Abstract
Background
Treatment of acute promyelocytic leukaemia has emerged as a major success in hemato-oncology. While literature from the developed world boasts of outstanding outcomes, there is a paucity of data from the developing world. This study aimed to assess complications and outcomes of acute promyelocytic leukaemia in a resource-constrained setting.
Methods
We retrospectively collected data from patients diagnosed with APL from January 2016 to December 2020.
Results
Sixty-four patients were treated-32 in both the Sanz high and low-risk groups. In the Sanz low-risk group, 12.5% of patients received ATRA with daunorubicin and 81.25% received ATRA with ATO. In the Sanz high-risk group, 18.8% of patients received ATRA with daunorubicin, 34.3% received ATRA with daunorubicin and ATO while 40.6% received ATRA with ATO. 56.25% of patients developed differentiation syndrome. The incidence was higher in Sanz high-risk group as compared to Sanz low-risk group. 57.4% of patients had an infection at the time of presentation. 62.5% of patients developed neutropenic fever during treatment. 17.2% of patients developed pseudotumor cerebri. The 4-year EFS and OS were 71.25 and 73.13%, respectively. Sanz low-risk group had a better 4-year EFS and OS as compared to the Sanz high-risk group. Haemoglobin at presentation and Sanz high-risk group were associated with poorer outcomes with a hazard ratio of 0.8 and 3.1, respectively. Outcomes in high-risk patients were better with the use of ATRA + ATO + daunorubicin.
Conclusion
In the Indian population, APL patients have a high incidence of differentiation syndrome, pseudotumor cerebri, and infections during induction. CR, EFS, and OS compared to the developed world can be achieved with optimal therapy. Low haemoglobin at presentation and Sanz high-risk group were associated with poorer outcomes. ATRA, ATO, and daunorubicin combination is the preferred protocol for treating high-risk patients.
Background
Acute Promyelocytic Leukaemia (APL) treatment has emerged as a major success in hemato-oncology, with long-term survival improving from a dismal 30% to over 90% [Citation1–3]. This is attributed to treatment with targeted therapies and timely and effective management of coagulopathy.
The introduction of All Trans Retinoic Acid (ATRA), which induces differentiation of leukemic promyelocytes into mature cells by targeting the pathogenic PML/RARA fusion protein, led to complete remission (CR) rates of 85% [Citation4]. The addition of arsenic trioxide (ATO) to regimens with a backbone of ATRA and chemotherapy has led to CR rates of 95–100% with long-term survival exceeding 90% [Citation2].
While literature from the trials in the developed world boasts of outstanding outcomes, there is a paucity of data on management strategies, complications, and outcomes from developing countries including India. With a large population still residing in rural areas, high infection rates at presentation and during neutropenic phase, limited access to healthcare and resulting delays in the presentation to hematologist/oncologist and starting treatment, arranging adequate financial as well as transfusion support are major hurdles to optimum outcomes of APL in the developing world.
Material and methods
We retrospectively reviewed the data of consecutive patients with newly diagnosed APL between 1 January 2016 and 31 December 2020 at the Department of Haematology, All India Institute of Medical Sciences, New Delhi, a tertiary care referral centre in India. A presumptive diagnosis of APL was made based on the presence of abnormal promyelocytes in peripheral blood or bone marrow morphology and flow cytometry (CD13, CD33 positive, HLA DR, CD34 negative). Patients were included in the study after confirmation of diagnosis by the presence of PML-RARA fusion gene by reverse transcriptase polymerase chain reaction (RT-PCR). To ensure the completeness of the study, inpatient records and laboratory immunophenotyping records were extracted. Patients were followed till 1 November 2021 and the median duration of follow-up was 678 days.
Treatment
Protocol (prior to 2018)—ATRA + daunorubicin
Induction—daunorubicin for three days and ATRA till remission induction was achieved or day +35 whichever is later. Consolidation—three cycles of daunorubicin for three days and ATRA for 2 weeks.
Maintenance—Eight 3-monthly cycles of ATRA from day 1 to 15, methotrexate once a week, and six Mercaptopurine once a day for the remainder of each cycle.
Protocol (2018 onwards)
Induction—ATRA + ATO protocol for low-risk patients: ATO and ATRA till remission induction was achieved or day +35 whichever is later. Daunorubicin + ATRA + ATO for high-risk patients: daunorubicin for three days intravenously, ATRA from day 1, and ATO from day 4 onwards continued till complete remission was achieved or day +35 whichever is later.
Consolidation—ATO 5 days/week for 4 weeks every 8 weeks for a total of four cycles + ATRA for 2 weeks every 4 weeks for a total of seven cycles.
Maintenance—Patients with low-risk disease who had received ATRA + daunorubicin-based induction and all patients with high-risk disease received maintenance therapy for 2 years, as described above.
Drug doses—ATO 0.15 mg/kg (maximum 10 mg per day), ATRA 45 mg/m2/day (25 mg/m2/day in paediatric age group), daunorubicin 60 mg/m2/day, Methotrexate 10–15 mg/m2, six Mercaptopurine 50 mg/m2.
Deviation from standard protocols—Standard anthracycline-containing protocols use idarubicin 12 mg/m2 on day 2, 4, 6, and 8 in addition to ATRA with or without ATO. We preferred to use daunrubicin 60 mg/m2 on day 1, 2, and 3. Due to greater experience with daunorubicin at our institution and ease of availability in/around the hospital as well as cost issues. The dose and duration of ATRA and ATO used in our protocols match that of published protocols.
Supportive care
Hydration at 3 L/m2 and allopurinol 300 mg OD for 7 days were given for the prevention of tumour lysis syndrome (TLS). Patients also received rasburicase 0.2 mg/kg for upto 5 days in case of TLS or total leukocyte count (TLC) ≥100,000/μl. Cryoprecipitate or fresh frozen plasma was administered to maintain fibrinogen ≥150 mg/dl. Platelets were transfused to keep platelet count ≥30,000/μl and ≥1,00,000/μl in case of intracranial bleeding. All patients received prophylactic acyclovir 400 mg PO BID, levofloxacin 500 mg PO OD, and syrup posaconazole 200 mg TIDPediatric patients received cefixime PO 8 mg/kg/day, intravenous liposomal amphotericin B 1 mg/kg/day and acyclovir 200–400mg PO BID.
Febrile neutropenia (FN) was treated as per department policy. Patients received dexamethasone 10 mg twice a day if they developed differentiation syndrome (DS). Tapering and discontinuation of dexamethasone therapy were decided based on clinical improvement. ATRA and ATO were discontinued if DS was severe or as per physician discretion and reintroduced gradually. Patients receiving ATO were monitored to maintain QTc <500 ms and potassium >4 mEq/L and magnesium ≥1.8 mg/dl.
Definitions
DS: Presence of at least two of the following findings: breathlessness, unexplained fever, pulmonary infiltrates, weight gain, pleuro-pericardial effusion, and renal failure. DS was defined as severe if severe dyspnoea, hypotension, or renal dysfunction were present.
Pseudotumor cerebri was ‘suspected’ if the patient developed severe headache, nausea/vomiting, papilledema, retinal haemorrhages, visual changes, and/or vision loss.
Drug-induced hepatotoxicity: increase in serum bilirubin or alkaline phosphatase or ALT >3 times upper limit of normal.
Event free survival (EFS): time to any event (relapse or death) from the time of diagnosis.
Overall Survival (OS) was defined as the duration from the time of diagnosis to the time of death due to any cause. Symptoms included fever, bleeding manifestations, bone pains, and fatigue.
Statistical analysis
Categorical variables were presented as number and percentage (%) and continuous variables as mean ± standard deviation and median values with range. The data normality was checked by using Kolmogorov–Smirnov test. Quantitative variables were analysed using Mann-Whitney Test (for not normally distributed data). Qualitative variables were analysed using Fisher’s Exact test. Kaplan Meier survival analysis curve was used to find the EFS and OS, log-rank test was used for comparison. Univariate and multivariate cox proportional hazard regression was used to find out significant risk factors of events. The final analysis was done with the use of Statistical Package for Social Sciences (SPSS) software ver 21.0. p-Value of <0.05 was considered as significant.
Results
Patient characteristics
At presentation, 57.4% () of patients had an infection, the most common focus being lungs in 27 patients, skin and soft tissue infection in four patients, and bacteraemia in two patients. The median duration of symptoms was 30 days, significantly shorter amongst high-risk patients (15 days vs. 30 days, p-value 0.002). Comparison of characteristics of patients who presented with TLC <50,000/µl and ≥ 50,000/µl is mentioned in Supplementary Table S1.
Table 1. Patient characteristics.
Therapy modifications
Thirteen patients with high-risk disease had an infection at presentation thus daunorubicin was avoided and they were administered ATRA + ATO regimen. One patient received only ATO. Two patients died within a day of starting therapy with differentiating agents, due to intracranial haemorrhage.
Complications during therapy
More than half of the patients (56.25%, n = 36) developed DS at a median of 4 (range 0–14) days of therapy. The incidence was higher among patients in the Sanz high-risk group as compared to the Sanz-low risk group (68.8 vs. 43.2%, p-value 0.044). Severe DS was seen in 72% (n = 26) patients, while 28% (n = 10) developed moderate DS. Patients with hyperleukocytosis developed DS earlier as compared to those without hyperleukocytosis (day 2 vs. day 5, p-value 0.011). All patients responded well to therapy except one who died due to severe DS.
The incidence of new episodes of FN during therapy was 62.5%; the incidence was higher in high-risk group as compared to low-risk group (75 vs. 50%, p-value 0.039). There were 12 instances of pneumonia, three of FN and bacteraemia but no obvious site of infection, and two instances each of cutaneous infections, dental infection, typhlitis, tuberculosis. One case each of C. difficile diarrhoea, sinusitis, ventilator-associated pneumonia, splenic abscess, otitis externa, and 5 cases in which the focus could not be determined.
ATRA-associated desquamation was seen in 14% of patients and was managed with emollients. Two patients developed genital ulceration for which ATRA was withheld temporarily in both till the lesions improved clinically. Pseudotumor cerebri was seen in 17.2% of patients and treated with acetazolamide; in non-responding six patients, the dose of ATRA was lowered to 25 mg/m2. Venous thrombosis occurred in three patients including one patient with superior sagittal sinus thrombosis at the time of presentation and two patients who developed central line-related thrombosis during induction therapy.
Hepatotoxicity: 10 patients developed hepatic dysfunction on therapy. Three patients out of these had underlying chronic liver disease. The remaining had a normal liver echotexture on ultrasound abdomen and viral markers repeated were normal, the liver dysfunction in them was attributed to drug-induced liver injury.
Cardiotoxicity: one patient who received a daunorubicin-based protocol developed Paroxysmal Supraventricular Tachycardia and was treated with adenosine. Another patient developed accelerated hypertension and congestive cardiac failure and was managed symptomatically.
Induction outcomes
CR rate was 75% and induction mortality was 25%. Nine patients died secondary to an infection, six due to bleeding, and one mortality occurred due to DS. Induction mortality was 34.4% in Sanz high-risk group and 15.6% in Sanz low-risk group. Two patients relapsed at a median follow-up of 26 months and both belonged to the Sanz high-risk category. One patient had received ATRA + daunorubicin. The other patient who had a biochemical relapse had received ATRA + daunorubicin + ATO but her remission induction was complicated by multiple episodes of infections and she had received inadequate therapy. They both received reinduction with ATRA and ATO, while the first patient succumbed to pancreatitis during induction, the other attained CR2 and is currently on maintenance therapy.
Survival outcomes
The EFS () and OS () at the end of 4 years was 71.25% (95% CI 59.97–82.53%) and 73.13% (95% CI 62.16–84.09%), respectively. Patients in Sanz low-risk group had a better 4-year EFS (84.38 vs. 58.33%, p-value 0.026) as well as better OS (84.38 vs. 62.5%, p-value 0.044) as compared to the Sanz high-risk group. 4-year EFS among patients with hyperleukocytosis (defined as TLC ≥50,000/µl) than without hyperleukocytosis was 81.82 vs. 68.31% (p-value:0.597). For patients with TLC of ≥100,000/µl vs. those <100,000/µl 4-year EFS was 87.5 vs. 67.99% (p-value 0.385) and OS was 87.5 vs. 74.65% (p-value 0.488), respectively.
Figure 1. Kaplan Meier survival analysis curve to compare event free survival between low and high-risk APL.
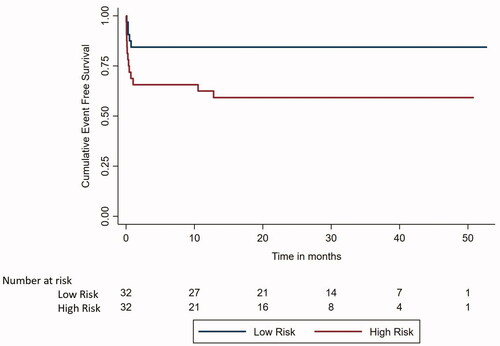
Figure 2. Kaplan Meier survival analysis curve to compare overall survival between low and high-risk APL.
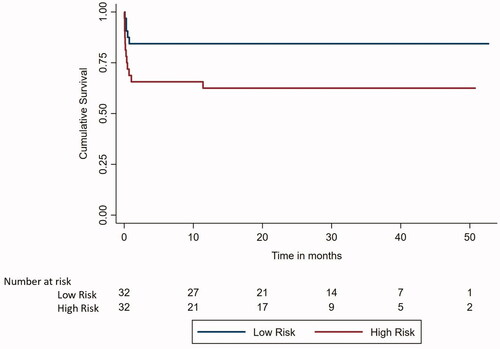
On univariate and multivariate cox proportional hazard regression analysis (Supplementary Tables S2 and S3), haemoglobin at presentation and Sanz high-risk group were associated with poorer event free survival and had a hazard ratio of 0.80 (p-value 0.016, 95% CI for hazard ratio 0.67–0.96) and 3.13 (p-value of 0.031, 95% CI for hazard ratio 1.11–8.81), respectively.
Discussion
The incidence of infection at presentation and during therapy in our group was 57.4 and 62.5%, respectively. These results were lower than those reported by Dayama et al. (91%) [Citation5], Bajpai et al. (64%) [Citation6] as well as the APML4 trial (76%) [Citation2] but higher than those reported by Kapoor et al. (50%) [Citation7]. Sanz high-risk group had a higher incidence of new infections during induction therapy (75 vs. 50%, p-value 0.039) despite lower days to count recovery and neutropenia (28.8 vs. 35.7 days in the low-risk group). Steroids for the prevention and treatment of DS in high-risk group and greater myelosuppression due to anthracycline use could be contributory factors. The UK-AML17 trial had a higher duration of antibiotic usage in the ATRA + idarubicin (IDA) + gemtuzumab (GO) arm (19.2 days) as compared to the ATRA + ATO + GO arm (9.3 days) (p < 0.0001) [Citation8].
The incidence of DS was 56.25% and was significantly higher in the Sanz high-risk group as compared to low-risk group (68.8 vs. 43.8%, p-value: 0.044). Although most studies report the incidence of around 20%, Indian studies have consistently reported >50% incidence of DS [Citation5,Citation7,Citation9,Citation10]. The median day of onset of differentiation in our patients was 4 days in our study as compared to 7.5, 5.6, and 9 days in previous studies [Citation6,Citation9,Citation11]. It is unclear if high infection rates, demographic factors, or genetic factors are responsible for the higher incidence of DS and its early development in the Indian population.
Out of 64 patients, 48 (75%) patients survived at the end of induction, and all attained CR. Early death (ED) rates in clinical trials range from 0 to 16%, however, EDs captured by clinical trials only represent the tip of the iceberg [Citation3,Citation12–14]. Trials often exclude patients with high risk of ED, such as those with a poor PS, older age, multiple comorbidities, active bleeding, and severe infection at presentation. Population-based and hospital-based studies often report a much higher rate of ED of 10–32% [Citation15,Citation16]. Our patient population consisted of all patients of APL who presented to our department including those with poor performance status, active infections, early death, as well as patients whose diagnosis was confirmed posthumously. Over-representation of the high-risk group (50%) also likely contributed to the high mortality. In a study by Österoos et al. [Citation17] that evaluated risk factors for ED, the odds ratio for age was 1.5 (95% CI 1.24–1.83) with an increased risk of ED with age >50 years. The median age of our group was 28 years which is much lower than that reported in other studies. While age was not a significant factor for ED on logistic regression, our study is limited by a small cohort. Nine patients died secondary to an infection, six from bleeding and one mortality occurred due to DS. This is consistent with the most common causes of ED being haemorrhage, infections, DS, and multiorgan failure [Citation18].
The 4-year EFS was 71.25%. Patients in Sanz low-risk group had a better 4-year EFS as compared to the Sanz high-risk group (84.38 vs. 58.33%, p-value 0.026). The relapse rate was 3%, all from high-risk group. Relapse rates in previous studies have ranged from 0% in a study by Varma et al. to 49.7% [Citation9] in the AIDA 0493 trial [Citation19]. The incorporation of ATO and ATRA has resulted in a significant reduction in relapse rates [Citation2,Citation20,Citation21]. In our study, there was a trend towards a superior 4-year EFS among patients with hyperleukocytosis (defined as TLC ≥50,000/µl) than without hyperleukocytosis (81.82 vs. 68.31%, p-value: 0.597). In a study by Daver et al. there was a trend towards inferior 3-year disease free survival (DFS) (69 vs. 80%; p = 0.057) and inferior 3-year OS (74 vs. 92%; p = 0.2) in the hyperleukocytosis group (defined as TLC ≥50,000/µl). Patients who had received ATRA, ATO, and cytoreduction with GO or IDA had a 3-year OS of 100% while patients who only received ATRA with cytoreduction had a far inferior 3-year OS of 35% [Citation22]. Similarly, the 5-year DFS and cumulative incidence of relapse in high-risk group in the APML4 trial were 95 and 5%, respectively [Citation2]. This was significantly superior to the APML3 which used the AIDA protocol and achieved a 4-year DFS of 79% and the difference could be attributed to the addition of ATO in the therapy regimen [Citation23].
Incorporation of ATO in treatment protocols for both low-risk as well as high-risk patients is associated with favourable outcomes with manageable toxicity and no increase in secondary malignancies [Citation24]. The major limitations of our study are its retrospective nature and deviations from the prescribed protocol. However, strict adherence to the treatment protocol is often difficult in real-world scenarios, especially when patients present with life-threatening infection and bleeding and admission is delayed for various reasons. High incidence of infections at presentation and DS are major problems seen in the study population and contributed to both morbidities as well as mortality.
| Abbreviations | ||
| APL | = | acute promyelocytic leukaemia |
| ATRA | = | all trans retinoic acid |
| CR | = | complete remission |
| ATO | = | arsenic trioxide |
| TLS | = | tumour lysis syndrome |
| TLC | = | total leukocyte count |
| FN | = | febrile neutropenia |
| DS | = | differentiation syndrome |
| EFS | = | event free survival |
| OS | = | overall survival |
| IDA | = | idarubicin |
| GO | = | gemtuzumab ozogamicin |
| ED | = | early death |
| DFS | = | disease free survival |
Supplemental Material
Download MS Word (18.7 KB)Supplemental Material
Download MS Word (23.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, MA. The data are not publicly available because it could compromise the privacy of research participants.
References
- Bernard J, Weil M, Boiron M, et al. Acute promyelocytic leukemia: results of treatment by daunorubicin. Blood. 1973;41(4):489–496.
- Iland HJ, Bradstock K, Supple SG, et al. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood. 2012;120(8):1570–1580.
- Lo-Coco F, Avvisati G, Vignetti M, et al. Retinoic acid and arsenic trioxide for acute promyelocytic leukemia. N Engl J Med. 2013;369(2):111–121.
- Tallman MS, Andersen JW, Schiffer CA, et al. All-trans-retinoic acid in acute promyelocytic leukemia. N Engl J Med. 1997;337(15):1021–1028.
- Dayama A, Dass J, Seth T, et al. Clinico-hematological profile and outcome of acute promyelocytic leukemia patients at a tertiary care center in North India. Indian J Cancer. 2015;52(3):309–312.
- Bajpai J, Sharma A, Kumar L, et al. Acute promyelocytic leukemia: an experience from a tertiary care centre in North India. Indian J Cancer. 2011;48(3):316–322.
- Kapoor J, Mirgh SP, Agrawal N, et al. High risk acute promyelocytic leukemia – an enigma for hematologists: optimizing treatment with APML-4 protocol. Indian J Hematol Blood Transfus. 2022;38(2):394–402.
- Burnett AK, Russell NH, Hills RK, et al. Arsenic trioxide and all-trans retinoic acid treatment for acute promyelocytic leukaemia in all risk groups (AML17): results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2015;16(13):1295–1305.
- Varma S, Yanamandra U, Khadwal A, et al. Paper: high risk Apml treated successfully with four cycles of ATO and ATRA combination in resource constrained settings. 57th Annual Meeting & Exposition, ASH; 2015.
- Montesinos P, Sanz MA. The differentiation syndrome in patients with acute promyelocytic leukemia: experience of the pethema group and review of the literature. Mediterr J Hematol Infect Dis. 2011;3(1):e2011059.
- Sanz MA, Martín G, Rayón C, et al. A modified AIDA protocol with anthracycline-based consolidation results in high antileukemic efficacy and reduced toxicity in newly diagnosed PML/RARalpha-positive acute promyelocytic leukemia. PETHEMA group. Blood. 1999;94(9):3015–3012.
- de la Serna J, Montesinos P, Vellenga E, et al. Causes and prognostic factors of remission induction failure in patients with acute promyelocytic leukemia treated with all-trans retinoic acid and idarubicin. Blood. 2008;111(7):3395–3402.
- Rahmé R, Thomas X, Recher C, et al. Early death in acute promyelocytic leukemia (APL) in French centers: a multicenter study in 399 patients. Leukemia. 2014;28(12):2422–2424.
- Di Bona E, Avvisati G, Castaman G, et al. Early haemorrhagic morbidity and mortality during remission induction with or without all-trans retinoic acid in acute promyelocytic leukaemia. Br J Haematol. 2000;108(4):689–695.
- McClellan JS, Kohrt HE, Coutre S, et al. Treatment advances have not improved the early death rate in acute promyelocytic leukemia. Haematologica. 2012;97(1):133–136.
- Jácomo RH, Melo RA, Souto FR, et al. Clinical features and outcomes of 134 Brazilians with acute promyelocytic leukemia who received ATRA and anthracyclines. Haematologica. 2007;92(10):1431–1432.
- Österroos A, Maia T, Eriksson A, et al. A real-world based score to predict early death in acute promyelocytic leukemia. Haematology. 2022;107(7):1528–1537.
- Lehmann S, Deneberg S, Antunovic P, et al. Early death rates remain high in high-risk APL: update from the Swedish Acute Leukemia Registry 1997–2013. Leukemia. 2017;31(6):1457–1459.
- Avvisati G, Lo-Coco F, Paoloni FP, et al. AIDA 0493 protocol for newly diagnosed acute promyelocytic leukemia: very long-term results and role of maintenance. Blood. 2011;117(18):4716–4725.
- Sanz MA, Montesinos P, Vellenga E, et al. Risk-adapted treatment of acute promyelocytic leukemia with all-trans retinoic acid and anthracycline monochemotherapy: long-term outcome of the LPA 99 multicenter study by the PETHEMA group. Blood. 2008;112(8):3130–3134.
- Lo-Coco F, Avvisati G, Vignetti M, et al. Italian GIMEMA cooperative group. Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults younger than 61 years: results of the AIDA-2000 trial of the GIMEMA group. Blood. 2010;116(17):3171–3179.
- Daver N, Kantarjian H, Marcucci G, et al. Clinical characteristics and outcomes in patients with acute promyelocytic leukaemia and hyperleucocytosis. Br J Haematol. 2015;168(5):646–653.
- Iland H, Bradstock K, Seymour J, et al. Results of the APML3 trial incorporating all-trans-retinoic acid and idarubicin in both induction and consolidation as initial therapy for patients with acute promyelocytic leukemia. Haematologica. 2012;97(2):227–234.
- Eghtedar A, Rodriguez I, Kantarjian H, et al. Incidence of secondary neoplasms in patients with acute promyelocytic leukemia treated with all-trans retinoic acid plus chemotherapy or with all-trans retinoic acid plus arsenic trioxide. Leuk Lymphoma. 2015;56(5):1342–1345.
