTc]Tc-MDP WBS, SPECT, and SPECT/CT (all p < .001), regardless of the analysis scenario. Notably, the absolute benefit in sensitivity of Na[18F]F PET/CT over [99mTc]Tc-MDP WBS and SPECT/CT was 44% and 30%, respectively, and higher than the improvements with Na[18F]F PET/CT seen in a general cancer population compared to WBS (+30%) or SPECT/CT (+12%) as reported previously [Citation26,Citation27]. In a general population, double-bed SPECT/CT of the trunk improves the sensitivity and specificity of [99mTc]Tc-MDP bone scan by identifying unseen lesions and reclassifying areas of uptake associated with nonmalignant pathology, with impact on patient management [Citation13,Citation29,Citation30]. In our study, the sensitivity of [99mTc]Tc-MDP SPECT (61%; nominal p = .125) and SPECT/CT (66%; nominal p = .031) was higher compared to WBS (52%), but the residual 30% gap in sensitivity with Na[18F]F PET/CT (96%) remains clinically relevant (illustrative cases shown in Supplementary Figures 2 and 3). Evidently, the SPECT/CT technique cannot fully compensate for the loss of count statistics and resolution compared to Na[18F]F PET/CT in the morbidly obese [Citation31]. Possible explanations for this observation may include lower quality of SPECT images in obese patients due to the high scatter in soft tissues and increased photon attenuation in a larger body mass. Also, due to constraints in the upper limit of activity that can be administered, the required activity based on body weight (MBq/kg) necessary for an optimal SPECT/CT acquisition may not be achieved in morbidly obese patients [Citation32].
The finding of a superior sensitivity of Na[18F]F PET/CT was strengthened by the pairwise comparisons of the other diagnostic test characteristics, independent of using a patient-based or lesion-based analysis. Except for the specificity between Na[18F]F PET/CT (96%) and [99mTc]Tc-MDP SPECT/CT (92%) in the optimistic analysis scenario, all traditional diagnostic test characteristics of Na[18F]F PET/CT were significantly higher compared to those of [99mTc]Tc-MDP WBS, SPECT, and SPECT/CT. The similarity in the specificity between Na[18F]F PET/CT and [99mTc]Tc-MDP SPECT/CT was also reported by Fonager et al. [Citation33]. A possible explanation may be that both hybrid modalities improve their specificity mainly through accurate localization and characterization using CT [Citation34,Citation35]. In line with this hypothesis, the specificity of [99mTc]Tc-MDP SPECT/CT (92%) was significantly higher than that of [99mTc]Tc-MDP SPECT (81%; nominal p = .021) in the present study. However, it is also recognized that particular expertise is required in reading Na[18F]F studies because benign Na[18F]F uptake is commonly found in joints, vertebral endplates, osteophytes, and tendon insertions.
Even though the higher bone-to-background ratio of Na[18F]F improves the detection of photopenic lesions, smaller lytic bone metastases were missed in two patients on Na[18F]F PET/CT in our study. The lower sensitivity for strictly osteolytic or bone marrow disease is a well-known limitation of bone-targeted tracers, which require some degree of osteoblastic response for deposition at the site of metastases. For these lesions, the use of [18F]FDG is usually considered superior [Citation36].
It has been noted that the estimated effective dose and average cost-effective ratio were poorer for Na[18F]F PET/CT than for [99mTc]Tc-MDP bone scan [Citation18]. The radiation exposure from Na[18F]F administration was estimated to range from 2.7 to 15 mSv (270 to 1500 mrem) and significantly higher than that from [99mTc]Tc-MDP 4.2 to 5.7 mSv (420 to 570 mrem) [Citation18]. In this study, the exposure from Na[18F]F 4.7 mSv (470 mrem) compared favorably with that of [99mTc]Tc-MDP 6.3 mSv (630 mrem).
Considering the impact on patient management, the findings on Na[18F]F PET/CT changed the management in 38% of patients in this study, which is higher than reported in general populations (6%−12%) [Citation37]. In the U.S. National PET Oncology Registry Na[18F]F PET/CT impacted management in 12.2% of prostate cancer patients [Citation38,Citation39]. The higher impact on patient management in morbidly obese patients supports the priority use of Na[18F]F PET/CT in this subgroup and may improve its cost-effectiveness. In a crude analysis, the cost-effectiveness ratio of Na[18F]F PET/CT was $1,508 and within the range of $1,250 to $1,877 (inflated to 2021 USD) for Na[18F]F PET(/CT) reported in the updated meta-analysis by Tateishi et al [Citation40]. Interestingly, [99mTc]Tc-MDP SPECT/CT and Na[18F]F PET/CT had comparable ICERs (approximately $3,500) when compared to a scenario of [99mTc]Tc-MDP WBS. In contrast, a SPECT-only approach would result in higher costs and worse diagnostic performance. It should kept in mind that in cost effective analysis the prices are country and even center dependent and results may change accordingly. Further cost-effectiveness analyses are warranted to investigate the health-economic impact in morbidly obese patients fully.
Finally, the self-reported patient acceptance strongly favored Na[18F]F PET/CT in our study, with the vast majority of patients (89%) and technologists (84%) preferring this modality. In general, the Na[18F]F PET/CT was better tolerated than [99mTc]Tc-MDP SPECT/CT in morbidly obese patients when considering patient preparation, the acquisition time and the total time spent.
The following limitations of this study should be taken into account. Although the study used a prospective design, the single-center nature may have resulted in referral bias beyond our control. For the reference standard, we relied on available imaging and clinical follow-up as deemed appropriate by the treating physician. Therefore, not all patients received the same subsequent imaging studies to confirm PET/CT and SPECT/CT findings, which can introduce confirmation bias. While we studied the impact on patient management of Na[18F]F PET/CT findings, it is unknown whether this change resulted in improved humanistic outcomes that are traditionally considered the ultimate metric in healthcare technology assessments. Lastly, the methodology used to assess the cost-effectiveness of Na[18F]F PET/CT does not constitute a complete cost-effectiveness analysis and is merely presented for comparison purposes with the other published results using this method [Citation18,Citation41]. Lastly, we acknowledge that using ICRP conversion coefficients to estimate effective dose may result in an overestimation in obese patients [Citation42].
Conclusions
The results of this prospective study provide evidence that Na[18F]F PET/CT may be the preferred imaging modality for skeletal staging in morbidly obese patients. The technique provides excellent diagnostic test characteristics that are superior to [99mTc]Tc-MDP bone scan (including SPECT/CT) and impacts patient management. It has an acceptable radiation exposure profile, is a well-tolerated procedure and is preferred by patients compared to bone scan. Preliminary data suggest that the cost-effectiveness of Na[18F]F PET/CT is comparable to that of [99mTc]Tc-MDP SPECT/CT but with superior sensitivity and specificity in the studied population but further research is warranted to confirm subsequent improvements in clinical outcomes.
Ethical approval
All procedure performed in studies involving human participants were in accordance with the ethical standard of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
The local ethics committee approved the study and informed consent was obtained from each patient.
Supplemental Material
Download MS Word (24.4 MB)Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- WHO. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation. WHO Technical Report Series 894. Geneva: World Health Organization; 2000.
- World Health Organization. Obesity and Overweight. 2021. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- Prevalence of Obesity. World Obesity Federation. 2021. https://www.worldobesity.org/about/about-obesity/prevalence-of-obesity
- Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010;15(6):556–565.
- Sinicrope FA, Dannenberg AJ. Obesity and breast cancer prognosis: weight of the evidence. J Clin Oncol. 2011;29(1):4–7.
- Donohoe KJ, Cohen EJ, Giammarile F, et al. Appropriate use criteria for bone scintigraphy in prostate and breast cancer: Summary and excerpts. J Nucl Med. 2017;58(4):14N–17N.
- Weiner GM, Jenicke L, Mueller V, et al. Artifacts and non-osseous uptake in bone scintigraphy: imaging reports of 20 cases. Radiol Oncol. 2001;35:185–191.
- Usmani S, Marafi F, Ahmed N, et al. Diagnostic challenge of staging metastatic bone disease in the morbidly obese patients: a primary study evaluating the usefulness of 18F-Sodium fluoride (NaF) PET-CT. Clin Nucl Med. 2017;42(11):829–836.
- WHO expert consultation Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. The Lancet. 2004;363(9403):157–163.
- Segall G, Delbeke D, Stabin MG, et al. SNM practice guideline for sodium 18F-fluoride PET/CT bone scans 1.0. J Nucl Med. 2010;51(11):1813–1820.
- Beheshti M, Mottaghy FM, Paycha F, et al. 18 F-NaF PET/CT: EANM procedure guidelines for bone imaging. Eur J Nucl Med Mol Imaging. 2015;42(11):1767–1777.
- Van den Wyngaert T, Strobel K, Kampen WU, et al. The EANM practice guidelines for bone scintigraphy. Eur J Nucl Med Mol Imaging. 2016;43(9):1723–1738.
- Palmedo H, Marx C, Ebert A, et al. Whole-body SPECT/CT for bone scintigraphy: diagnostic value and effect on patient management in oncological patients. Eur J Nucl Med Mol Imaging. 2014;41(1):59–67.
- Hamaoka T, Costelloe CM, Madewell JE, et al. Tumour response interpretation with new tumour response criteria vs the world health organisation criteria in patients with bone-only metastatic breast cancer. Br J Cancer. 2010;102(4):651–657.
- Radiation dose to patients from radiopharmaceuticals. Addendum 3 to ICRP publication 53.ICRP publication 106. Approved by the commission in October 2007. Ann ICRP. 2008;38(1-2):1–197.
- ICRP Radiation dose to patients from radiopharmaceuticals (addendum to ICRP publication 53). ICRP publication 80. Ann ICRP. 1998;28:1–126.
- Christner J, Kofler J, McCollough CH. Estimating effective dose for CT using dose-length product compared with using organ doses: consequences of adopting international commission on radiological protection publication 103 or dual-energy scanning. AJR Am J Roentgenol. 2010;194(4):881–889.
- Tateishi U, Morita S, Taguri M, et al. A eta-analysis of 18F-fluoride positron emission tomography for assessment of metastatic bone tumor. Ann Nucl Med. 2010;24(7):523–531.
- Roldán-Nofuentes JA. Compbdt: an R program to compare two binary diagnostic tests subject to a paired design. BMC Med Res Methodol. 2020;20(1):143.
- DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845.
- Shim SR, Kim SJ, Lee J. Diagnostic test accuracy: application and practice using R software. Epidemiol Health. 2019;41:e2019007.
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc. Ser B (Methodol). 1995;57(1):289–300.
- Azad GK, Taylor B, Rubello D, et al. Molecular and functional imaging of bone metastases in breast and prostate cancers: an overview. Clin Nucl Med. 2016;41(1):e44– 50–e50.
- Schirrmeister H, Guhlmann A, Elsner K, et al. Sensitivity in detecting osseous lesions depends on anatomic localization: planar bone scintigraphy versus 18F PET. J Nucl Med. 1999;40(10):1623–1629.
- Even-Sapir E, Metser U, Mishani E, et al. The detection of bone metastases in patients with high-risk prostate cancer: 99mTc-MDP planar bone scintigraphy, single- and multi-field-of-view SPECT, 18F-fluoride PET, and 18F-fluoride PET/CT. J Nucl Med. 2006;47:287–297.
- Chakraborty D, Bhattacharya A, Mete UK, et al. Comparison of 18F fluoride PET/CT and 99mTc-MDP bone scan in the detection of skeletal metastases in urinary bladder carcinoma. Clin Nucl Med. 2013;38(8):616–621.
- Liu Y, Sheng J, Dong Z, et al. The diagnostic performance of 18F-fluoride PET/CT in bone metastases detection: a meta-analysis. Clin Radiol. 2019;74(3):196–206.
- Sheikhbahaei S, Jones KM, Werner RA, et al. 18F-NaF-PET/CT for the detection of bone metastasis in prostate cancer: a meta-analysis of diagnostic accuracy studies. Ann Nucl Med. 2019;33(5):351–361.
- Guezennec C, Keromnes N, Robin P, et al. Incremental diagnostic utility of systematic double-bed SPECT/CT for bone scintigraphy in initial staging of cancer patients. Cancer Imaging. 2017;17(1):16.
- Rager O, Nkoulou R, Exquis N, et al. Whole-Body SPECT/CT versus planar bone scan with targeted SPECT/CT for metastatic workup. Biomed Res Int. 2017;2017:7039406.
- Keidar Z, Israel O, Krausz Y. SPECT/CT in tumor imaging: technical aspects and clinical applications. Semin Nucl Med. 2003;33(3):205–218.
- Schirrmeister H, Glatting G, Hetzel J, et al. Prospective evaluation of the clinical value of planar bone scans, SPECT, and (18)F-labeled NaF PET in newly diagnosed lung cancer. J Nucl Med. 2001;42(12):1800–1804.
- Fonager RF, Zacho HD, Langkilde NC, et al. Diagnostic test accuracy study of (18)F-sodium fluoride PET/CT, (99 m)Tc-labelled diphosphonate SPECT/CT, and planar bone scintigraphy for diagnosis of bone metastases in newly diagnosed, high-risk prostate cancer. Am J Nucl Med Mol Imaging. 2017;7:218–227.
- Even-Sapir E, Keidar Z, Bar-Shalom R. Hybrid imaging (SPECT/CT and PET/CT)–improving the diagnostic accuracy of functional/metabolic and anatomic imaging. Semin Nucl Med. 2009;39(4):264–275.
- Strobel K, Burger C, Seifert B, et al. Characterization of focal bone lesions in the axial skeleton: performance of planar bone scintigraphy compared with SPECT and SPECT fused with CT. AJR Am J Roentgenol. 2007;188(5):W467–W474.
- Basu S, Alavi A. Bone marrow and not bone is the primary site for skeletal metastasis: critical role of [18F] fluorodeoxyglucose positron emission tomography in this setting. J Clin Oncol. 2007;25(10):1297–1299.
- Gauthé M, Aveline C, Lecouvet F, et al. Impact of sodium 18F-fluoride PET/CT, 18F-fluorocholine PET/CT and whole-body diffusion-weighted MRI on the management of patients with prostate cancer suspicious for metastasis: a prospective multicentre study. World J Urol. 2019;37(8):1587–1595.
- Hillner BE, Siegel BA, Hanna L, et al. Impact of 18F-fluoride PET in patients with known prostate cancer: initial results from the national oncologic PET registry. J Nucl Med. 2014;55(4):574–581.
- Hillner BE, Siegel BA, Hanna L, et al. Impact of (18)F-fluoride PET on intended management of patients with cancers other than prostate cancer: results from the national oncologic PET registry. J Nucl Med. 2014;55(7):1054–1061.
- Tateishi U, Morita S, Inoue T. Diagnostic accuracy of 18F-fluoride PET and PET/CT in patients with bone metastases: a systematic review and meta-analysis update. Clin Transl Imaging. 2013;1(2):123–134.
- Hetzel M, Arslandemir C, König HH, et al. F-18 NaF PET for detection of bone metastases in lung cancer: accuracy, cost-effectiveness, and impact on patient management. J Bone Miner Res. 2003;18(12):2206–2214.
- Liang B, Gao Y, Chen Z, et al. Evaluation of effective dose from CT scans for overweight and obese adult patients using the virtual dose software. Radiat Prot Dosimetry. 2017;174(2):216–225.