Abstract
Background
Consolidation radiotherapy for advanced Hodgkin lymphoma (AHL) is controversial. Precise knowledge of the most likely relapse location is crucial for radiotherapy planning. We performed detailed patterns of relapse analyses and evaluated if initial bulky disease, initial 18F-fluoro-deoxy-glucose (FDG)-avidity and/or a residual mass on computed tomography (CT)-scan after chemotherapy are sites with a high risk of relapse. This information could provide guidance for optimal use of radiotherapy in AHL.
Material and methods
We included 133 patients treated with curatively intended chemotherapy for AHL. 23 patients received consolidation radiotherapy. For relapsed patients, imaging from diagnosis, response evaluation, relapse, and any radiotherapy planning, were retrieved and co-registered to determine the exact site(s) of relapse relative to initial site(s), residual mass(es) and to any irradiated volumes. Size and FDG-avidity of initial sites with later relapse, and residual CT-abnormalities after chemotherapy in these sites were registered. Survival analyses were done using the Kaplan–Meier method.
Results
Nine (6.8%) patients relapsed, eight in initially involved sites. One relapse was in an initially irradiated site (as well as other sites). Initial bulky disease, high initial FDG-uptake, and/or residual masses on CT-scan after chemotherapy did not predict sites with a high risk of relapse. Overall survival was 79.6% (95% CI, 72.7–86.5%) and 70.6% (95% CI, 62.4–78.8%) at 5 and 10 years, respectively. Time to progression analysis showed 91.8% (95% CI, 86.9–96.7%) and 90.7% (95% CI, 85.4–96.0%) without progression at 5 and 10 years, respectively.
Conclusion
Current treatment strategies for AHL provide excellent disease control. Neither initial bulk, high initial FDG-uptake, nor a residual CT-abnormality post-chemotherapy seem to indicate sites with a high risk of relapse.
Introduction
In patients with advanced Hodgkin lymphoma (AHL) radiotherapy is used as a consolidation after effective systemic treatment. Currently, consolidation radiotherapy is used in two clinical scenarios: for residual positron emission tomography (PET) positive lymphoma masses and/or for initial bulky disease, under the assumption that these volumes are at a high risk of relapse.
From randomized studies, it is documented that patients with interim or residual PET positive lymphoma and/or bulky disease have a higher risk of relapse [Citation1–4]. However, it is not known in which exact site(s) they relapse, nor if it is possible to predict upfront which parts of the lymphoma volume have the highest risk of relapse after chemotherapy alone. As the aim of consolidation radiotherapy is to secure local disease control, defining which sites are at the highest risk of relapse is essential in order to determine which volumes may benefit from radiotherapy, thereby guiding a more rational use of radiotherapy for AHL.
Here, we present patterns of relapse analyses at the individual patient level, in an unselected 10-year cohort of patients treated according to modern guidelines with effective systemic therapy with or without radiotherapy. We performed a detailed analysis of the exact anatomical site(s) of relapse and evaluated if initial disease characteristics such as bulk or 18F-fluoro-deoxy-glucose (FDG)-avidity, or a residual mass on computed tomography (CT)-scan after chemotherapy indicate specific sites with a high risk of relapse that could possibly be prevented by radiotherapy. Additionally, we analyzed the long-term outcome of this patient cohort.
Material and methods
Patients
We included patients with stage III and IV classical Hodgkin lymphoma (HL) treated at Rigshospitalet, University of Copenhagen, in a 10-year period from 2005 to 2014. Patients were identified through the Danish National Lymphoma Registry (LYFO) [Citation5]. Patient characteristics, treatment details and follow-up information were retrieved from LYFO and verified through review of individual medical records. Patients with primary refractory disease (progression of initially involved sites or new sites during or just after chemotherapy), patients who received no treatment or palliative treatment only, and patients treated according to pediatric regimens were not included (see Figure S1 in Supplementary material).
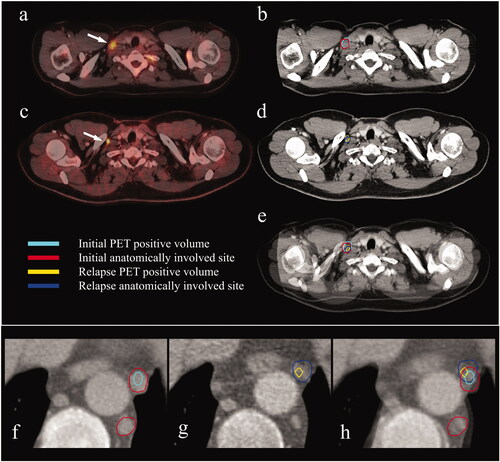
Figure 1. Rigid co-registration of the staging- and relapse scans for two patients (a–e and f–h). (a) Staging PET/CT-scan. Arrow pointing to an initially involved PET positive supraclavicular lymph node. (b) CT part of the staging PET/CT-scan. Delineation of the PET positive (cyan) and the anatomically involved volume (red). (c) Relapse PET/CT-scan. Arrow pointing to a PET positive relapsed supraclavicular lymph node. (d) CT part of the relapse PET/CT-scan. Delineation of the PET positive (yellow) and the anatomically involved volume (blue). (e) Fusion of the staging- and the relapse PET/CT-scans. The relapse is in the exact same lymph node as initially involved. (f) CT part of the staging PET/CT-scan. Delineation of a PET positive (cyan) and two anatomically involved mediastinal lymph nodes (red). (g) CT part of the relapse PET/CT-scan. Delineation of a PET positive (yellow) and anatomically involved (blue) mediastinal lymph node. (h) Fusion of the staging- and the relapse scans. The relapse is in the exact same lymph node as initially involved.CT: computed tomography; PET: positron emission tomography.
Permits
Permission to collect patient data was obtained from the Danish Patient Safety Authority and from the Danish Data Protection Agency. Permission from the Ethics Committee was not required for this retrospective study according to Danish law.
Staging, treatment, response assessment, and follow-up
Initial workup included physical examination, blood tests, and a CT- or whole-body PET/CT-scan. Patients were staged using the Ann Arbor staging classification [Citation6], and the International Prognostic Score (IPS) was used for risk stratification [Citation7].
Chemotherapy regimens consisted of adriamycin, bleomycin, vinblastine, dacarbazine (ABVD) or bleomycin, etoposide, adriamycin, cyclophosphamide, oncovin, procabazine, prednisone (escalated BEACOPP), exceptions were patients enrolled in study protocols.
Radiotherapy was delivered to patients who did not achieve complete response (CR) after chemotherapy (evaluated either by PET/CT-scan or CT-scan alone) and in some cases also to patients with initial bulky disease (≥10 cm in the largest diameter [Citation8]). All patients who had PET positive (defined according to the consensus recommendations of the International Harmonization Project [Citation9] (before 2009), and according to the Deauville score ≥4 [Citation10] (after 2009)) residual masses were irradiated. The target volume encompassed the residual lymphoma after chemotherapy (including both PET positive and PET negative parts of the lymphoma mass) and/or the initial bulky disease modified to account for anatomical changes after chemotherapy according to the involved site radiotherapy (ISRT) principles [Citation11]. Doses were 30–36 Gray (Gy), delivered in 1.8 Gy fractions, 5 fractions per week.
Response assessment with physical examination, blood tests, and CT- or PET/CT-scan (virtually all patients had a PET/CT) was done two months after the end of treatment.
During follow-up, a PET/CT-scan was only performed if a relapse was suspected. Patients were followed clinically for a total of five years. Thereafter follow-up information was obtained via registry data. Patients treated on protocol were followed accordingly.
Patterns of relapse analysis
For patients who relapsed, the staging CT- or PET/CT-scan, the post-chemotherapy CT- or PET/CT-scan and the PET/CT-scan at relapse were retrieved. For patients who had radiotherapy, the planning CT-scan and treatment plan were also retrieved. The initially involved sites, both metabolically active sites (PET positive) and anatomically involved sites (seen on CT), were delineated on the staging scan, and the relapse sites were delineated on the relapse scan. Rigid co-registration of the staging scan with the relapse scan was done to assess the exact location of the relapse site(s) relative to the initially involved site(s) (see ). For the patient who received radiotherapy, co-registration with the planning CT-scan and treatment plan was also done, to assess if the relapse was located anatomically in a previously irradiated site. Sometimes more than one co-registration was necessary to achieve satisfactory soft tissue fit in each involved area.
For patients with a relapse in an initially involved site(s), the largest diameter of the initially involved site (measured in three planes) was registered to evaluate if a relapse preferentially occurred in initial bulky sites. Also, the maximum Standardized Uptake Value (SUVmax) of the initially involved site(s) with a later relapse was measured to evaluate if relapses occurred preferentially in sites with a high initial FDG-uptake. Finally, registration of any residual mass on CT-scan post-chemotherapy was done to evaluate if relapses occurred primarily in sites that did not achieve complete structural remission (see ).
Table 1. Details of relapse locations.
Statistical analyses
The close-out date for follow-up was 31 December 2019. Overall survival (OS) and time to progression (TTP) were calculated using the Kaplan–Meier method [Citation12]. OS was defined as time from the date of diagnosis until the date of death from any cause. TTP was defined as time from the date of diagnosis until the date of relapse or the date of death due to HL. TTP was chosen as the relevant measure of disease control, as progression free survival (PFS) includes patients dying from other causes, and therefore not contributing with information on relapse localization. Differences in OS and TTP between IPS risk groups were tested with the log-rank test [Citation13]. Multivariate analysis was performed according to the Cox regression model [Citation14]. Analyses were performed in IBM® SPSS® statistical software (version 25) and R® (SPSS Inc., Chicago, IL, USA).
Results
Patient and treatment characteristics
Of 175 patients identified from LYFO, a total of 133 patients were included. Forty-two patients were excluded due to: early-stage disease (1), uncertainty as to whether the patient was treated for primary or relapsed disease (1), palliative intent (3), no treatment (5), treatment according to a pediatric regimen (22) or primary refractory disease (10) (see Figure S1 in Supplementary material). Patient and treatment characteristics are shown in and .
Table 2. Baseline characteristics.
Table 3. Treatment characteristics.
Twenty-three (17.3%) patients received radiotherapy to a residual lymphoma volume following chemotherapy, nine (39.1%) patients due to residual PET positive lymphoma and 14 (60.9%) due to residual lymphoma on CT-scans (nine of these had a PET negative interim scan). Seven (30.4%) irradiated patients also had initial bulky disease.
Patterns of relapse
A total of nine (6.8%) patients relapsed. The median time from diagnosis to relapse was 25 months (range: 9–82). Eight patients relapsed in initially involved sites. Of these, five relapsed in both previously involved and uninvolved sites. One patient relapsed in an initially uninvolved site only.
One patient relapsed in an initial bulky site which had not been irradiated. The remaining seven patients relapsed in initially involved non-bulky sites, and four of these patients had other initially involved sites larger than the one(s) with a later relapse.
Initial SUVmax of the sites with a later relapse varied in and between relapsed patients, and only one patient relapsed in the site with the highest initial SUVmax. All other relapsed patients had initially involved sites with a higher SUVmax than in the one(s) with a later relapse.
Three patients relapsed in a site with a residual mass on CT-scan after chemotherapy; none of them had any other post-chemotherapy masses on CT-scans (see ).
Only one of the relapsed patients had received consolidation radiotherapy. For this patient, the relapse occurred in two initially involved sites as well as in previously uninvolved sites. However, only one of the initially involved sites with a later relapse received the full dose of radiotherapy (36 Gy). The other initially involved site was at the margin of the treatment field, in an area that received at most a total dose of 4 Gy (see ).
Survival
A total of 38 patients died at a median age of 61 years (range: 18–85), three from HL (two during treatment, one from relapsed disease). Two patients were lost to follow-up.
The median follow-up time for all patients was 112 months (range: 0–179) and the median follow-up time for patients still alive was 121 months (range: 31–179). For the whole group, the OS was 79.6% (95% CI, 72.7–86.5%) and 70.6% (95% CI, 62.4–78.8%) at five and ten years, respectively, with a significantly better OS for patients with IPS 0–3 compared to patients with IPS 4–7 (p = 0.00017) (see ). Multivariate analyses of the seven individual factors in IPS showed that only age had independent significance (see Table S1 in Supplementary material).
Figure 2. Overall survival (a) and time to progression (b) for all patients. Overall survival (c) and time to progression (d) stratified on IPS groups. Four patients could not be stratified to a group due to missing values in the IPS and were left out of the stratified analyses. IPS: International Prognostic Score.
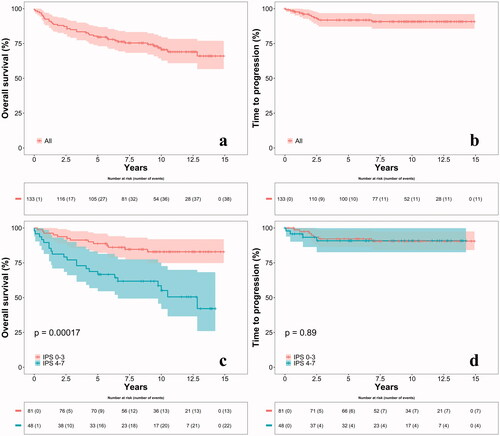
TTP analyses showed 91.8% (95% CI, 86.9–96.7%) and 90.7% (95% CI, 85.4–96.0%) without progression at 5 and 10 years, respectively. Median time of follow-up for all patients and for patients without an event was 108 months (range: 0–179) and 113 months (range: 0–179), respectively. No significant difference (p = 0.89) in TTP between the two IPS groups was seen (see ). Multivariate analyses were not possible due to the low number of events in TTP.
Discussion
Knowledge of the exact site(s) of relapse in relation to initially involved sites is important for determining the target volume for radiation therapy in order to prevent relapses while irradiating the smallest possible volume. If we can predict upfront which anatomical sites are at a high risk of relapse, a more rational and limited radiotherapy can be planned. Hence, a pattern of relapse study as we performed could be informative in designing the optimal radiotherapy for AHL patients. This study is, to our knowledge, the first to perform a detailed analysis of the patterns of relapse following modern imaging and treatment protocols in AHL [Citation11,Citation15]. We found that for the (few) patients who later relapsed neither initial bulky disease, initial FDG-avidity, nor residual lymphoma on CT-scan after chemotherapy predicted sites with a high risk of later relapse. Hence, these factors did not seem useful in determining which volumes should be irradiated in AHL.
The disease control was excellent with a relapse rate at 5 and 10 years, respectively of only of 6.6% and 7.7% in patients who achieved CR with first line treatment including radiotherapy (if given). This was lower than relapse rates reported in earlier studies [Citation1,Citation16,Citation17], and comparable to recent randomized studies [Citation4,Citation18,Citation19]. In accordance with other studies [Citation1,Citation20,Citation21], eight (89%) of nine relapses were confined to initially involved sites. Importantly, there was no indication of relapses being more common in sites of initial bulky disease. Moreover, patients did not necessarily relapse in the largest – albeit non-bulky – site. Likewise, there was no indication that a high initial SUVmax was associated with a preferential site of relapse. Relapses, except for one, did not occur in the sites with the highest initial SUVmax. Finally, a residual mass on CT-scan after chemotherapy also did not indicate a preferential site of relapse. However, relapses were few and conclusions should be made with caution.
Radiotherapy for patients with AHL remains controversial. Restricting consolidation radiotherapy to patients with residual masses/PET positive masses after effective chemotherapy seems efficient [Citation2,Citation18,Citation22–24]. In the European Organization for Research and Treatment of Cancer (EORTC) H34 study [Citation1], involved field radiotherapy (IFRT) to all initial disease did not improve outcome in patients who achieved CR (evaluated on CT-scan) after MOPP-ABV chemotherapy (mechlorethamine, vincristine, procarbazine, prednisone, doxorubicin, bleomycin, vinblastine). However, all patients with a partial response (PR) after chemotherapy received IFRT and had outcomes similar to patients in CR. This was interpreted as an indirect indication of benefit from radiotherapy to residual masses. The EORTC H34 also demonstrated that non-irradiated patients most often relapsed in initially involved sites. A subsequent relapse analysis in patients with PR who received IFRT [Citation22] demonstrated that most patients had only one nodal area with residual disease, suggesting that radiotherapy for lymphoma remnants only could be relevant. However, these patients were not evaluated with PET scans. Our results demonstrate that a residual mass on CT scan did not indicate a preferential site of relapse.
The German Hodgkin Study Group (GHSG) studies HD 6, 9, 12 and 15 [2, 16, 18, 25] administered radiotherapy as consolidation to residual masses, hence the irradiated volumes were reduced compared to the EORTC H34. In the GHSG HD6 [16] and HD9 [25] studies, consolidation radiotherapy was given to all patients to sites of residual disease (sites >2 cm in HD6, any residual tumor in HD9). However, none of the studies were designed to evaluate the effect of radiotherapy. The GHSG HD12 [18] study evaluated the impact of consolidation radiotherapy to sites of residual disease (≥1.5 cm) in patients responding to intensive BEACOPP chemotherapy and confirmed that consolidation radiotherapy could not be omitted in patients with residual disease (evaluated on CT-scan). The GHSG HD15 and HD18 studies [Citation2,Citation4,Citation23] delivered consolidation radiotherapy after intensive BEACOPP chemotherapy to patients with PET positive residual lymphoma ≥2.5 cm with excellent results. However, patients with residual masses <2.5 cm did not have a PET-scan; hence it is not known if some patients with smaller residual disease on CT-scan had PET positive residual masses. Generally, none of the studies evaluated the exact patterns of relapse. Hence, it is not known if relapses occurred exactly in the sites of residual disease, and it is therefore difficult to assess the actual contribution of radiotherapy in relation to the site of relapse.
Findings from our study support the current approach of restricting radiotherapy to PET positive residual lymphoma. In our cohort, nine patients had radiotherapy for residual PET positive lymphoma, only one of these patients relapsed. The relapse was in a residual PET positive site as well as other sites. Of the remaining eight patients who relapsed none had residual PET positive lymphoma after chemotherapy. We did not have data on the results of the non-relapsing patients’ post-chemotherapy scans. However, the policy throughout the period was to irradiate PET-positive residual disease, so they are unlikely to have been positive.
Consolidation radiotherapy to sites of initial bulky disease is continually debated. The FIL HD0801 study [Citation26] randomized patients (n = 116) with initial bulky disease and complete metabolic response (CMR) after ABVD to either consolidation radiotherapy to initial bulky sites or no further treatment. Per protocol analyses showed a PFS benefit of 10.3% and 7.5% at 3 and 5 years, respectively, for patients treated with consolidation radiotherapy. However, the difference was insignificant, probably due to small sample size and few events. In the GHSG HD 6, 9, and 12 studies [Citation16,Citation18,Citation25], consolidation radiotherapy was also administered to sites of initial bulky disease (≥5 cm). However, only the HD12 allowed an evaluation of the effect of radiotherapy. Subgroup analyses showed that radiotherapy did not improve freedom from treatment failure in patients with initial bulky disease and CR (evaluated on CT-scan), suggesting that radiotherapy could be safely omitted in these patients. Likewise, the GITIL/FIL HD0706 Trial [Citation27] showed no difference in PFS between patients treated with or without consolidation radiotherapy to initial bulky sites following CMR after ABVD chemotherapy. These findings are in accordance with our results demonstrating that relapses did not preferentially occur in initial bulky sites. In our material, 14 patients had bulky disease, seven of these patients were irradiated and did not relapse. Two patients with initial bulky disease and not treated with radiotherapy relapsed (only one of them in the initial bulky site). Hence, there might be some benefit from radiotherapy, but the numbers are small and no definitive conclusions can be made. As stated previously, relapse does not seem to happen more often in bulky sites.
Few studies evaluated the patterns of relapse. In the GHSG HD15 study [Citation3], relapses were analyzed, but only in irradiated patients and with the aim of determining whether relapses occurred in the irradiated target volume and if the definition of local radiotherapy used by the GHSG was adequate. In order to define which sites need to be irradiated patterns of relapse analyses in patients who did not receive radiotherapy are more relevant. In the SWOG S0816 study [Citation17], no radiotherapy was administered. Patterns of relapse analysis showed 32% of relapses in initially involved sites, 6% in initially involved and new sites and 53% in new sites only. Furthermore, only two patients relapsed in initial bulky sites (>10 cm). The authors concluded that the omission of radiotherapy contributed little to relapses, as sites of initial bulk, the most obvious target for radiotherapy, were rarely sites of relapse. This is in accordance with our study.
The current treatment strategies control the disease very well, reflected by the high TTP. The IPS was not significant for TTP, likely because treatment was tailored according to the IPS. The IPS was significantly associated with OS, which was lower than in the randomized studies [Citation1,Citation2,Citation4]. The low OS is related to the fact that our study is a population based study of an unselected cohort of patients with a higher median age at diagnosis (44 years, range 16–81 years) than in the randomized studies [Citation1,Citation2,Citation4,Citation18,Citation25]. Also, median age at death was 61 years (range: 18–85) and only three patients died from HL, the remaining from causes not related to their lymphoma. Hence, the OS primarily reflects a population of older people not dying from HL.
The interpretation of our study is limited by the low number of relapses, which is always a problem in studies of the primary treatment of HL, due to the excellent prognosis. The retrospective design and the variation in chemotherapy regimens are also limitations, although all patients received anthracycline containing chemotherapy. The heterogeneity, nevertheless, reflects the daily clinical reality. The strengths are that it is a study of a real life cohort with long follow-up time and detailed analyses of the patterns of relapse in unselected patients treated according to modern principles with effective chemotherapy with or without limited radiotherapy. These very detailed patterns of relapse analyses provide an opportunity to guide an even more rational use of radiotherapy for AHL. However, further studies, ideally international multicenter studies with inclusion of more patients are needed.
Conclusion
Patients with AHL treated according to modern principles have a high chance of permanent cure. Neither initial bulk, high initial FDG-uptake, nor a residual mass on CT-scan after chemotherapy seem to be sites with a high risk of relapse and therefore cannot guide the use of radiotherapy for AHL. Residual PET positive lymphoma in AHL remains the only target for radiotherapy supported by the clinical data.
Ethics approval
The study was approved by the Danish Patient Safety Authority (3-3013-1590/1) and the Danish Data Protection Agency (RH-2016-113).
By Danish law, no ethical approval was required for this retrospective study.
Supplemental Material
Download PDF (289.8 KB)Supplemental Material
Download PDF (82.6 KB)Acknowledgements
Acknowledgements to the Danish Cancer Society, Arvid Nilsson Foundation, and the Department of Oncology, Rigshospitalet, University of Copenhagen, Denmark.
Disclosure statement
Dr Nielsen reports grant from Arvid Nilsson Foundation, during the conduct of the study.
Dr Brown reports personal fees from Roche, Takeda, and BMS, and grants from Novartis, outside the submitted work.
Dr Vogelius reports grants from Danish Cancer Society, during the conduct of the study; grants from Varian Medical Systems and ViewRay and other from Varian Medical Systems, outside the submitted work.
Dr Specht reports grants from Danish Cancer Society, during the conduct of the study; personal fees from Takeda, Kyowa Kirin and MSD, grants from Varian and ViewRay, outside the submitted work.
The remaining authors report no conflicts of interest.
Data availability statement
The data generated during the current study are not publicly available due to regulations on personal data protection but are available from the corresponding author on reasonable request.
Additional information
Funding
References
- Aleman BMP, Raemaekers JMM, Tirelli U, et al. Involved-field radiotherapy for advanced Hodgkin’s lymphoma. N Engl J Med. 2003;348(24):2396–2406.
- Engert A, Haverkamp H, Kobe C, et al. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin’s lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet. 2012;379(9828):1791–1799.
- Kriz J, Reinartz G, Dietlein M, et al. Relapse analysis of irradiated patients within the HD15 trial of the German Hodgkin study group. Int J Radiat Oncol Biol Phys. 2015;92(1):46–53.
- Borchmann P, Goergen H, Kobe C, et al. PET-guided treatment in patients with advanced-stage Hodgkin’s lymphoma (HD18): final results of an open-label, international, randomised phase 3 trial by the German Hodgkin study group. Lancet. 2017;390(10114):2790–2802.
- Arboe B, Josefsson P, Jørgensen J, et al. Danish national lymphoma registry. Clin Epidemiol. 2016;8:577–581.
- Carbone PP, Kaplan HS, Musshoff K, et al. Report of the committee on Hodgkin’s disease staging classification. Cancer Res. 1971;31:1860–1861.
- Hasenclever D, Diehl V. A prognostic score for advanced Hodgkin’s disease. International prognostic factors project on advanced Hodgkin’s disease. N Engl J Med. 1998;339(21):1506–1514.
- Cheson BD, Fisher RI, Barrington SF, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3067.
- Juweid ME, Stroobants S, Hoekstra OS, et al. Use of positron emission tomography for response assessment of lymphoma: consensus of the imaging subcommittee of international harmonization project in lymphoma. J Clin Oncol. 2007;25(5):571–578.
- Meignan M, Gallamini A, Meignan M, et al. Report on the first international workshop on interim-PET scan in lymphoma. Leuk Lymphoma. 2009;50(8):1257–1260.
- Specht L, Yahalom J, Illidge T, ILROG, et al. Modern radiation therapy for Hodgkin lymphoma: field and dose guidelines from the international lymphoma radiation oncology group (ILROG). Int J Radiat Oncol Biol Phys. 2014;89(4):854–862.
- Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–481.
- Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br J Cancer. 1977;35(1):1–39.
- Cox DR. Regression models and life-tables. J R Stat Soc Ser B. 1972;34(2):187–220.
- Eichenauer DA, Aleman BMP, André M, et al. Hodgkin lymphoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol off J Eur Soc Med Oncol. 2018;29:iv19–iv29.
- Sieber M, Tesch H, Pfistner B, et al. Treatment of advanced Hodgkin’s disease with COPP/ABV/IMEP versus COPP/ABVD and consolidating radiotherapy: final results of the German Hodgkin’s lymphoma study group HD6 trial. Ann Oncol. 2004;15(2):276–282.
- Press OW, Li H, Schöder H, et al. US intergroup trial of response-adapted therapy for stage III to IV Hodgkin lymphoma using early interim fluorodeoxyglucose-positron emission tomography imaging: southwest oncology group S0816. J Clin Oncol. 2016;34(17):2020–2027.
- Borchmann P, Haverkamp H, Diehl V, et al. Eight cycles of escalated-dose BEACOPP compared with four cycles of escalated-dose BEACOPP followed by four cycles of baseline-dose BEACOPP with or without radiotherapy in patients with advanced-stage Hodgkin’s lymphoma: final analysis of the HD12 trial o. J Clin Oncol. 2011;29(32):4234–4242.
- Gallamini A, Tarella C, Viviani S, et al. Early chemotherapy intensification with escalated BEACOPP in patients with advanced-stage Hodgkin lymphoma with a positive interim positron emission tomography/computed tomography scan after two ABVD cycles: long-term results of the GITIL/FIL HD 0607 trial. J Clin Oncol. 2018;36(5):454–462.
- Dhakal S, Biswas T, Liesveld JL, et al. Patterns and timing of initial relapse in patients subsequently undergoing transplantation for Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 2009;75(1):188–192.
- Dharmarajan KV, Friedman DL, Schwartz CL, et al. Patterns of relapse from a phase 3 study of response-based therapy for intermediate-risk Hodgkin lymphoma (AHOD0031): a report from the children’s oncology group. Int J Radiat Oncol Biol Phys. 2015;92(1):60–66.
- Aleman BMP, Raemaekers JMM, Tomiŝiĉ R, et al. Involved-field radiotherapy for patients in partial remission after chemotherapy for advanced Hodgkin’s lymphoma. Int J Radiat Oncol Biol Phys. 2007;67(1):19–30.
- Kobe C, Dietlein M, Franklin J, et al. Positron emission tomography has a high negative predictive value for progression or early relapse for patients with residual disease after first-line chemotherapy in advanced-stage Hodgkin lymphoma. Blood. 2008;112(10):3989–3994.
- Lockney NA, Yang JC. Radiation therapy for advanced-stage Hodgkin lymphoma. Adv Radiat Oncol. 2020;5(5):809–816.
- Diehl V, Franklin J, Pfreundschuh M, et al. Standard and increased-dose BEACOPP chemotherapy compared with COPP-ABVD for advanced Hodgkin’s disease. N Engl J Med. 2003;348(24):2386–2395.
- Ricardi U, Levis M, Evangelista A, et al. OC-0502 role of consolidation RT to bulky lesions of advanced Hodgkin lymphoma: results of FIL HD0801 trial. Radiother Oncol. 2019;133:S258–S259.
- Gallamini A, Rossi A, Patti C, et al. Consolidation radiotherapy could be safely omitted in advanced Hodgkin lymphoma with large nodal mass in complete metabolic response after ABVD: final analysis of the randomized GITIL/FIL HD0607 trial. J Clin Oncol. 2020;38(33):3905–3913.
