Abstract
Background
The role of adjuvant radiotherapy (RT) following gross total resection (GTR) in atypical meningioma (AM) is not well established and its benefit remains unclear. We aim to evaluate the survival benefit of adjuvant RT in AM following GTR.
Methods
We searched biomedical databases for studies published between January 1964-February 2021 and included studies reporting primary outcomes of 5-year PFS, 5-year OS and had survival curves for restricted mean survival time (RMST) calculations. Data extracted from survival curves were pooled and analyzed using a random-effects model. Hazard ratio (HR) was calculated for sensitivity analysis.
Results
We included 12 non-randomized studies comprising 1,078 patients. 803 (74.5%) patients were treated with GTR alone and 275 (25.5%) patients received adjuvant RT. In 9 studies, RT included 3 D conformal RT, intensity modulated RT, or fractionated stereotactic radiotherapy); in 3 studies, stereotactic radiosurgery was also used. Median dose of RT was 59.4 Gy. Adjuvant RT resulted in an increase of 3.9 months for restricted mean PFS truncated at 5 years (95% CI 0.23–7.72; p = 0.037) and a 22% reduction in the hazard of disease progression or death (hazards ratio 0.78; 95% CI 0.46–1.33; p = 0.370). Restricted mean OS, truncated at 5 years, was improved with adjuvant RT by 1.1 months (95% CI 0.37–1.81; p = 0.003) and a 21% reduction in the hazard of death from any cause (HR 0.79; 95% CI 0.51–1.24; p = 0.310). Meta-regression analysis of the RMST of EBRT dose did not reveal any significant difference in PFS or OS between studies reporting median dose of <59.4 Gy vs. ≥ 59.4 Gy.
Conclusion
Adjuvant RT following GTR in patients with AM improved restricted mean PFS and OS. While we await the results from ongoing randomized controlled trials, adjuvant RT should be recommended.
Background
Meningiomas are the most common form of primary intracranial tumors with an estimated incidence of ∼8 in 100,000 persons per year [Citation1,Citation2]. The World Health Organization (WHO) classifies and grades meningiomas as benign (grade 1), atypical (grade 2), or anaplastic/malignant (grade 3). WHO grade 2 or atypical meningioma (AM) represents approximately 30–35% of all cases of meningioma [Citation3]. Since it was first introduced by WHO in 1993, the diagnostic criteria for AM has been redefined. This include mitotic count of ≥ 4 and additive criteria of 3 of the other 5 histological features: spontaneous necrosis, sheeting (loss of whorling or fascicular architecture), prominent nucleoli, high cellularity and small cells (tumor clusters with high nuclear:cytoplasmic ratio) [Citation4]. The 2007 classification suggested brain invasion as an indicator of increased risk of recurrence, however it was only in 2016 that brain invasion was formally introduced as an independent histological criterion for atypical (grade 2) meningioma [Citation4,Citation5]. These changes have broadened the diagnostic criteria of atypical meningioma resulting in the increased frequency of its diagnosis from 5% to 35% [Citation6–9]. With AM being such a heterogeneous category, clinical behavior and response to treatment may be variable. The most recent WHO classification in 2021 recommends changing all CNS WHO tumor grades to Arabic numerals and in view of higher likelihood of recurrence, chordoid and clear cell meningioma have been assigned to WHO grade 2 meningioma [Citation10]. The use of molecular biomarkers have also been recognized in the classification and grading of meningioma with some being used as a prognostic factor in guiding management decisions.
To date, there is no consensus guideline on the role of adjuvant radiotherapy (RT) in atypical (grade 2) meningioma. Decisions are often at the discretion of individual physician after taking into consideration patient’s age, performance status, co-morbidity, location of tumor, likelihood of recurrence, and feasibility of repeating surgery should recurrence occur [Citation11]. Gross total resection (GTR), usually defined as Simpson grades 1 to 3, is the surgical goal for atypical meningioma. However, recurrences still occur despite macroscopic clearance. Previous studies have reported recurrence rates in the range of 30–50% with surgery alone [Citation5,Citation12–14]. Whilst the benefit of adjuvant RT following subtotal resection is well established, survival data on the benefit of adjuvant RT following GTR have been conflicting with some reporting improved local control (LC), progression free survival (PFS) and/or overall survival (OS) and others reporting no benefit with adjuvant RT [Citation14–18]. The average time to recurrence with GTR alone and adjuvant RT has been reported as 45 months and 67 months, respectively [Citation19,Citation20]. As recurrences are challenging to treat, and may involve critical structures, establishing a treatment regimen to ensure the best possible outcome should be the ultimate aim in the management of atypical meningioma at first diagnosis.
Previous meta-analysis and systematic reviews have evaluated the role of adjuvant RT in atypical meningioma with hazards ratio (HR), however this assumes the ratio between the two hazard functions remain proportional during the study period. Restricted mean survival time (RMST) is the average time to an event (e.g., recurrence or death) and has been shown to be more suitable for analysis of a time-to-event outcome on a standard time scale [Citation21,Citation22]. Our study aims to evaluate the role of adjuvant RT in atypical (WHO Grade 2) meningioma following GTR using RMST, by comparing the PFS and OS of patients who underwent immediate post-operative RT.
Methods
A systematic review was conducted in accordance to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [Citation23,Citation24].
Search strategy
Biomedical databases such as Medline, EMBASE and CENTRAL were searched for studies published between January 1964 to February 2021. In addition, manual searching of references were performed in included studies. All studies retrieved were imported into EndNote X9. Duplicates were removed accordingly.
Inclusion criteria and exclusion criteria
Inclusion criteria were: 1) studies, either prospective or retrospective, which reported primary outcomes (5-year PFS and 5-year OS) of patients with atypical meningioma (WHO grade 2). We specifically selected studies which included survival curves so RMST could be calculated to provide an average event-free survival period up to a pre-specified time which is 60 months for our study. 2) studies comparing the effects of adjuvant RT following GTR versus GTR alone; 3) randomized controlled trials (RCTs) and cohort studies.
Exclusion criteria were [Citation1]: studies involving patients with metastasis of meningioma [Citation2]; studies where patients underwent GTR or STR and separate outcomes were not reported [Citation3]; studies of patients receiving systemic treatment; and [Citation4] articles that were not original studies such as commentaries and reviews.
Data extraction and assessment of quality
Two reviewers (CW, BV) independently reviewed all included studies to extract data using a pre-designed data sheet. The following data was extracted from each study [Citation1]: general information: author, publication year, title, source, country of origin and language [Citation2]; study characteristics: study design and follow-up duration [Citation3]; inclusion and exclusion criteria for study participants, number of study arms, size of each study arm, and baseline characteristics of each study arms [Citation4]; prescription of adjuvant RT with doses normalized using the linear quadratic model [Citation5]; techniques of external beam RT utilized (3 D conformal RT, intensity modulated RT, fractionated stereotactic radiotherapy (SRT) and stereotactic radiosurgery (SRS) [Citation6]; outcomes of interest.
Quality assessment were independently assessed by the authors (CW, BV) using the Robins-I tool to evaluate the risk of bias in non-randomized studies [Citation25].
Outcome measures
The primary outcomes were PFS and OS. PFS was defined as the time from surgical resection to disease progression or death from any cause. OS was defined as time from surgical resection to death.
Statistical analysis
Extracted data was analyzed using random-effects meta-analysis of time to event using the DerSimonian and Laird (DL) methods. It is a reliable approximation as the number of studies is large.
For the analysis of PFS and OS, we estimated the HRs and 95% confidence interval (CI) directly or indirectly from the given data. RMST was used to compare survival outcomes as we anticipated the proportional hazards assumption for the Cox Proportional Hazard model analysis of the PFS and OS outcomes would be violated in majority of the individual studies. Hence, we used HR as supplementary analysis. All calculations and synthesis of data were conducted using Stata version 15.0.
Results
Results of literature search
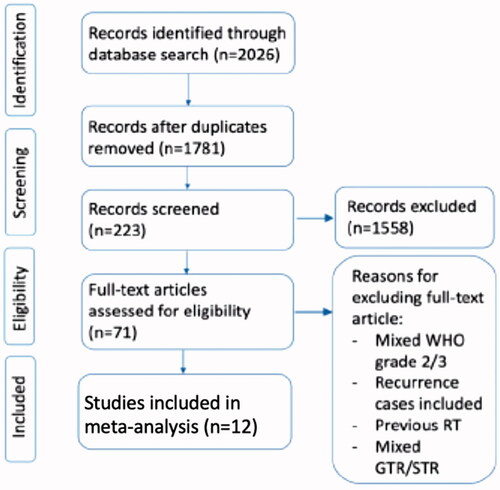
Initial literature search through Pubmed, Medline, and Embase identified 2026 studies (see PRISMA flow diagram in ). After removing 245 duplicate studies and excluding 1558 studies based on their titles, we screened through the abstracts of 223 studies, of which 71 studies met our study criteria for full-text article review. We excluded 59 studies due to various reasons including studies which combined the analysis of atypical meningioma with other grades, recurrences, previous RT, mixed cases GTR & STR. Studies with no survival curves available were also excluded as our statistical analysis requires data extraction from survival curves. After all the above exclusions, we finally included a total of 12 retrospective studies in our meta-analysis (see ).
Characteristics of included studies
Twelve studies comprising of 1,078 patients met our inclusion criteria and had study period ranging from 1988 to 2017. All 12 studies were of retrospective nature with only WHO grade 2/AM in their study population. WHO 2007 classification criteria for AM was used in 7 studies [Citation26–32], one study used both 2000 and 2007 classification [Citation33], one study used the 2016 classification [Citation34]. Two studies applied the WHO classification in place at the time of diagnosis [Citation15,Citation35] and one at the time of surgery [Citation36]
Median follow-up of these studies ranged from 31 to 104 months. Only two studies reported the intervals of the follow-up imaging; Dohm et al. reported that patients had 6-monthly MRI for the first 2 years then annually thereafter [Citation26]. Shakir et al. stated that all patients had follow-up imaging ranging from 3- to 6-monthly in the first 3 years, then 10- to 12-monthly thereafter [Citation31]. GTR was the only treatment received in 803 (74.5%) patients and adjuvant RT following GTR was given in 275 (25.5%) patients. Eight studies defined GTR as resection with Simpson grade 1–3 (15, 26, 29–32, 35, 36), two studies only included those with Simpson grade 1–2 as having GTR [Citation28,Citation33], one study strictly defined GTR as Simpson grade 1 only [Citation27], and in another study, GTR was defined as ‘absence of nodular enhancement at first post-operative magnetic resonance imaging’ [Citation34].
In 9 studies, adjuvant RT was delivered in the form of external beam RT including 3 D conformal RT (3 D-CRT), intensity modulated RT (IMRT), fractionated stereotactic radiotherapy (SRT) [Citation15,Citation27–29,Citation31–35]. In the remaining 3 studies, RT was given in the form of stereotactic radiosurgery (SRS) and/or fractionated RT [Citation26,Citation30,Citation36].
Survival outcomes
We included studies which reported 5-year PFS and/or 5-year OS (see ) and obtained the RMST up to 5 years. Hazards ratio was calculated for the purpose of supplementary analysis.
Table 2. Survival outcomes of GTR alone vs. GTR with adjuvant RT.
Progression free survival
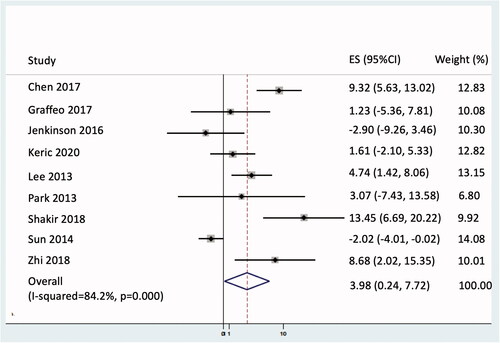
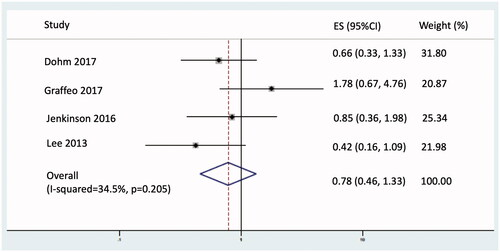
Adjuvant RT resulted in an increase of 3.9 months for restricted mean PFS truncated at 5 years (95% CI 0.23–7.72; p = 0.037; see ) and a non-statistically significant 22% reduction in the hazard of disease progression or death (hazards ratio 0.78; 95% CI 0.46–1.33; p = 0.370; see ).
Meta-regression analysis of the RMST of EBRT dose (< 59.4 Gy vs. ≥ 59.4 Gy) did not reveal any significant effect modification on PFS (p = 0.073) or OS (p = 0.85).
Overall survival
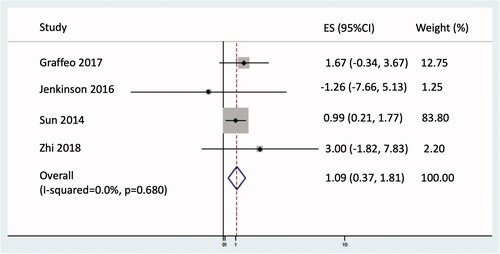
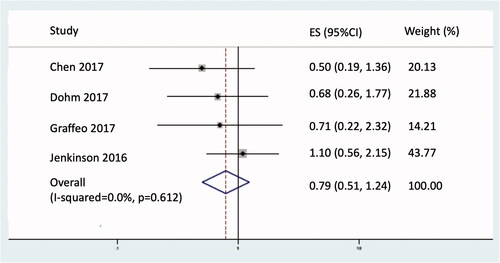
Restricted mean OS, truncated at 60 months, was improved with adjuvant RT by 1.1 months (95% CI 0.37–1.81; p = 0.003; see ) and a non-significant 21% reduction in the hazard of death from any cause (HR 0.79; 95% CI 0.51–1.24; p = 0.310; see ).
Radiation toxicities
Radiation treatment-related toxicities were not reported in all studies. One study reported late toxicity with radiation necrosis in 4 patients (10%) receiving 46–54 Gy to the surgical bed at a median of 10 months after adjuvant EBRT [Citation32]. Three out of those 4 patients underwent debulking surgery and had histological confirmation of radiation necrosis.
Risk of bias
The risk of bias in non-randomized studies of interventions (ROBINS-I) tool was used to evaluate the quality of these studies [Citation25]. The overall risk of bias was assessed as serious in 8 studies [Citation27–30,Citation32,Citation34–36], and critical in 4 studies [Citation15,Citation26,Citation31,Citation33]. Please refer to Supplementary Appendix 1 for the ROBINS-I tool evaluation of the studies included in our meta-analysis.
Discussion
We evaluated the role of adjuvant RT in AM following GTR in 12 studies which met our eligibility criteria and compared 5-year PFS and OS in those with and without adjuvant RT. We found that adjuvant RT was beneficial for patients with AM who had undergone GTR in that it significantly improved PFS by 3.9 months and OS by 1.1 month. Fewer data points were available to determine the reduction in hazard in PFS and OS. There was a non-significant reduction, of approximately 20%, in both these endpoints. Meta-regression analysis of EBRT dose did not show any significant difference between studies with median dose of < 59.4 Gy vs. ≥ 59.4 Gy.
The benefit of adjuvant RT following GTR in atypical meningioma remains debatable as class I evidence is not available. RT is the only form of adjuvant treatment that is currently known to reduce the risk of recurrence, and therefore it should be offered upfront to patients who are at higher risk with GTR alone [Citation37]. Recurrences are likely to cause neurological dysfunction, detriment in Quality of Life, and necessitate a repeat resection, if feasible [Citation8]. Such a risk-stratified approach is currently recommended by many society guidelines – based on the WHO grade and extent of resection [Citation37]. However, patients with WHO grade 2 meningioma who have undergone GTR fall within a grey zone, as the risks of recurrence are balanced with the risk of adjuvant RT (e.g., cognitive decline, radiation necrosis, pituitary dysfunction) [Citation32]. Further, advocates of the observation and early salvage strategy point to the lack of survival benefit with adjuvant RT. However, multiple surgeries for recurrent meningioma pose its own set of risks. A phase 2 non-randomized study (RTOG 0539), reported initial outcomes of 3-year PFS of 96% with local failure of 2.0% in 52 patients with intermediate risk meningioma who received adjuvant RT 54 Gy in 30 fractions [Citation38]. In this study, intermediate risk meningioma include patients with WHO grade 2 meningioma who had GTR (36 patients) and patients with recurrent WHO grade 1 meningioma (16 patients). There was no difference in PFS between these two subgroups [Citation38]. Another phase 2 trial (EORTC 22042–26042) reported 3-year PFS of 88.7% and 3-year OS of 98.2% with adjuvant RT following GTR [Citation39]. In both trials, adjuvant RT was well tolerated with no significant effect on memory or cognitive function [Citation38,Citation39].
Over the years, meta-analysis and systematic reviews have reported the effectiveness of adjuvant RT in atypical meningioma following GTR to varying degrees (see ). Hasan et al. reported that adjuvant RT after GTR (Simpson grade I-III) may improve LC but there was no significant benefit in the 5-year OS and no analysis on PFS was carried out [Citation40]. Pereira et al. reported no difference in recurrence rate with or without adjuvant RT [Citation41]. More recently, He et al. specifically selected studies that defined AM using the WHO classification criteria from year 2000 onwards and found that adjuvant RT after GTR significantly improved LC and OS but not PFS [Citation42]. Zoli et al. reported improvement in PFS, which were seen particularly at 3 and 5 years [Citation43]. Song et al. reported significant improvement in PFS and OS with adjuvant RT in patients with GTR (Simpson grade I-III) however subsequent subgroup analysis revealed that only those with Simpson grade III had significant benefit from adjuvant RT [Citation44].
Table 3. Comparison of meta-analysis and systematic reviews on post-operative RT in atypical meningioma.
The latest European Association of Neuro-Oncology (EANO) guidelines recommend adjuvant RT for Simpson 4–5 resection. Recommendation for Simpson 1–3 is uncertain and observation may be considered appropriate as no randomized trials are available to date with only level IV evidence currently available for this group [Citation37]. Two highly anticipated phase 3 randomized controlled trials comparing adjuvant RT to observation following GTR in AM are currently in progress and their results are much awaited to help shed a light on the benefit of adjuvant RT in these cases. The ROAM/EORTC-1308 (Radiotherapy versus Observation following surgical resection of Atypical Meningioma/European Organization for Research and Treatment of Cancer-1308) trial will be the first multi-centre randomized controlled trial to evaluate the role of adjuvant RT in AM following GTR [Citation45]. The NRG-BN003 study on observation versus irradiation for a gross totally resected grade 2 meningioma (clinicaltrials.gov NCT No: NCT03180268) is a phase 3 trial which randomized newly diagnosed AM patients with GTR to observation or early RT with photons or protons.
The benefit of adjuvant RT in our study was numerically small (PFS 3.9 months, OS 1.1 month) and still leaves unanswered questions about whether it should be offered to all patients with atypical meningioma undergoing GTR. The morphological classification of meningioma may soon give way to molecular classification. This may help to risk-stratify patients, aiding physicians to better select patients for adjuvant RT, where the benefit may be more pronounced. The group from Princess Margaret Hospital in Toronto have developed a new molecular classification based on the DNA methylation, somatic copy number aberration, point mutation and gene expression data from 121 patients [Citation46]. They proposed 4 groups: MG1(immunogenic), MG2(benign NF2 wild-type), MG3(hypermetabolic) and MG4(proliferative). Other groups have suggested treatment algorithms considering other morphological features such as brain invasion, mitotic index and MIB-1. Adjuvant RT is recommended for AM with GTR where there is brain invasion as it carries a recurrence rate of up to 60% in 5 years with ∼25% mortality rate. In cases where there is no brain invasion, adjuvant RT should be offered in AM with MIB-1 index of over 4.2% or more than 4 mitoses/10 HPF as this is deemed to be more aggressive with recurrence rate of more than 50% in 5 years [Citation47,Citation48]. A recent meta-analysis on the prognostic role of Ki-67 in meningioma reported worse OS in patients with high expression levels of Ki-67 (HR = 1.56; 95% CI: 1.217–2.013) [Citation49].
All 12 studies included in our meta-analysis were of retrospective nature with limited number of patient population. This is in part due to our strict inclusion criteria, where only studies which reported outcomes of GTR with or without adjuvant RT and included survival curves were selected. The long duration of study inclusion (on average about 10 years) also meant that the WHO classification for atypical meningioma have changed over that period of time, hence affecting the consistency of the diagnostic criteria used in the studies. GTR was not uniformly defined as most studies included Simpson grades 1–3 as GTR, however others only considered Simpson grades 1–2 or 1 only as GTR. With no standardized guidelines in place, there might have been some degree of bias in the selection of patients who were offered adjuvant RT following GTR as this depended on individual clinician’s recommendation.
Conclusion
The use of adjuvant RT in patients with AM who have undergone GTR provided a modest PFS benefit (based on restricted mean survival). RT reduces the relative risk of recurrence and should be discussed with all patients. However, in view of the small OS benefit, there may be an option for close surveillance and early salvage for patients where repeat surgical resection can be easily performed. As such, no strong recommendation can be made for, or against, adjuvant RT. We anticipate better clarity with the ongoing RCTs addressing this question, and the increased use of molecular studies to better risk-stratify patients.
Authors contributions
All authors contributed to the study conception and design. Data collection and analysis were performed by Caryn Wujanto, Balamurugan Vellayappan, and Soon Yu Yang. The first draft of the manuscript was written by Caryn Wujanto and Tabitha Chan and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript
Supplemental Material
Download MS Word (17.8 KB)Acknowledgement
The authors thank Senior Librarian (National University of Singapore, Medical Library), Ms Annelissa Chin for assisting with the literature review and search strategy.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available online from their respective journal website (please refer to for a list of all the included studies and their references).
Table 1. Baseline characteristics of included studies.
Additional information
Funding
References
- Ostrom QT, Gittleman H, Farah P, et al. BTRUS statistical report: Primary brain and Central nervous system tumors diagnosed in the United States in 2006–2010. Neuro Oncol. 2013;15 (Suppl 2):ii1–56.
- Holleczek B, Zampella D, Urbschat S, et al. Incidence, mortality and outcome of meningiomas: a population-based study from Germany. Cancer Epidemiol. 2019;62:101562.
- Backer-Grøndahl T, Moen BH, Torp SH. The histopathological spectrum of human meningiomas. Int J Clin Exp Pathol. 2012;5(3):231–242.
- Louis DN, Perry A, Reifenberger G, et al. The 2016 world health organization classification of tumors of the Central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820.
- Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tumours of the Central nervous system. Acta Neuropathol. 2007;114(2):97–109.
- Alghamdi M, Li H, Olivotto I, et al. Atypical meningioma: Referral patterns, treatment and adherence to guidelines. Can J Neurol Sci. 2017;44(3):283–287.
- Willis J, Smith C, Ironside JW, et al. The accuracy of meningioma grading: a 10-year retrospective audit. Neuropathol Appl Neurobiol. 2005;31(2):141–149.
- Rogers L, Gilbert M, Vogelbaum MA. Intracranial meningiomas of atypical (WHO grade II) histology. J Neurooncol. 2010;99(3):393–405.
- Jääskeläinen J, Haltia M, Servo A. Atypical and anaplastic meningiomas: radiology, surgery, radiotherapy, and outcome. Surg Neurol. 1986;25(3):233–242.
- Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the Central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251.
- Jenkinson MD, Weber DC, Haylock BJ, et al. Atypical meningoma: current management dilemmas and prospective clinical trials. J Neurooncol. 2015;121(1):1–7.
- Engenhart-Cabillic R, Farhoud A, Sure U, et al. Clinicopathologic features of aggressive meningioma emphasizing the role of radiotherapy in treatment. Strahlenther Onkol. 2006;182(11):641–646.
- Pearson BE, Markert JM, Fisher WS, et al. Hitting a moving target: evolution of a treatment paradigm for atypical meningiomas amid changing diagnostic criteria. Neurosurg Focus. 2008;24(5):E3.
- Aghi MK, Carter BS, Cosgrove GR, et al. Long-term recurrence rates of atypical meningiomas after gross total resection with or without postoperative adjuvant radiation. Neurosurgery. 2009;64(1):56–60; discussion.
- Jenkinson MD, Waqar M, Farah JO, et al. Early adjuvant radiotherapy in the treatment of atypical meningioma. J Clin Neurosci. 2016;28:87–92.
- Durand A, Labrousse F, Jouvet A, et al. WHO grade II and III meningiomas: a study of prognostic factors. J Neurooncol. 2009;95(3):367–375.
- Komotar RJ, Iorgulescu JB, Raper DM, et al. The role of radiotherapy following gross-total resection of atypical meningiomas. J Neurosurg. 2012;117(4):679–686.
- Hug EB, Devries A, Thornton AF, et al. Management of atypical and malignant meningiomas: role of high-dose, 3D-conformal radiation therapy. J Neurooncol. 2000;48(2):151–160.
- Palma L, Celli P, Franco C, et al. Long-term prognosis for atypical and malignant meningiomas: a study of 71 surgical cases. J Neurosurg. 1997;86(5):793–800.
- Mair R, Morris K, Scott I, et al. Radiotherapy for atypical meningiomas. J Neurosurg. 2011;115(4):811–819.
- Perego C, Sbolli M, Specchia C, et al. Utility of restricted mean survival time analysis for heart failure clinical trial evaluation and interpretation. JACC Heart Fail. 2020;8(12):973–983.
- Uno H, Claggett B, Tian L, et al. Moving beyond the hazard ratio in quantifying the between-group difference in survival analysis. J Clin Oncol. 2014;32(22):2380–2385.
- Moher D, Liberati A, Tetzlaff J, PRISMA Group, et al. Group P. Preferred reporting items for systematic reviews and Meta-analyses: the PRISMA statement. Int J Surg. 2010;8(5):336–341.
- Moher D, Altman DG, Liberati A, et al. PRISMA statement. Epidemiology. 2011;22(1):128. author reply
- Sterne JAC, Savović J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898.
- Dohm A, McTyre ER, Chan MD, et al. Early or late radiotherapy following gross or subtotal resection for atypical meningiomas: Clinical outcomes and local control. J Clin Neurosci. 2017;46:90–98.
- Hammouche S, Clark S, Wong AH, et al. Long-term survival analysis of atypical meningiomas: survival rates, prognostic factors, operative and radiotherapy treatment. Acta Neurochir (Wien). 2014;156(8):1475–1481.
- Keric N, Kalasauskas D, Freyschlag CF, et al. Impact of postoperative radiotherapy on recurrence of primary intracranial atypical meningiomas. J Neurooncol. 2020;146(2):347–355.
- Lee KD, DePowell JJ, Air EL, et al. Atypical meningiomas: is postoperative radiotherapy indicated? Neurosurg Focus. 2013;35(6):E15.
- Pant S, Tonse R, Kannan S, et al. Impact of timing of radiation therapy on outcomes in atypical meningioma: a clinical audit. Pract Radiat Oncol. 2018;8(5):e275–e284.
- Shakir SI, Souhami L, Petrecca K, et al. Prognostic factors for progression in atypical meningioma. J Neurosurg. 2018;129(5):1240–1248.
- Sun SQ, Kim AH, Cai C, et al. Management of atypical cranial meningiomas, part 1: predictors of recurrence and the role of adjuvant radiation after gross total resection. Neurosurgery. 2014;75(4):347–354. discussion 54-5; quiz 55.
- Park HJ, Kang HC, Kim IH, et al. The role of adjuvant radiotherapy in atypical meningioma. J Neurooncol. 2013;115(2):241–247.
- Graffeo CS, Leeper HE, Perry A, et al. Revisiting adjuvant radiotherapy after gross total resection of world health organization grade II meningioma. World Neurosurg. 2017;103:655–663.
- Zhi M, Girvigian MR, Miller MJ, et al. Long-Term outcomes of newly diagnosed resected atypical meningiomas and the role of adjuvant radiotherapy. World Neurosurg. 2019;122:e1153–e1161.
- Chen WC, Magill ST, Wu A, et al. Histopathological features predictive of local control of atypical meningioma after surgery and adjuvant radiotherapy. J Neurosurg. 2019;130(2):443–450.
- Goldbrunner R, Stavrinou P, Jenkinson MD, et al. EANO guideline on the diagnosis and management of meningiomas. Neuro Oncol. 2021;23(11):1821–1834.
- Rogers L, Zhang P, Vogelbaum MA, et al. Intermediate-risk meningioma: initial outcomes from NRG oncology RTOG 0539. J Neurosurg. 2018;129(1):35–47.
- Weber DC, Ares C, Villa S, et al. Adjuvant postoperative high-dose radiotherapy for atypical and malignant meningioma: a phase-II parallel non-randomized and observation study (EORTC 22042-26042). Radiother Oncol. 2018;128(2):260–265.
- Hasan S, Young M, Albert T, et al. The role of adjuvant radiotherapy after gross total resection of atypical meningiomas. World Neurosurg. 2015;83(5):808–815.
- Pereira BJA, de Almeida AN, Paiva WS, et al. Impact of radiotherapy in atypical meningioma recurrence: literature review. Neurosurg Rev. 2019;42(3):631–637.
- He L, Zhang B, Zhang J, et al. Effectiveness of postoperative adjuvant radiotherapy in atypical meningioma patients after gross total resection: a Meta-Analysis study. Front Oncol. 2020;10:556575.
- Zoli M, Della Pepa GM, Carretta A, et al. Adjuvant radiotherapy in grossly total resected grade II atypical meningiomas: a protective effect on recurrence? J Neurosurg Sci. 2022;66(3):240–250.
- Song D, Xu D, Han H, et al. Postoperative adjuvant radiotherapy in atypical meningioma patients: a Meta-Analysis study. Front Oncol. 2021;11:787962.
- Jenkinson MD, Javadpour M, Haylock BJ, et al. The ROAM/EORTC-1308 trial: Radiation versus observation following surgical resection of atypical meningioma: study protocol for a randomised controlled trial. Trials. 2015;16:519.
- Nassiri F, Liu J, Patil V, et al. A clinically applicable integrative molecular classification of meningiomas. Nature. 2021;597(7874):119–125.
- Modha A, Gutin PH. Diagnosis and treatment of atypical and anaplastic meningiomas: a review. Neurosurgery. 2005;57(3):538–550. discussion -50.
- Perry A, Stafford SL, Scheithauer BW, et al. The prognostic significance of MIB-1, p53, and DNA flow cytometry in completely resected primary meningiomas. Cancer. 1998;82(11):2262–2269.
- Liu N, Song SY, Jiang JB, et al. The prognostic role of Ki-67/MIB-1 in meningioma: a systematic review with Meta-analysis. Medicine. 2020;99(9):e18644.
- Chun SW, Kim KM, Kim MS, et al. Adjuvant radiotherapy versus observation following gross total resection for atypical meningioma: a systematic review and Meta-analysis. Radiat Oncol. 2021;16(1):34.