Xeroderma pigmentosum (XP) is a rare autosomic recessive disorder of DNA repair pathways that confers photosensitivity along with an increased incidence of ultraviolet (UV)-induced cutaneous cancer [Citation1–3]. Eight XP complementation groups (XP-A to XP-G) and an XP variant (XP-V) related to mutations in different genes involved in DNA repair of UV-induced DNA lesions have been described. We described here a patient with a locally advanced and metastatic cutaneous squamous cell carcinoma (SCC) revealing an XP-V successfully treated with PD-1 antibody, pembrolizumab.
Observation
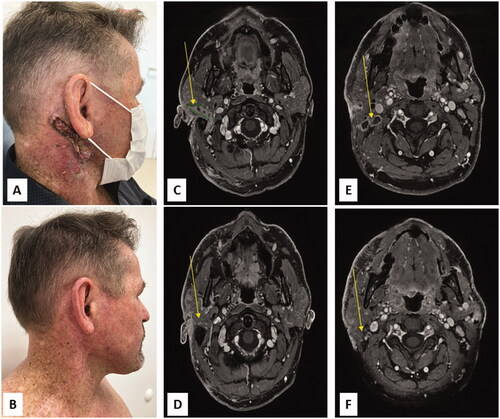
A 58-year-old man was referred for a locally advanced and metastatic cutaneous SCC. His medical history included an inflammatory bowel disease (IBD) treated by methotrexate, an AJCC 8th stage IA melanoma of the forehead treated by wide excision and a 20-year long history of recurrent cutaneous SCCs of the face treated with multiple iterative surgeries (lower lip, nasofrontal area, scalp, both ears). In 2000, he developed a right cervical mass. Neck dissection revealed an SCC lymph node metastasis with capsular effraction out of 3 lymph nodes, along with an SCC metastasis of the parotid gland. The patient was then lost to follow-up. In May 2021, he presented a recurrence of a right cervical mass surrounding the right mastoid and parotid associated with cervical necrotic lymph nodes (). Lymph node SCC metastasis was confirmed by ultrasound-guided biopsy. A total body CT-scan did not show any other suspicious lesions. Methotrexate was discontinued and the patient was treated with pembrolizumab (200 mg every 3 weeks). The patient presented a rapid reduction in tumor mass after the second infusion of pembrolizumab that was still maintained 15 months later () after 19 cycles of pembrolizumab. No flare-up of IBD and other immune-related adverse event were observed. In addition, clinical examination at baseline revealed a skin type II with a striking and severe helioderma (), contrasting with a low cumulative UV-exposure reported by the patient. He worked as a watchmaker, did not travel out of France, did not use any tanning beds nor phototherapy nor photosensitizing drugs. Furthermore, knowing his fair skin type and aware of his recurrent skin cancer history, he reported regular sun protection measures. He had one sister and one uncle with similar skin alteration. No familial history of skin cancer was reported. His parents were not consanguineous. A molecular genetic analysis was then performed and demonstrated an homozygotous POLH gene C.225_227 deletion and p.(Leu77Del) mutation leading to the diagnosis of XP-V.
Figure 1. Tumor response of advanced cutaneous squamous cell carcinoma to pembrolizumab with clinical photography (A, B) and cervical MRI images (C-F): Right ulcerative parotid mass, severe helioderma (A) and right surrounding mastoid and parotid mass (C), associated with multiple cervical necrotic lymph nodes (E) before treatment. Major clinical response with complete healing of the skin (B), decrease of the parotid mass (D) and disappearance of several cervical necrotic lymph nodes (F), 3 months after pembrolizumab introduction.
Discussion
XP-V is characterized by a late onset (after 30 years of age) of skin cancers. XP-V patients gradually develop freckling at sun exposed sites and hypo- and/or hyperpigmentation leading to premature skin aging [Citation1–3]. In contrast to XP groups A-G, XP-V cells have normal nucleotide excision repair, but mutations at POLH gene lead to a deficiency in translesion synthesis [Citation3]. As a consequence, UV-induced DNA lesions are replicated by a more error-prone polymerase that produces extra mutations, therefore allowing skin cancers to occur [Citation1–3].
The efficacy of anti-PD1 therapy for the treatment of unresectable advanced SCC in XP patients () have been scarcely reported [Citation4–9]. The rapid clinical response to anti-PD1 starting after few infusions may be due to the higher mutational burden in the cutaneous neoplasms because of DNA repair defect. A recent study [Citation10] demonstrated indeed that cumulative sun exposure related to skin location and molecular UV signature were relevant biomarkers directly linked to tumor mutational burden and response to anti-PD1 therapy. In conclusion, our observation confirms the rapid effectiveness of anti-PD1 drugs for the treatment of cutaneous advanced SCC in a patient suffering from XP-V.
Table 1. XP patients with cutaneous neoplasms response to immune checkpoint inhibitor.
Acknowledgements
The authors thank the patient for granting permission to publish this information and Elisabeth Homassel for her technical assistance.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- Leung AK, Barankin B, Lam JM, et al. Xeroderma pigmentosum: an updated review. DIC. 2022;11:1–17.
- Opletalova K, Bourillon A, Yang W, et al. Correlation of phenotype/genotype in a cohort of 23 xeroderma pigmentosum-variant patients reveals 12 new disease-causing POLH mutations. Hum Mutat. 2014;35(1):117–128.
- Martens MC, Emmert S, Boeckmann L. Xeroderma pigmentosum: Gene variants and splice variants. Genes (Basel). 2021;12(8):1173.
- Hauschild A, Eichstaedt J, Möbus L, et al. Regression of melanoma metastases and multiple non-melanoma skin cancers in xeroderma pigmentosum by the PD1-antibody pembrolizumab. Eur J Cancer. 2017;77:84–87.
- Deinlein T, Lax SF, Schwarz T, et al. Rapid response of metastatic cutaneous squamous cell carcinoma to pembrolizumab in a patient with xeroderma pigmentosum: Case report and review of the literature. Eur J Cancer. 2017;83:99–102.
- Salomon G, Maza A, Boulinguez S, et al. Efficacy of anti-programmed cell death-1 immunotherapy for skin carcinomas and melanoma metastases in a patient with xeroderma pigmentosum. Br J Dermatol. 2018;178(5):1199–1203. 59.
- Steineck A, Krumm N, Sarthy JF, et al. Response to pembrolizumab in a patient with xeroderma pigmentosum and advanced squamous cell carcinoma. J Clin Oncol Precis Oncol. 2019;3:PO.19.00028.
- Ameri AH, Mooradian MJ, Emerick KS, et al. Immunotherapeutic strategies for cutaneous squamous cell carcinoma prevention in xeroderma pigmentosum. Br J Dermatol. 2019;181(5):1095–1097.
- Rubatto M, Merli M, Avallone G, et al. Immunotherapy in xeroderma pigmentosum: a case of advanced cutaneous squamous cell carcinoma treated with cemiplimab and a literature review. Oncotarget. 2021;12(11):1116–1121.
- Dousset L, Poizeau F, Robert C, et al. Positive association between location of melanoma, ultraviolet signature, tumor mutational burden, and response to anti-PD-1 therapy. J Clin Oncol Precis Oncol. 2021;5:PO.21.00084.
