Abstract
Objective
Triple-negative breast cancer (TNBC) is a subtype of breast cancer with a poor prognosis that seriously threatens women’s health. There is still a lack of effective therapeutic targets for TNBC treatment. We conducted a meta-analysis to evaluate the efficacy and safety of programmed cell death protein 1 (PD-1)/programmed death protein ligand 1 (PD-L1) inhibitors in combination with chemotherapy for TNBC patients.
Methods
We searched PubMed, EMBASE, Cochrane Library, and Web of Science for randomized controlled trials (RCTs) related to PD-1/PD-L1 inhibitors combined with chemotherapy. Literature conforming to the research content was identified according to the inclusion and exclusion criteria. The endpoints of efficacy were pathological complete response (pCR), event-free survival (EFS), progression-free survival (PFS), and overall survival (OS). Safety outcomes included adverse events (AEs) of any grade, AEs of grade ≥3, serious AEs, and the incidence of various AEs. We obtained odds ratios (OR), hazard ratio (HR), and 95% confidence interval (CI) for the included studies. Data analysis was performed using Review Manager software (version 5.3).
Results
A total of 4468 patients from eight RCTs were analyzed. PD-1/PD-L1 inhibitors in combination with chemotherapy significantly improved pCR (OR, 1.59; 95% CI, 1.28 − 1.98, p < 0.0001), EFS (HR, 0.66; 95% CI, 0.48 − 0.91, p = 0.01), and OS (HR, 0.72; 95% CI, 0.52 − 0.99, p = 0.05) in patients with TNBC compared to chemotherapy alone or placebo in combination with chemotherapy. Furthermore, we found that the pCR rate was almost identical in the PD-L1 positive group (OR, 1.65; 95% CI, 1.26 − 2.16, p = 0.0002) and the PD-L1 negative group (OR, 1.56; 95% CI, 1.04 − 2.33, p = 0.03). Among patients with advanced-stage TNBC, PFS (HR, 0.82; 95% CI, 0.74 − 0.90, p < 0.0001) was longer in the combination therapy group than in the chemotherapy group. There were no statistically significant differences between the experimental and control groups in OS (HR, 1.03; 95% CI, 0.74 − 1.42, p = 0.87). In terms of safety, we found that the combination therapy group had a significantly higher incidence of hyperthyroidism in patients with early and advanced TNBC (OR, 5.76; 95% CI, 2.38 − 13.95, p = 0.0001) (OR, 7.86; 95% CI, 2.65 − 23.29, p = 0.0002).
Conclusions
The combination of PD-1/PD-L1 inhibitors and chemotherapy could improve the survival and prognosis of patients with early and advanced TNBC. Combination treatment may be harmful to the thyroid; therefore, active surveillance and regular follow-up are necessary during treatment.
Background
Triple-negative breast cancer (TNBC) accounts for approximately 15 − 20% of all breast cancers and has a poor prognosis. Since the estrogen receptor, progesterone receptor, and human epidermal growth factor receptor-2 (HER-2) are all negative, there is a lack of effective therapeutic targets; therefore, endocrine therapy and targeted therapy are not applicable. TNBC is a malignant tumor with a high rate of recurrence and metastasis. Chemotherapy is still the fundamental therapy for patients with TNBC, while the median survival of patients with TNBC who are not sensitive to chemotherapy is only 10 − 12 months after recurrence or metastasis [Citation1,Citation2]. Therefore, there is an urgent need to identify novel therapeutic targets. The development of immunotherapy in recent years has increased the number of patients with TNBC. Tumor immunotherapy is the process of restoring, rebuilding, and enhancing the immune system so that it can recognize abnormal tumor cells and produce effector cells to kill tumor cells [Citation3,Citation4]. Immune checkpoint inhibitors (ICIs), such as anti-programmed cell death protein 1 (PD-1) and anti-programmed death protein ligand 1 (PD-L1), are the current popular treatment measures for TNBC. PD-L1 binds to the PD-1 protein. Binding of the PD-1 protein to T cells and the PD-L1 ligand to tumor cells inhibits the normal immune activity of T cells, leading to an immune system that is incapable of functioning. Through the combination of anti-PD-1/PD-L1 antibody with PD-1/PD-L1, the signal pathway of PD-1/PD-L1 is blocked, thus reducing the immune escape of tumor cells. Consequently, T cells can play their normal immune role and achieve the effect of killing cancer cells [Citation5,Citation6].
Although PD-1/PD-L1 inhibitors have been shown to achieve better efficacy in nonsmall cell lung cancer, malignant melanoma, and uroepithelial cancer, the efficacy and safety of ICIs in patients with TNBC are controversial [Citation7–9]. Only 10 − 30% of breast cancer patients have been reported to benefit from PD-1/PD-L1 inhibitors, and some patients may develop drug resistance and uncontrollable adverse events (AEs) as a result of the complexity of immune mechanisms [Citation4,Citation10]. Several clinical trials that focus on immunotherapy in combination with chemotherapy have achieved promising outcomes in TNBC; however, the results of these clinical trials have been inconsistent. IMpassion130 [Citation11] and Keynote-355 [Citation12] suggested that the combination of immunotherapy with chemotherapy could increase efficacy in patients with advanced TNBC compared with chemotherapy alone. However, IMpassion131 [Citation13] showed that atezolizumab combined with chemotherapy did not improve survival in patients with advanced TNBC. Therefore, it was essential to perform a meta-analysis of these studies. Previous meta-analyses have focused on TNBC in early stages [Citation14–16]. In this meta-analysis, we included all available randomized controlled trials (RCTs) to investigate early- and advanced-stage TNBC. This meta-analysis aimed to evaluate the efficacy and safety of PD-1/PD-L1 inhibitors combined with chemotherapy and to provide effective clinical diagnosis and treatment strategies.
Materials and methods
Data sources and search strategy
This systematic review and meta-analysis was reported following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) Statement and registered with INPLASY (registration number: 202250128) [Citation17]. We searched PubMed, EMBASE, Cochrane Library, and Web of Science in November 2021. The search strategy combines subject words and free words; it included ‘triple-negative breast neoplasms’ and free words, ‘immune checkpoint inhibitors,’ and free words. Furthermore, specific drug names of PD-1/PD-L1 inhibitors were searched simultaneously, such as ‘pembrolizumab,’ ‘atezolizumab,’ ‘durvalumab,’ ‘avelumab,’ and ‘nivolumab.’ According to McMaster University's search formula, the retrieval strategy of study type was ‘randomized controlled trial’ OR ‘randomized’ OR ‘placebo.’ The search was carried out with an ‘or’ between the subject words and free words; ‘and’ was used to connect between the subject words. Moreover, we screened references from reviews and meta-analyses related to ICIs to ensure the inclusion of all currently available RCTs. The literature search process was performed independently by two investigators. If there was disagreement, a third researcher decided until the results were unified.
Inclusion and exclusion criteria
The following inclusion criteria were used in this study: (1) patients with confirmed TNBC based on pathology; (2) the type of study was RCT; (3) the experimental group consisted of PD-1/PD-L1 inhibitors in combination with chemotherapy, and chemotherapy combined with or without placebo was administered to the control group; (4) the endpoints were pathological complete response (pCR), event-free survival (EFS), progression-free survival (PFS), or overall survival (OS); and 5) AEs associated with ICIs plus chemotherapy could be extracted.
Exclusion criteria were as follows: (1) non-RCTs; (2) patients had other subtypes of breast cancer; (3) studies included systematic reviews or animal studies; (4) available data and relevant outcomes could not be extracted; (5) studies were incomplete or were ongoing trials; and (6) full text was not available.
Data extraction
First, general information about the studies was extracted from the included RCTs, including experimental name, trial phase, first author, year of publication, number of experimental and control groups, tumor stage, study arm, control arm, dose and duration of PD-1/PD-L1 inhibitor, and positive rate of PD-L1 (PD-L1 positive was defined as PD-L1-expressing tumor-infiltrating immune cells covering 1% or more of the tumor area). Second, we extracted outcome indicators such as pCR, EFS, PFS, OS, hazard ratio (HR), 95% confidence interval (CI), and occurrence of AEs. Data extraction was performed independently by two investigators. A third researcher resolved the disagreements if necessary.
Literature quality evaluation
We perform a quality evaluation of the RCTs using the Cochrane Collaboration tool [Citation18]. The process was completed using Review Manager, version 5.3. The criteria used to assess the quality of the literature were as follows: (1) random sequence; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcomes; (5) incomplete outcome data; (6) selective reporting, and (7) other biases: ‘low risk,’ ‘high risk,’ and ‘unclear’ were made for each item. Two researchers independently assessed the quality of the literature and resolved differences through discussion until the results were unified.
Statistical analysis
Data analysis was performed using Review Manager software (version 5.3). Odds ratios (OR) and 95% CI were used to compare pCR and AEs between the two groups. We used HR and 95% CI to assess EFS, PFS, and OS. A P-value <0.05 was considered statistically significant. We judged heterogeneity according to the Cochran’s Q and I2 values. Cochran Q p < 0.1 or I2 ≥ 50% indicated heterogeneity among the included studies. We further performed subgroup or sensitivity analysis; otherwise, a random-effects model was used. Furthermore, the fixed-effects model was adopted when Cochran Q p > 0.1 and I2 < 50% [Citation19,Citation20].
Results
Literature search results
We retrieved 54, 283, 400, and 171 articles from PubMed, EMBASE, Cochrane Library, and Web of Science, respectively. A total of 908 studies were obtained. After eliminating the duplicate 364 articles in the literature, we further screened the remaining 544 studies. A total of 82 studies did not meet these requirements (animal studies, reviews, meta-analyses, etc.). Furthermore, another 326 articles were removed according to their irrelevant titles or abstracts, and 136 studies were downloaded in full text to determine whether they could be selected. Finally, we included eight RCTs after a comprehensive analysis and evaluation of the full text (eFigure 1 in the supplementary files).
Study characteristics and quality assessment
The characteristics of the eight studies [Citation11–13,Citation21–25] that were included in this study were shown in . A total of 4468 patients with TNBC were included, five RCTs used neoadjuvant chemotherapy (NACT), and three RCTs used advanced-stage treatment. Four studies used atezolizumab (PD-L1 inhibitor) and one RCT used durvalumab (PD-L1 inhibitor). The remaining three studies used pembrolizumab (PD-1 inhibitor). Two RCTs were open-label, and the remaining six RCTs were blinded. The pooled efficacy and safety endpoints were listed in . All studies were of high quality (eFigure 2 in the supplementary files).
Table 1. Characteristics of included studies.
Table 2. Pooled the endpoints of efficacy and safety.
Efficacy in early-stage TNBC
pCR
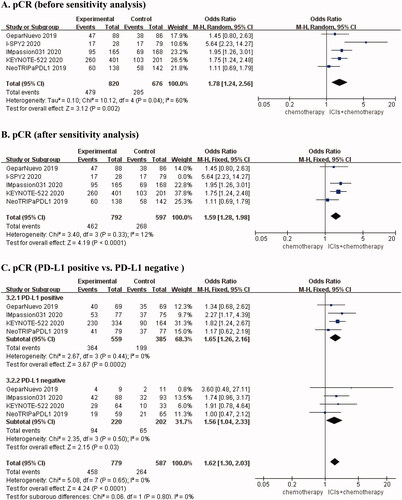
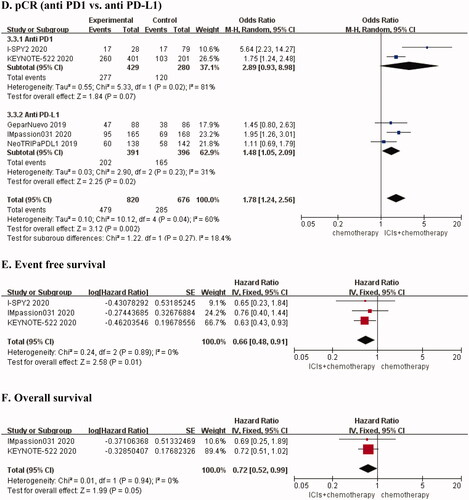
Pooled frequencies for pCR were shown in eTable 1. We clearly found that the pCR rate of the combination group was higher than that of the control group (eTable 1 in the supplementary files). Five RCTs [Citation21–25] reported pCR rates for combination therapy compared with chemotherapy alone, and the results suggested that there was obvious heterogeneity (I2 = 60%, p = 0.04) (), followed by sensitivity analysis. There was no heterogeneity (I2 = 12%, p = 0.33) after removing the I-SPY2 trial [Citation23]. We chose the fixed-effects model for the analysis. The results showed that the pCR rate was higher in the combination therapy group than in the chemotherapy group (OR, 1.59; 95% CI, 1.28 − 1.98, p < 0.0001) (). Furthermore, we performed a subgroup analysis based on the expression status of PD-L1 and the type of ICI. We found that there was no heterogeneity in the PD-L1 positive (I2 = 0%, p = 0.44) and PD-L1 negative groups (I2 = 0%, p = 0.50), and the fixed effect model was selected. Furthermore, we found that the PCR rate was almost identical in the PD-L1 positive group (OR, 1.65; 95% CI, 1.26 − 2.16, p = 0.0002) and the PD-L1 negative group (OR, 1.56; 95% CI, 1.04 − 2.33, p = 0.03) (). Additionally, we chose the random-effects model for statistical analysis in subgroups of different ICIs types. The results suggested that the anti-PD-1 group could significantly improve the pCR of patients with early TNBC (OR, 2.89; 95% CI, 0.93 − 8.98, p = 0.07). In the anti-PD-1 subgroup, the pCR of ICIs combined with chemotherapy was 2.89 times that of the control group. ().
Figure 1. Forest plots for outcomes in early triple-negative breast cancer. (A) OR for PCR (before sensitivity analysis), (B) OR for PCR (after sensitivity analysis), (C) Subgroup analysis for PCR according to different PD-L1 status (positive versus negative), (D) Subgroup analysis for PCR according to different types of interventions (anti PD1 versus anti PD-L1), (E) HR for EFS, (F) HR for OS.
EFS and OS
We found that a total of three RCTs [Citation22–24] studied the EFS of NACT without statistical heterogeneity (I2 = 0%, p = 0.89). The results showed that PD-1/PD-L1 inhibitors in combination with chemotherapy had better EFS than chemotherapy alone (HR, 0.66; 95% CI, 0.48 − 0.91, p = 0.01) (). OS was reported in two studies [Citation22,Citation24] on early-stage TNBC, and there was no heterogeneity (I2 = 0%, p = 0.94). We found that the risk of death in the combination treatment group was 72% of that of the chemotherapy alone group (HR, 0.72; 95% CI, 0.52 − 0.99, p = 0.05) ().
Efficacy in advanced TNBC
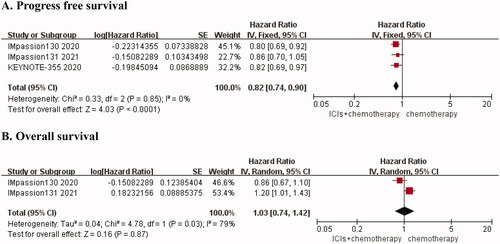
PFS and OS
Three of the included studies [Citation11–13] reported PFS rates in patients with advanced TNBC. There was no heterogeneity among the studies (I2 = 0, p = 0.85). We chose the fixed-effects model for the meta-analysis. To some extent, combination therapy can reduce disease progression in advanced TNBC. The risk of death or disease progression in the PD-1/PD-L1 inhibitor plus chemotherapy group was 82% of that in the chemotherapy group (HR, 0.82; 95% CI, 0.74 − 0.90, p < 0.0001) (). We found that two studies assessed OS in patients with advanced TNBC [Citation11,Citation13]. The heterogeneity analysis found I2 = 79%, p = 0.03, using the random-effects model for the pooled analysis. The results indicated that the combination of PD-1/PD-L1 inhibitors and chemotherapy did not improve OS in patients with advanced TNBC (HR, 1.03; 95% CI, 0.74 − 1.42, p = 0.87). The results were shown in .
Safety in early-stage TNBC
We analyzed the safety of PD-1/PD-L1 inhibitors in combination with chemotherapy compared with chemotherapy alone in patients with early-stage TNBC. The combination therapy group had a significantly increased incidence of hypothyroidism (OR, 4.59; 95% CI, 2.73 − 7.72, p < 0.00001), hyperthyroidism (OR, 5.76; 95% CI, 2.38 − 13.95, p = 0.0001), infusion reaction (OR, 1.66; 95% CI, 1.19 − 2.30, p = 0.003), and rash (OR, 1.33; 95% CI, 1.03 − 1.70, p = 0.03). Additionally, we found that grade ≥3 AEs in the combination therapy group were 1.31 times higher than those in the chemotherapy group (OR, 1.31; 95% CI, 1.05 − 1.64, p = 0.02) ().
Table 3. Pooled odds ratio for toxicities in early triple-negative breast cancer.
Safety in advanced TNBC
The incidence of AEs of any grade (OR, 1.48; 95% CI, 1.09 − 2.02, p = 0.01), grade ≥3 AEs (OR, 1.39; 95% CI, 1.15 − 1.70, p = 0.0009), hypothyroidism (OR, 0.05; 95% CI, 2.82 − 5.82, p = 0.00001), hyperthyroidism (OR, 7.86; 95% CI, 2.65 − 23.29, p = 0.0002), neutropenia (OR, 1.27; 95%CI 1.02 − 1.59, p = 0.04), and pneumonitis (OR, 3.77; 95% CI, 1.91 − 7.43, p = 0.0001) was higher in the combination group than in the chemotherapy group in patients with advanced TNBC (). We summarized the frequencies of toxicities and the results were shown in eTable 2 (eTable 2 in the supplementary files).
Table 4. Pooled odds ratio for toxicities in advanced triple-negative breast cancer.
Discussion
Current systemic therapies for TNBC are dominated by chemotherapy, such as paclitaxel, anthracyclines, and platinum. Currently, clinical treatments are limited, with few effective drugs available, and there is a lack of effective therapeutic targets for patients with TNBC who are not sensitive to chemotherapy or for advanced breast cancer patients with recurrence and metastasis. In recent years, with the advent of immunotherapy, more clinical trials of PD-1/PD-L1 inhibitors have been conducted [Citation26,Citation27]. PD-L1 is not expressed in normal epithelial tissues but is expressed in breast cancer cells and tumor-infiltrating lymphocytes (TIL). Furthermore, the expression of PD-L1 in TNBC is positively correlated with TILs [Citation28,Citation29]. TNBC has the highest expression of PD-L1 (approximately 20%) compared to other molecular subtypes. Relevant studies have also reported a correlation between PD-L1 expression and the efficacy of ICIs in various solid tumors, including breast cancer [Citation30]. Our study included all RCTs that met the criteria to investigate the efficacy and safety of PD-1/PD-L1 inhibitors plus chemotherapy compared to chemotherapy alone.
pCR and EFS in patients with early-stage TNBC
The CTNeoBC meta-analysis suggested that patients with TNBC who achieved pCR after NACT had a better prognosis than non-pCR patients using the same regimen, indicating that pCR is an important reference for chemotherapy sensitivity and prognosis in patients with TNBC [Citation31]. Our results showed that the pCR rates of PD-1/PD-L1 inhibitors combined with chemotherapy were higher than those of chemotherapy alone or chemotherapy combined with placebo in TNBC. In our initial meta-analysis of pCR, we found that the results produced strong heterogeneity and sensitivity analysis suggested that I-SPY2 [Citation23] was the main reason for this heterogeneity. We found that the small sample size of patients with TNBC included in this clinical trial might have impacted the results. We also performed a subgroup analysis of the pCR rates, splitting them into positive and negative groups according to the expression status of PD-L1, and the results indicated that the pCR rates did not correlate with the PD-L1 status. We found similar results in the studies by Michal et al. [Citation32] and Xin et al. [Citation14]. However, in a meta-analysis that included five RCTs of early TNBC that required NACT conducted by Paolo et al. [Citation16], the subgroup analysis showed that pCR rates were significantly related to PD-L1 status and PD-L1 positive patients could benefit from immunotherapy in combination with chemotherapy (OR, 1.65; 95% CI, 1.06 − 2.57), while the effect was not significant in the PD-L1 negative group. Similarly, we also performed a subgroup analysis of different types of ICIs, and the results showed that the use of PD-L1 inhibitors could improve pCR in the combined treatment group. No statistically significant differences were found between them in the anti-PD-1 group. Furthermore, subgroup analyses have shown that patients with TNBC with positive axillary lymph nodes would have a higher pCR rate with a regimen of ICIs combined with chemotherapy [Citation14]. From the included literature, the NeoTRIPaPDL1 trial [Citation21] indicated that the combination of atezolizumab, carboplatin, and nab-paclitaxel did not improve pCR rates, while IMpassion031 and KEYNOTE-522 showed positive results [Citation22,Citation24]. NeoTRIPaPDL1 did not add anthracycline to fundamental chemotherapy regimens, which may explain the difference in efficacy. Relevant studies have indicated that EFS has a prognostic value in patients with TNBC. Our meta-analysis included three RCTs and the results emphasized that the EFS was higher in the group treated with ICIs in combination with chemotherapy. Michal et al. [Citation32] and Xin et al. [Citation14] obtained similar findings. Whether pCR or EFS was used as the observed outcome, these results provided a new therapeutic target for NACT in patients with early-stage TNBC and found a new direction for chemotherapy-insensitive patients.
PFS and OS in advanced TNBC patients
Based on previous meta-analyses [Citation14–16,Citation32], none of them have analyzed the drug efficacy of advanced TNBC. The present analysis of three RCTs on advanced TNBC revealed that the efficacy of immunotherapy in combination with chemotherapy was stronger than that of chemotherapy in terms of PFS. Furthermore, it is suggested that ICIs combined with chemotherapy are valuable in the treatment of advanced TNBC and could provide a new therapeutic target for clinicians. Furthermore, in terms of OS, we realized that IMpassion131 [Citation13] was the main reason for the unstable outcomes. IMpassion131 was studied in unresectable locally advanced or metastatic triple-negative breast cancer (mTNBC), and the results showed that, compared to the application of paclitaxel alone, paclitaxel combined with atezolizumab did not affect patients' PFS. Moreover, the OS data showed a negative trend. In contrast, IMpassion130 [Citation11] showed that atezolizumab plus nab-paclitaxel improved PFS and OS in PD-L1 positive patients. The significant difference in the results of these two RCTs was the class of paclitaxel application and the use of conventional paclitaxel in the clinic required corticosteroids, which could be the main reason for the efficacy of immunotherapy. Our study showed that PD-1/PD-L1 inhibitors combined with chemotherapy could effectively improve PFS in patients with advanced TNBC. In patients with early-stage TNBC, the combination group showed improved OS, but in patients with advanced-stage TNBC, there were no statistically significant differences in OS between the two groups. Therefore, more clinical trials are needed to show the effect of immunotherapy on OS.
Safety of immunotherapy
Regarding safety, our results showed that immunotherapy combined with chemotherapy had the greatest effect on thyroid function in patients with early and advanced-stage TNBC. Therefore, it is important to monitor thyroid function when administering immunotherapy in combination with chemotherapy. Patients who develop endocrine disease may be irreversible and require long-term treatment [Citation33,Citation34]. Furthermore, the combination group was more likely to have pneumonitis, which was similar to the results of a meta-analysis by Zhang et al. [Citation35]. In addition, hepatitis, adrenal insufficiency, skin reactions, infusion reaction and pyrexia also deserve our attention in the combination therapy. Therefore, a comprehensive assessment of tumor conditions in cancer patients and the underlying physical fitness is necessary. Under the premise of ensuring safety, we should fully improve the ability of anticipated treatment to prevent withdrawal of patients due to serious AEs in the clinic.
Clinical implications
In this meta-analysis, the efficacy and safety of combination chemotherapy with PD-1/PD-L1 inhibitors were investigated and the results were promising. A combination of multiple ICIs and clinical trials of immunotherapy combined with radiotherapy or targeted therapy is being carried out, and the results are equally worthy of our expectations. With the advent of precision therapy, the classification of breast cancer is a future direction, and we believe that immunotherapy will provide a good prospect for cancer patients. To date, there is no standardized test for PD-L1 expression. Furthermore, the threshold for determining PD-L1 positivity has been inconsistent. It is a question we should consider which chemotherapeutic agents are used in combination with immunotherapy to achieve optimal efficacy. However, there are still many challenges in immunotherapy.
Study limitations
This study had some limitations. First, only eight studies were included, and the number of studies was limited. With the continuous development of clinical trials on ICIs plus chemotherapy, there will be more data for our research in the future. Second, due to the insufficient number of studies, publication bias was not evaluated. Despite these limitations, this meta-analysis provides objective evidence for the clinical treatment of the combination of chemotherapy with PD-1/PD-L1 inhibitors in patients with TNBC.
Conclusions
In conclusion, compared with chemotherapy alone, PD-1/PD-L1 inhibitors plus chemotherapy in NACT improved pCR rates, EFS, and OS in early-stage TNBC. Similarly, PD-1/PD-L1 inhibitors combined with chemotherapy can increase PFS in patients with advanced TNBC. Additionally, more attention should be paid to thyroid function in patients treated with PD-1/PD-L1 inhibitors.
Supplemental Material
Download MS Word (27.9 KB)Supplemental Material
Download MS Word (79 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Marra A, Viale G, Curigliano G. Recent advances in triple negative breast cancer: the immunotherapy era. BMC Med. 2019;17(1):90.
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–249.
- Keenan TE, Tolaney SM. Role of immunotherapy in Triple-Negative breast cancer. J Natl Compr Canc Netw. 2020;18(4):479–489.
- Andrews LP, Yano H, Vignali DAA. Inhibitory receptors and ligands beyond PD-1, PD-L1 and CTLA-4: breakthroughs or backups. Nat Immunol. 2019;20(11):1425–1434.
- Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov. 2018;8(9):1069–1086.
- Lotfinejad P, Kazemi T, Mokhtarzadeh A, et al. PD-1/PD-L1 axis importance and tumor microenvironment immune cells. Life Sci. 2020;259:118297.
- Tarhoni I, Wakefield CJ, Kollipara R, et al. Relationship between circulating tumor-associated autoantibodies and clinical outcomes in advanced-stage NSCLC patients receiving PD-1/-L1 directed immune checkpoint inhibition. J Immunol Methods. 2021;490:112956.
- Ibrahim T, Mateus C, Baz M, et al. Older melanoma patients aged 75 and above retain responsiveness to anti-PD1 therapy: results of a retrospective single-institution cohort study. Cancer Immunol Immunother. 2018;67(10):1571–1578.
- Bellmunt J, de Wit R, Vaughn DJ, KEYNOTE-045 Investigators, et al. Pembrolizumab as Second-Line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026.
- Hwang SY, Park S, Kwon Y. Recent therapeutic trends and promising targets in triple negative breast cancer. Pharmacol Ther. 2019;199:30–57.
- Schmid P, Rugo HS, Adams S, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Oncology. 2020;21(1):44–59.
- Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. The Lancet. 2020;396(10265):1817–1828.
- Miles D, Gligorov J, Andre F, IMpassion131 investigators, et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann Oncol. 2021;32(8):994–1004.
- Xin Y, Shen G, Zheng Y, et al. Immune checkpoint inhibitors plus neoadjuvant chemotherapy in early triple-negative breast cancer: a systematic review and Meta-analysis. BMC Cancer. 2021;21(1):1261.
- Marinelli D, Mazzotta M, Pizzuti L, et al. Neoadjuvant immune-checkpoint blockade in triple-negative breast cancer: current evidence and literature-based meta-analysis of randomized trials. Cancers (Basel. 2020;12(9):2497.
- Tarantino P, Gandini S, Trapani D, et al. Immunotherapy addition to neoadjuvant chemotherapy for early triple negative breast cancer: a systematic review and Meta-analysis of randomized clinical trials. Crit Rev Oncol Hematol. 2021;159:103223.
- Moher D, Liberati A, Tetzlaff J, PRISMA Group, et al. Preferred reporting items for systematic reviews and Meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–1012.
- Higgins JP, Altman DG, Gøtzsche PC, Cochrane Statistical Methods Group, et al. The cochrane collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928.
- DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28(2):105–114.
- Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560.
- Gianni L, Huang C-S, Egle D, et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple negative, early high-risk and locally advanced breast cancer. NeoTRIPaPDL1 Michelangelo randomized study. Can Res. 2020;80(4_Supplement):GS3-04–04.
- Mittendorf EA, Zhang H, Barrios CH, et al. Neoadjuvant atezolizumab in combination with sequential nab-paclitaxel and anthracycline-based chemotherapy versus placebo and chemotherapy in patients with early-stage triple-negative breast cancer (IMpassion031): a randomised, double-blind, phase 3 trial. The Lancet. 2020;396(10257):1090–1100.
- Nanda R, Liu MC, Yau C, et al. Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic complete response in women with Early-Stage breast cancer: an analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial. JAMA Oncol. 2020;6(5):676–684.
- Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early Triple-Negative breast cancer. N Engl J Med. 2020;382(9):810–821.
- Loibl S, Untch M, Burchardi N, et al. A randomised phase ii study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol. 2019;30(8):1279–1288.
- Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. 2020;11(1):3801.
- Zhu H, Du C, Yuan M, et al. PD-1/PD-L1 counterattack alliance: multiple strategies for treating triple-negative breast cancer. Drug Discov Today. 2020;25(9):1762–1771.
- Mittendorf EA, Philips AV, Meric-Bernstam F, et al. PD-L1 expression in triple-negative breast cancer. Cancer Immunol Res. 2014;2(4):361–370.
- Lotfinejad P, Asghari Jafarabadi M, Abdoli Shadbad M, et al. Prognostic role and clinical significance of Tumor-Infiltrating lymphocyte (TIL) and programmed death ligand 1 (PD-L1) expression in Triple-Negative breast cancer (TNBC): a systematic review and Meta-Analysis study. Diagnostics (Basel). 2020;10(9):704.
- AiErken N, Shi HJ, Zhou Y, et al. High PD-L1 expression is closely associated with Tumor-Infiltrating lymphocytes and leads to good clinical outcomes in chinese triple negative breast cancer patients. Int J Biol Sci. 2017;13(9):1172–1179.
- Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172.
- Sternschuss M, Yerushalmi R, Saleh RR, et al. Efficacy and safety of neoadjuvant immune checkpoint inhibitors in early-stage triple-negative breast cancer: a systematic review and Meta-analysis. J Cancer Res Clin Oncol. 2021;147(11):3369–3379.
- Elia G, Ferrari SM, Galdiero MR, et al. New insight in endocrine-related adverse events associated to immune checkpoint blockade. Best Pract Res Clin Endocrinol Metab. 2020;34(1):101370.
- Delaunay M, Caron P, Sibaud V, et al. [Toxicity of immune checkpoints inhibitors]. Rev Mal Respir. 2018;35(10):1028–1038.
- Zhang Y, La B, Liang B, et al. Treatment-related adverse events with PD-1 or PD-L1 inhibitors: a systematic review and meta-analysis. Life (Basel). 2021;11(11):1277.