Abstract
Introduction
The aim of this study was to investigate phase IV efficacy, of the PD-1 inhibitor nivolumab among an unselected and unbiased national cohort of recurrent/metastatic Head and Neck Squamous Cell Carcinoma (rmHNSCC) patients.
Material and Methods
Inclusion criteria included histologically confirmed rmHNSCC and nivolumab as a second-line palliative treatment. Data were collected from patient files at the five Danish head and neck cancer centers and from the DAHANCA database. The iRECIST criteria were used for treatment evaluation.
Endpoints were response rate (RR), overall survival (OS), and progression-free survival (PFS), calculated from the start of treatment to the date of event/censoring by the KM-method. Descriptive statistics were used to describe patients and treatment. Analyses were two-sided, with p < .05 considered significant.
Results
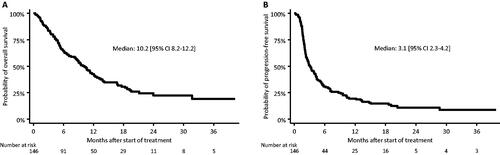
A total of 146 patients were identified in the period 2017–2020. They had a RR of 14%, median OS of 10.2 months [95% CI: 8.2–12.2] and median PFS of 3.1 months [95% CI: 2.3–4.2]. Patient age (≥ 70 years) or comorbidity did not significantly affect outcome. WHO performance status (PS) =1 was associated with an increased risk of death (HR: 2.1 [95% CI: 1.2–4.0], p = .02) and progression (HR: 1.9 [95% CI: 1.2–3.2], p = .01). Concomitant glucocorticoid-treatment during immunotherapy (≥ 50% of treatment time) appeared important for risk of death (HR: 6.4 [95% CI: 2.3–17.8], p < .001) and risk of progression (HR: 4.8 [95% CI: 1.8–12.5], p = .001). PD-L1 expression ≥ 20% was associated with a lowered risk of progression (HR: 0.5 [95% CI: 0.3–0.7], p = .001), but not lowered risk of death.
Conclusion
In this unselected national cohort, outcome of second-line treatment reflects data from the registration studies. Furthermore, the results suggest that immunotherapy should be used with great care in treatment of rmHNSCC in patients with poor performance.
Background
Until recently, the preferred systemic treatment option for recurrent or metastatic head and neck squamous cell carcinoma (rmHNSCC) was based on the use of classic anti-neoplastic drugs, but this was often associated with acute side effects that affected the patient’s quality of life [Citation1] and with low response rates [Citation2]. In Denmark the treatment of choice as first-line systemic palliative treatment is paclitaxel in combination with capecitabine [Citation3], whereas the second-line treatment is nivolumab for PD-L1 positive tumors and chemotherapy after the physicians choice, or cetuximab in PD-L1 negative tumors.
Treatment with PD-1 inhibitors is a new and promising palliative treatment for rmHNSCC often with lower morbidity than classical chemotherapy [Citation4,Citation5]. Common side effects related to PD-1 inhibition include fatigue, nausea, rash, decreased appetite, and pruritus [Citation4]. However, the available studies investigating the efficacy of PD-1 inhibitors have only included a highly selected group of relatively young patients in good general condition, and these do not necessarily reflect the typical real-life patient. Such patients are often older, present with substantial comorbidity, as well as impaired WHO performance status [Citation6]. Furthermore, the difference in treatment response appears to differ greatly between patients [Citation4]. For this reason, identification of clinical, as well as biological, factors affecting the individual response to the treatment would be valuable for patient selection.
Thus, this study was designed to evaluate the benefit of the immune checkpoint inhibitor, nivolumab, as second-line treatment, in a national multicenter, unselected and unbiased population of Danish patients with recurrent or metastatic head and neck squamous cell carcinoma. The aim was to determine which clinical factors, if any, that affected the individual benefit of this treatment. The factors investigated in this study included patient age, WHO performance status, comorbidity, PD-L1 score, concomitant glucocorticoid treatment, and neutrophilia.
Material and methods
Inclusion
Patients were initially identified using the DAHANCA database. The cohort was then supplemented with additional patients identified by each institution through cross-referencing ICD-10 codes (describing diagnosis) with SKS-codes (describing treatment). Patients were eligible if they had histologically confirmed rmHNSCC and had received nivolumab as second-line treatment. The inclusion period was 2017, where nivolumab was introduced as treatment for rmHNSCC, through to 2020, where immunotherapy with pembrolizumab became the first-line systemic standard for PD-L1 positive tumors.
Treatment
Nivolumab was used as second-line treatment in palliative treatment of PD-L1 positive rmHNSCC. It was administered intravenously every 14 days with a dose of 3 mg/kg bodyweight.
Patients were expected to have received primary palliative treatment for rmHNSCC that typically included combination chemotherapy consisting of paclitaxel and capecitabine up to 6 treatment cycles or disease progression. In other rarer cases, patients received cisplatin, 5FU and cetuximab.
Radiological assessment
The best response achieved by the treatment (defined by iRECIST criteria [Citation7]), and the date when that occurred was determined using the routine follow-up status report CT scans (radiologist evaluated) performed roughly every 2 months during treatment. Furthermore, whether patients’ disease was metastatic or locally advanced at baseline was registered. Data relating to radiology was obtained through the radiology system on site (Impax™ by Agfa Healthcare Inc.).
PD-l1
The immunohistochemical PD-L1 expressions (predominantly evaluated as tumor proportion score; TPS) were obtained through the national online pathology system on site (Patobank). Pathologist reevaluations were completed in cases where a PD-L1 score was missing from the Patobank system and if tumor tissue was available. TPS was evaluated according to the instructions from the manufacturer (PD-L1 28-8 pharmDx kit from Agilent Technologies, Inc.).
Patient characteristics
Patient and treatment related data were retrospectively obtained from patient files at the five head and neck cancer centers in Denmark that utilized immune checkpoint inhibitors as treatment for rmHNSCC. Treatment related data were obtained on site through medical records and included date of initiation and discontinuation of immunotherapeutic treatment, number of cycles administered, reason for discontinuation of treatment and WHO performance status (WHO PS). Data regarding the initial treatment of the patients’ primary disease, and comorbidity, were obtained from the DAHANCA registries [Citation8]. Information on comorbidity, in relation to the Charlson comorbidity index [Citation9], at the time of primary disease was then cross-referenced with the patients’ medical journals to accurately describe comorbidity at time of starting immunotherapy.
Previous work have shown that not all parameters in the Charlson comorbidity index are of importance for the survival of head and neck cancer patients, since the index was originally developed for breast cancer patients. Thus, a modified version of the system has been developed to better fit the patients in question [Citation6]. The DAHANCA modified comorbidity index include fewer diseases, but all of which are proven to negatively affect overall survival [Citation10]. These six groups of comorbidities are congestive heart failure, cerebrovascular disease, chronic pulmonary disease, peptic ulcer disease, liver disease, and diabetes. Both Charlson indexes were utilized.
Since glucocorticoids are renowned for their inhibitory effects on the immune system, it can be hypothesized that glucocorticoids might limit the effect of immunotherapy.
Therefore, the number of days each patient had received glucocorticoids (independent of dosage) during the immunotherapeutic treatment was calculated from the medicine charts. Blood neutrophil granulocyte count at baseline was obtained from the electronic medical records systems on site, because it previously has been speculated to exhibit predictive properties in PD-1 treatment of other types of advanced cancer [Citation11].
Variables
When comparing patients in terms of age, the cohort was divided into two groups depending on whether the age at the start of immunotherapy was either < or ≥ 70 years of age. This specific age was chosen because patients can generally be considered elderly and frail after having reached this age. For comparing patients with regard to WHO PS at baseline, those that had WHO PS = 0 were compared to those with WHO PS = 1 and WHO PS ≥ 2.
To determine the influence of patient comorbidity at baseline, the patient cohort was divided into two groups, with one having little to no comorbidity at the start of immunotherapy (Charlson < 2) and the other having significant comorbidity (Charlson ≥ 2). This division was used for analyses with both versions of the Charlson comorbidity index.
The effects of a high versus a low PD-L1 score was investigated by comparing patients with a pretreatment biopsy PD-L1 score <20% with those ≥20%. The possible influence of concomitant glucocorticoid treatment during immunotherapy was determined by comparing patients who had received systemic treatment with glucocorticoids either < or ≥ 50% of the time they had received immunotherapy.
The importance of neutrophilia was investigated by comparing patients that were neutrophilic at baseline with those that were not. Neutrophilia was defined as a blood neutrophil count > 7.00*109/l, which is the generally accepted definition in Denmark.
Statistics
Descriptive statistics were used to describe patient population, tumor and treatment characteristics. The primary endpoint, overall survival (OS), was evaluated using the date treatment started with date of death or end of study (February 17. 2021). Progression-free survival (PFS) was evaluated using the same criteria, as well as time of disease progression - whichever came first. Survival curves were estimated by the Kaplan–Meier method, and the impact of potential prognostic variables on the primary endpoint was evaluated using the univariate Cox-regression model. A multivariate analysis was then carried out using the same variables as in the univariate analyses (excluding only the DAHANCA modified Charlson index). For all analyses, two-sided tests were performed and p-values <0.05 were considered statistically significant.
Ethics
Permission to conduct the study was granted by The Danish Health Data Board and the National Committee on Health Research Ethics [reference-ID: 1-10-72-65-20]. All data was treated confidentially and according to relevant regulations.
Results
Patients
From 2017 to 2020 a total of 146 Danish patients, who had received nivolumab as second-line treatment (outside clinical trials) of rmHNSCC, were identified. The patient cohort had a median age of 62 years (range 36–86) () and the median number of treatment cycles received was 5 (range 1–54) (cf. Supplementary Table 1 describing immunotherapeutic treatment and Supplementary Table 2 for data on primary treatment and site).
Table 1. Patient characteristics at baseline.
Overall efficacy
A median OS of 10.2 months [95% CI: 8.2–12.2] () and a median PFS of 3.1 months [95% CI: 2.3–4.2] () were observed. The iRECIST evaluated response rate (RR) was 14%, while the treatment effect (complete remission, partial remission or stable disease) was 46%.
Side effects
In 12 (8%) patients, treatment was discontinued due to side effects. The most frequent being colitis (n = 3), pneumonitis (n = 2), gastritis (n = 2), and arthralgia/myalgia (n = 2).
Patient age
Patients < 70 years of age had a median OS of 10.8 months [95% CI: 9.0–13.8], while for patients ≥ 70 years of age it was 5.9 months [95% CI: 4.5–11.3] (p = .03) (). Patients in the younger group had a median PFS of 3.8 months [95% CI: 2.5–4.6], while the older group had a median PFS of 2.5 months [95% CI: 1.8–3.4] (p = .03) (). In the multivariate analysis, patient age at start of immunotherapy appeared to have only a small significant impact on risk of death (p = .04) and risk of progression (p = .05) ().
Figure 2. Patient age at baseline: overall survival (A) and progression-free survival (B) and WHO PS at baseline: overall survival (C) and progression-free survival (D).
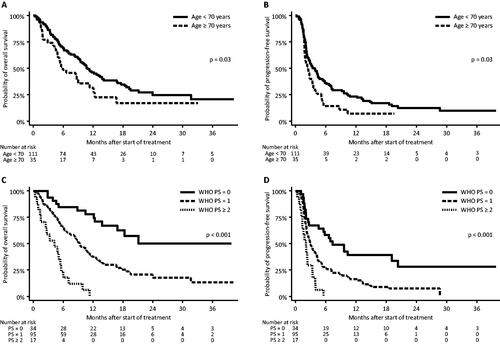
Table 2. Multivariate analysis.
Comorbidity
Comparing the two groups, no significant difference was found both in terms of OS and PFS (OS: p = .4/PFS: p = .4) (Supplementary Figure 1 A + 1B).
Re-doing the analyses, using the DAHANCA modified version of the index, did not change the results (OS: p = .4/PFS: p = .2) (Supplementary Figure 1 C + 1 D).
In the multivariate analysis, the influence of patient comorbidity (calculated using the traditional Charlson comorbidity index and the modified DAHANCA index) remained insignificant in both cases (p = .8/ p = .9) ().
WHO performance status
Patients having a WHO PS of 0 showed a 3-year OS of 8.8%. Median OS was not reached (), but we estimated it to be around 21 months. Patients with a WHO PS of 1 had a median OS of 9.3 months [95% CI: 7.6–11.4] and for patients with WHO PS of 2 or more the median OS was 4.2 months [95% CI: 1.3–5.6] (p < .001). In terms of progression, patients with WHO PS = 0 had a median PFS of 7.4 months [95% CI: 2.7–20.3], while for patients with WHO PS = 1 the median was 2.9 months [95% CI: 2.2–4.2] and 2.0 months [95% CI: 1.1–3.3] (p < .001) for patients with WHO PS ≥ 2 ().
In the multivariate analysis, patients with WHO PS = 1 presented an increased risk of both death (HR: 2.1 [95% CI: 1.2–4.0], p = .02) and progression (HR: 1.9 [95% CI: 1.2–3.2], p = .01), with risks being even higher for patients of WHO PS ≥ 2 (risk of death: HR: 6.5 [95% CI: 2.9–14.6], p < .001; risk of progression: HR: 3.3 [95% CI: 1.6–6.9], p = .001) (). The negative impact of WHO PS ≥ remained significant on both endpoints after correcting for multiple testing.
PD-l1
In terms of OS no difference was found (p = .4) (). In terms of PFS, patients with a PD-L1 score < 20% had a median PFS of 2.8 months [95% CI: 2.1–3.8], while those with a PD-L1 score ≥20% the median PFS was 4.2 months [95% CI: 2.3–7.4] (p = .003) ().
Figure 3. PD-L1 expression in the pretreatment biopsy: overall survival (A) and progression-free survival (B) and concomitant steroid treatment during immunotherapy: overall survival (C) and progression-free survival (D).
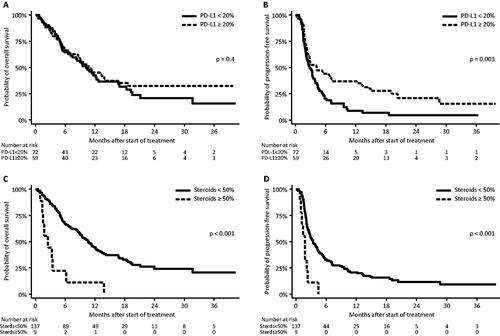
In the multivariate analysis, a PD-L1 expression ≥ 20% was associated with a lower risk of progression (HR: 0.5 [95% CI: 0.3–0.7], p = .001), but this did not influence the risk of death (p = .2). Correcting for multiple testing did not change the significance.
Concomitant steroid treatment and blood neutrophils
The group receiving concomitant corticosteroids for less than 50% of their immunotherapeutic treatment time had a median OS of 10.6 months [95% CI: 8.7–12.7] while the group that received it for 50% or more had a median OS of 2.5 months [95% CI: 1.0–6.2] (p < .001) (). Comparing these in terms of PFS, the < 50% group had a median PFS of 3.4 months [95% CI: 2.7–4.3], while the ≥ 50% group had a median PFS of 1.8 months [95% CI: 0.8–2.3] (p < .001) ().
In the multivariate analysis, concomitant steroid-treatment during immunotherapy (≥ 50% of the treatment time) still appeared to be of importance to both risk of death (HR: 6.4 [95% CI: 2.3–17.8], p < .001) and risk of progression (HR: 4.8 [95% CI: 1.8–12.5], p = .001) (). The effect of corticosteroid use remained significant on both endpoints after correcting for multiple testing.
A clear association between steroid treatment and increased blood neutrophil count (>7.00*109/l) was found (p = .009). Patients with a blood neutrophil count ≤ 7.00*109/l at baseline showed a median OS of 12.3 months [95% CI: 9.0–17.6], while those with an increased value at baseline presented with a median OS of 4.8 months [95% CI: 2.9–9.3] (p < .001) (Supplementary Figure 2 A). The same pattern emerged for PFS (Supplementary Figure 2B), where those without an increased cell count at baseline had a median PFS of 4.0 months [95% CI: 2.8–5.6], while for those with a cell count > 7.00*109/l it was 2.1 months [95% CI: 1.6–2.9] (p < .001).
In the multivariate analysis, blood neutrophil count at baseline remained significant (risk of death: HR: 2.0 [95% CI: 1.3–3.3], p = .003/risk of progression: HR: 1.8 [95% CI: 1.2–2.7], p = .007) ().
Discussion
Overall efficacy
In this study, a median OS of 10.2 months [95% CI: 8.2–12.2] and median PFS of 3.1 months [95% CI: 2.3–4.2] were observed. This is in line with the results from the phase III study that lead to FDA and EMA approval of nivolumab as second-line palliative treatment for rmHNSCC [Citation4] (median OS: 7.5 months [95% CI: 5.5–9.1]; median PFS: 2.0 months [95% CI: 1.9–2.1]). When comparing the efficacy of nivolumab to other second-line single-agent therapies (methotrexate, docetaxel or cetuximab), the median OS achieved using these drugs has been estimated to be 5.1 months [95% CI: 4.0–6.0] with a median PFS of 2.3 months [95% CI: 1.9–3.1] [Citation4]. Considering the confidence intervals, only the OS appears to be significantly higher. However, when comparing the PFS curves for nivolumab and standard therapy, the main difference appears to be the lack of long-term survivors in the standard therapy group, highlighting the importance of identifying predictive factors associated with PD-1 therapy to be better able to predict whether a patient will benefit from PD-1 inhibition.
The incidence of common mild side effects is not reported in the present study due to the retrospective nature of the study, which makes under-reporting of side effects very likely. However, side effects that lead to cessation of treatment is very likely to always be reported. Eight percent had their treatment terminated due to serious side effects which is less than reported with traditional chemotherapy [Citation3].
PD-L1 score
When comparing median PFS, split by the PD-L1 score of 20%, the data suggests a difference between the two groups (), although the overlapping confidence intervals of the medians, suggest they are likely to be equal (PD-L1 < 20% PFS: 2.8 months [95% CI: 2.1–3.8]; PD-L1 ≥ 20% PFS: 4.2 months [95% CI: 2.3–7.4], p = .003).
Variability in PD-L1 scoring procedure introduces some degree of imprecision. The data contain expressions reported predominantly by tumor proportion score, but also as combined positivity score. Since the combined positive score included the expression on both tumor cells as well as on immune cells, it will always be equal to, or higher than, the tumor positivity score. Generally, PD-L1 measurements are also associated with a certain degree of imprecision related to issues regarding the analytical validity of the PD-L1 staining, the spatial and temporal intra-tumoral heterogeneity (tendency for variation within the tumor and over time, respectively), as well as due to inter-observer variability [Citation12–14].
Due to the nature of this study, with a substantial part of data being gathered retrospectively from medical records, some imprecision is also to be expected with many of the other parameters, sometimes due to a certain degree of interpretation by the data collector.
General condition
A high WHO performance status was shown to have a high impact on OS and PFS in the multivariate analysis. Even though it might seem obvious that patients with a worse general condition generally have a higher mortality, this information is still of value. It indicates that a clinician should be selective when deciding whether a patient is eligible for immunotherapy despite the potential relative few side effects.
The same point can be made for both concomitant steroid usage and elevated blood neutrophil levels, which, in most cases, will arguably also be related to a worse general condition, and therefore, is associated with a poor treatment outcome. However, the fact that these remain significant in terms of both OS and PFS in the multivariate analysis, suggests that these factors might be involved in the immune response occurring with PD-1 therapy.
Perspectives
The low RR of 14%, alongside the median OS of 10 months, suggests a large variation in patients’ response to the treatment. While a small group of patients show a significant benefit from the treatment, a large number appears to experience no benefit at all (). Thus, more research is needed, due to the lack of well-proven prognostic and predictive factors. Furthermore, immunotherapy through PD-1 inhibition should not currently be considered a replacement for traditional chemotherapy, but instead a valuable alternative that can be offered to selected patients.
Conclusion
The present national cohort of patients treated with second-line nivolumab outside clinical trials showed a median OS of 10.2 months [95% CI: 8.2–12.2], a median PFS of 3.1 months [95% CI: 2.3–4.2], and a RR of 14%. These results are in line with what has been observed in phase III studies [Citation4]. Patient WHO PS at baseline was associated with treatment outcome and the results strongly suggest that immunotherapy should be used with great care in treatment of rmHNSCC in patients with a WHO PS of 2 or higher.
Supplemental Material
Download MS Word (49.3 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data available upon agreement with authors.
Additional information
Funding
References
- Vermorken JB, Mesia R, Rivera F, et al. Platinum-Based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–1127.
- Lala M, Chirovsky D, Cheng JD, et al. Clinical outcomes with therapies for previously treated recurrent/metastatic head-and-neck squamous cell carcinoma (R/M HNSCC): a systematic literature review. Oral Oncol. 2018;84:108–120.
- Bentzen JKD, Kristensen CA, Overgaard M, et al. A non platinum regimen for the treatment of recurrent or metastatic squamous cell carcinoma of the head and neck region. Results from an extended phase II study with paclitaxel and capecitabine. Front Oncol. 2018;8:243–243.
- Ferris RL, Blumenschein G, Fayette J, et al. Nivolumab for recurrent Squamous-Cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856–1867.
- Cohen EEW, Souliéres D, Tourneau CL, et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet. 2019;393(10167):156–167.
- Carlsen AH, Eriksen JG, Godballe C, et al. Impact of age, comorbidity, and WHO performance status on delay of treatment in patients undergoing fast-track work-up for head and neck cancer. J Geriatric Oncol. 2019;10(2):259–264.
- Seymour L, Bogaerts J, Perrone A, RECIST working group, et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–e152.
- Overgaard J, Jovanovic A, Godballe C, et al. The danish head and neck cancer database. Clin Epidemiol. 2016;8:491–496.
- Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619.
- Bøje CR, Dalton SO, Primdahl H, et al. Evaluation of comorbidity in 9388 head and neck cancer patients: a national cohort study from the DAHANCA database. Radiother Oncol. 2014;110(1):91–97.
- Soyano AE, Dholaria B, Marin-Acevedo JA, et al. Peripheral blood biomarkers correlate with outcomes in advanced non-small cell lung cancer patients treated with anti-PD-1 antibodies. J Immunother Cancer. 2018;6(1):129–129.
- Kim S, Koh J, Kwon D, et al. Comparative analysis of PD-L1 expression between primary and metastatic pulmonary adenocarcinomas. Euro J Cancer. 2017;75:141–149. (1990)
- Pinato DJ, Shiner RJ, White SDT, et al. Intra-tumoral heterogeneity in the expression of programmed-death (PD) ligands in isogeneic primary and metastatic lung cancer: Implications for immunotherapy. Oncoimmunology. 2016;5(9):e1213934-e1213934.
- Frank MS, Bødtger U, Høegholm A, et al. Re-biopsy after first line treatment in advanced NSCLC can reveal changes in PD-L1 expression. Lung Cancer. 2020;149:23–32.