Abstract
Background and purpose
Strategies for minimizing irradiation of organs at risk (OARs) from pathological inguinal lymph node (PILN) boosting are needed to minimize the risk of morbidity. Coverage probability (CovP) is a conformal planning strategy for simultaneously integrated boost (SIB). Our aim was to investigate if SIB of PILN using CovP can be delivered safely in vulvar cancer.
Materials and methods
Ten consecutive patients treated with definitive radiotherapy (RT) including SIB of PILN and with daily cone beam CT (CBCT) were included. Dose prescription was 51.2/32 fx to the elective target and 64 Gy/32 fx to the gross disease at the vulva and to positive lymph nodes (LN). PILN were contoured on both planning CT and MRI (GTV-N) and combined to form ITV-N. Each PILN GTV-N was contoured on every third CBCT, in total 11 CBCT for each patient. OARs were subcutaneous tissue (SC), inguinal vessels, skin rim, bowel, and body contour. Three plans were created for every patient: A) Standard CT-based planning; PTV-N based on GTV-NCT with a 10 mm isotropic margin. B) CT and MRI-based planning with smaller margins: PTV-N based on ITV-N with a 5 mm isotropic margin. C) CovP. The total delivered dose to GTV-Ns was estimated by accumulating dose across all fractions based on GTV-Ns contoured on CBCT.
Results
Thirty-five PILNs were boosted. There was no significant difference in accumulated GTV-N D98% between the three plans. CovP delivered a higher mean dose to the GTV-N D50% and D2% (p < 0.001). The planned mean doses to the OARs were reduced when applying CovP.
Conclusions
SIB of PILN in vulvar cancer based on CovP and a 5 mm PTV margin does not compromise target coverage during RT and reduces the dose to normal tissues in the groin.
Introduction
Vulvar cancer is the fourth most common gynecological cancer. There are 6100 new cases estimated per year in USA [Citation1]. Vulvar cancer is managed by one or more treatment modalities according to the stage. Operable cases can be treated by surgery. Radio (chemo) therapy can be the sole treatment modality or combined with surgery according to the stage and status of the patient. Radiotherapy (RT) for cancer vulva cases can be offered as adjuvant, definitive, or palliative RT.
Pathological inguinal lymph nodes (PILNs) are found in >50% of patients with vulvar cancer referred for RT. Lymph node (LN) involvement is considered the most important prognostic factor for survival in vulvar cancer [Citation2]. The five years overall survival is 86% and 54% for patients without and with LN involvement, respectively [Citation2]. A multi-center study of 502 patients with cancer vulva showed that recurrences developed in about 37% of cases and that the pattern of recurrence was: 53% perineal, 19% inguinal, and 6% pelvic, 8% distant; and 14% multiple [Citation3]. Positive LN involvement at diagnosis was statistically correlated with the risk of recurrence. The 5-year survival rate was 27% in patients with inguinal and pelvic recurrences [Citation3]. Hence, it is crucial to focus on proper management of involved positive inguinal LNs. Groins may be treated surgically with inguinal LN dissection or by definitive RT or combination of surgery and RT.
The GOG study by Stehman et al., studied inguinal LN surgical dissection vs. groin RT in N0-1 vulvar cancer patients. The study reported inferior results of progression-free interval and survival for RT [Citation4]. The criticism for that study was that it used a poor RT technique and did not secure proper dose to the nodal target volume [Citation5,Citation6]. The morbidity, e.g., wound infection and wound gap – were higher in the lymphadenectomy than the RT group, whereas both groups had 16% lymphedema reported [Citation5]. The results of GROINSS-V II showed that patients who reported lymphedema at 6 and 12 months after RT were 16.4% and 10.7%, respectively. The frequency of Grade 1–3 skin toxicity after RT was 21.3%, 14.8%, and 1.3%, respectively, at 4–6 weeks post RT and 7.2%, 0.7%, and 0.7%, respectively, at 6 months post RT [Citation7].
Some studies recommended RT dose escalation to achieve better pathological response and comparable survival to surgical approaches for locally advanced vulvar cancer [Citation8–11]. Some authors recommended delivering up to 70 Gy to involved inguinal LNs [Citation12,Citation13]. However, to deliver such higher doses we need a precise strategy with tight margins and high conformality. Strategies for minimizing irradiation of organs at risk (OARs) from pathological inguinal LN boosting are needed to minimize the risk of morbidity arising in the groin such as lymphedema. Currently, there is no clear consensus for margins needed for inguinal LN boosting for vulvar cancer patients. Some report a margin of 5−10 mm and others do not mention the margin used [Citation8–11]. Yet, there is paucity in the publications focusing on consensus regarding required margins for inguinal LN boosting for cancer vulva patients and the coverage of nodal boosts by the current RT techniques. Therefore, there is a need to investigate different RT techniques with regard to dose coverage of pathological LN targets and exposure of normal tissue.
CovP is a planning strategy clinically used for pelvic nodal boosting for cancer cervix patients with promising results [Citation14–16]. CovP takes into account the probabilities of geometric uncertainties of the target in relation to the PTV. During optimization, CovP allows a higher dose in the center of the node and a lower dose at the edge of the PTV which makes it possible to achieve sparing of OARs [Citation14,Citation17].
The aim of this work was to investigate if boosting the pathologically involved inguinal LN can be delivered safely and with adequate target coverage with the use of CovP. Furthermore, to assess if OARs could be spared when using CovP. In this study, we therefore compared different optimization and planning strategies in terms of nodal target coverage and normal tissue doses.
Materials and methods
One hundred and twenty patients with vulvar cancer treated at Department of Oncology at Aarhus University Hospital from 2013 to 2019 were reviewed. Thirty-five of them were diagnosed with inguinal LN involvement. Only patients with involved inguinal LNs who were treated with definitive RT were included in this study. This was in total 10 patients. A total number of 35 inguinal LNs in the 10 patients were analyzed. Out of the 35 boosted LNs, 20 were located in the right inguinal region and 15 were located in the left inguinal region. RT was delivered by VMAT. RT dose to the elective target was 51.2/32 fx and a simultaneously integrated boost (SIB) with 64 Gy/32 fx was administered to the gross disease at the vulva and to the positive LNs. Cone beam CT (CBCT) was acquired before each fraction of EBRT and rigid bony registration to the planning CT was done for the purpose of daily couch correction. Daily couch corrections included rotation and translation but not pitch and roll.
Delineation
Patients were scanned with planning CT and MRI in a supine frog-leg position. For fixation, the patient is placed supine in a vacuum-molded bag extending from the sacral region to the knees, with the legs in a frog position. The grossly involved LNs were contoured on the planning CT (GTV-N) as well as on the planning MRI. The images were rigidly registered. For each LN, the GTV-N contoured on MRI and CT were combined and named ITV-N.
For the purpose of this post-treatment analysis of different LN boosting strategies, the GTV-N of each boosted LN was contoured on every second or third CBCT for each patient. The total number of CBCTs used for delineation for each patient was 11.
The OARs list in this study was specially created to define the surrounding normal tissue in the groin region. For OARs, the following contours were delineated: inguinal vessels, skin (a rim of a 3 mm in thickness from outer body contour), bowel and body contour. In addition, a structure named subcutaneous tissue (SC) was defined to be all tissues beginning anterior to the femoral vessels, excluding the 3 mm skin rim structure, and bounded by the muscles of the femoral triangle laterally and the medial edge of the pubic bone medially excluding the GTV-N and the inguinal vessels. In this study, the doses from the LN boosts to the bladder and pelvic bones were negligible so were not included in the analysis.
Planning
For this study, three plans were created for every patient (Plans A–C). Varian Eclipse Treatment Planning System and calculation algorithm AcurosXB_15.6.05 was used All three dose plans were VMAT with three full 360° arcs with collimator angles 20°, 90°, and 340°. For patients with more than one boosted LN, the LNs were optimized individually to ensure that all boosted LNs received the prescribed dose (PD).
(A) Standard CT-based planning; PTV-N was GTV-N with a 10 mm isotropic margin added. GTV-N was defined as the appearance on the planning CT. The dose planning aims were PTV-N D98% > = 95% of the PD and the dose plans were normalized to mean PTV-N dose being 100% of PD. For patients with more than one boosted LN, the normalization was to the LN receiving the lowest dose and the mean dose in the other LNs were slightly above 100% of PD.
(B) CT and MRI-based planning with smaller margins: PTV-N was ITV-N with a 5 mm isotropic PTV margin. The dose planning aims and normalization strategy were the same as in strategy A.
(C) CT and MRI-based CovP planning: PTV-N was the same as in Plan B. CovP planning is not commercially available, and therefore, we used a dose optimization method developed by Ramlov et al. in the context of definite RT in locally advanced cervix cancer. This method generates plans which mimic CovP dose distributions in a commercial dose planning system [Citation14]. With this method, dose gradient are controlled to meet an aim to get as close to 90% of PD on the outer edge of PTV-N and full coverage (100% of PD) of the ITV-N. The dose planning aims were ITV-N D98% ≥ 100% PD and PTV D98% ≥ 90% of PD. The normalization was done such that all dose-to-target criteria are fulfilled [Citation14,Citation15].
Rigid registration was performed between CBCTs and the planning CT, thus all the CBCT delineations of GTV-Ns were propagated to the planning CT. For every GTV-N (from planning CT and from CBCTs) D98%, D50%, and D2% were extracted for each fraction and for each of the three plans on the planning CT. Dose accumulation for GTV-Ns (D98%, D50%, and D2%) was carried out as crude addition of DVH parameters from each fraction. We evaluated also GTV-N volume and PTV-N volume. Doses to OAR were evaluated on the planning CT. We evaluated the inguinal vessels D1 cm3, V55 Gy, and V60 Gy. For the skin rim of the inguinal region, we evaluated D2 cm3, D1 cm3, and D0.1 cm3. We also recorded V60 Gy and V55 Gy for the body, SC, bowel. We reported the V60 and V55 Gy since the PD was 64 Gy/32 fx to the positive LN s, consequently these volumes reflect the high dose volumes.
In addition, the evaluation of the volume of the GTV-Ns in relation to the fraction number was done by linear regression. Paired student t-test was used to assess the difference between the different plans. A p value of ≤ 0.05 was defined as statistically significant.
Results
Inguinal GTV-Ns were clearly visible on 91% of the CBCTs. In 9% of cases the GTV-N was not visible, mainly due to shrinkage of the LN. The median volume of GTV-N on the first CBCT was 0.6 cc with a range of (0.05–4.2). The median volume of GTV-Ns at the last CBCT (n = 32) was 0.3 cc (0.0−1.6). In relation to nodal volumes during treatment, we found that 49% of the LNs decreased significantly in size during treatment (p < 0.05), and reduction of the GTV-N volume from first to last fraction ranged between 31 and 95%. In addition, 11% showed a tendency of decreased volume (p < 0.09), with the reduction of the LN volume from first to last fraction ranged between 36 and 96%. In 6% of LNs, the volume increased significantly (p < 0.05), and the percentage increase ranged between 69 and 180%. The remaining LNs (34%) showed non-significant change in size during the treatment.
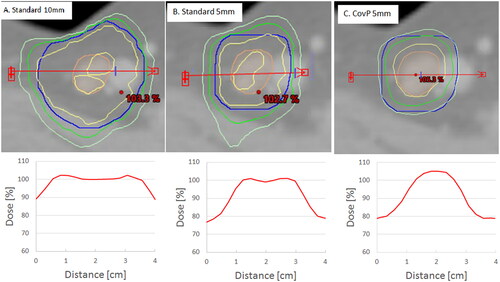
shows an example of the three planning approaches. With the standard plans, the GTV-N was covered by at least 95% of dose. For the CovP plan, at least 98% of the GTV-N was covered by 100% of PD.
Figure 1. Example of the three planning approaches. A: standard plan with 10 mm PTV-N margin. B: plan with 5 mm PTV-N margin. C: coverage probability plan (CovP) with 5 mm PTV-N margin. With the corresponding dose profile for each plan shown below each one. Orange contour is GTV-N, Blue contour is the PTV-N. Yellow contour is the 100% isodose. Green contour is 95% isodose. Light green contour is 90% isodose. The red arrow represents the dose profile seen below each figure.
summarizes the PTV-N volumes and the planned doses for the PTV-N and the GTV-N for the three plan strategies The mean planned GTV-N D98%, D50%, and D2% was significantly higher with the CovP plan as compared to plan A and plan B (p < 0.001). The planned doses for the OAR are shown in . shows that the planned mean doses to the surrounding SC, skin, body, and inguinal vessels were reduced when applying the CovP plan compared to the other two planning strategies. For the inguinal vessels, the D1 cm3 was below 60 Gy for all patients for the CovP plans while above 60 Gy in 69% and 25% of cases for the 10 mm plan and the 5 mm plan strategy, respectively. For the SC, there was a significant reduction in the V55 Gy by a mean of 23 cc and 9 cc when applying the CovP vs. Plans A and B, respectively (p < 0.001). Furthermore, there was a significant reduction of the SC V60 Gy by a mean of 20 cc and 8 cc when applying the CovP vs. Plans A and B, respectively (p < 0.001). Applying the CovP plan significantly reduced the Rt inguinal vessels D1cm3 by a mean of 8 and 3 Gy, and the Lt inguinal vessels D1cm3 by 6 and 2 Gy vs. Plans A and B, respectively (p < 0.001).
Table 1. PTV volumes and the planned doses for the PTV-N and the GTV-N as follows: D98%, D50%, and D2% for the three plans strategies.
Table 2. Summary of the planned mean doses to the organs at risk by the three plans.
compares doses delivered (mean + SD) to the GTV-Ns. There was no statistically significant difference in accumulated D98% to the GTV-N between the three plans, with a p value of 0.72, 0.74, and 0.91 when testing the difference for Plan A vs. CovP, Plan B vs. CovP, and Plans A vs. B, respectively. CovP plans delivered a statistically significant higher mean dose to the GTV-N D50% and D2% compared to Plans A and B (p < 0.001).
Table 3. Comparison between the accumulated delivered GTV-N doses (mean and SD) relative to PD by the three plans strategies.
Regarding the numbers of nodes with accumulated delivered D98% below 95% of the PD, they were 2, 1, and 4 for the 10 mm PTV-N, 5 mm PTV-N plan, and CovP plan, respectively. One node received below 95% with all planning strategies for which the delivered D98% was 89.6, 90.2, 89.1% for the 10 mm PTV-N, 5 mm PTV-N plan, and CovP plan, respectively. For the other three nodes with coverage below 95% with CovP plan, the coverage ranged between 93.5 and 94.8% with the CovP plan.
Discussion
This study evaluated accumulated LN doses throughout vulva cancer RT. Three different planning techniques were compared with different sizes of margins and planning approach.
We introduced the CovP plan method in delivering RT with concomitant boost to positively involved inguinal LN as a method for improving inguinal LN boosting and evaluated the technique through assessing doses to the target and OARs. The CovP plan assigns a volume in the center of the LN to be covered with high probability with higher doses than the periphery of the PTV-N volume [Citation14]. The concept of CovP dose planning is based on the assumption that the movement around the center of mass is random. Having at least 100% of PD in ITV-N can compensate if part of the GTV-N moves out of the 95% isodose during one fraction as it may the next fraction move into the area with 100% of PD and in that way even out the low dose it received during an earlier fraction. This is illustrated in our results by which shows that for the CovP plan the 100% of the GTV-N is covered by 100% of the dose. Given that the accumulated dose to the GTV-N is similar with the CovP strategy as compared to the standard planning approaches, we are not expecting more groin recurrences with CovP. CovP was investigated for treatment of nodal boost for cancer cervix patients with promising results [Citation14]. As reported in the results section, in our cohort few GTV-Ns received accumulated delivered D98% below 95% of the PD, mainly due to movement. One LN had compromised coverage by all three planning approaches and that was due to the LN being in close proximity to the skin. This indicates the importance of daily CBCT for monitoring LN position and groin swelling [Citation18].
CovP was also implemented in the international study EMBRACE II for boosting the LN s in the treatment of cancer cervix patients, with a subsequent significant reduction in the high dose volume V50 Gy [Citation19].
In our study, we demonstrated that the near-minimum dose to GTV-N’s remained the same for CovP strategy while statistically significantly reducing dose to OARs. At the same time, the CovP plan delivered a higher mean dose to the D50% and D 2%than the standard plan by about 3 and 4 Gy, respectively (p < 0.001). Generally, for the target, the three plans have an acceptable target coverage. The CovP allowed dose escalation in the center of the GTV-Ns and controlled lower doses at the edge of the PTV-Ns. This resulted in better sparing for the vascular and nearby skin and SC (). For the OARs, RT should in general aim for doses as low as possible, and therefore, it makes sense to apply a technique that is safe from target perspective and achieves significantly less dose to OARs. According to our knowledge, there are currently no international guidelines on contouring of specific OAR located in the groin regions. On the other hands, there are clinically observed groin toxicity, e.g., skin ulceration, edema, and fibrosis. We attempted to create representative OARs for the groin area in the form of skin, SC, and inguinal vessels, which are at risk for acute and late toxicity. Our aim was to match the spectrum of toxicities with relevant regions of interest and relevant DVH parameters. For the skin, according to literature, using the whole organ volume is not representative for a clinically relevant endpoint [Citation20,Citation21]. Therefore, specification of a skin volume was proposed, as it is easily measured. For the vessels, we used D1cm3, since this has been used for major vessels in previous studies (although not specifically for inguinal vessels) [Citation22].
A limitation of this study is that dose accumulation was carried out with crude addition of DVH parameters and not through deformable registration and 3D addition of doses. However, the main dosimetric endpoint was GTV D98. GTV D98 which is a ‘near minimum dose’. Since crude addition of DVH parameters is in reality a ‘worst case assumption’ that assumes that the ‘cold spot’ coincides in all fractions, our crude addition represents a conservative estimate of D98 (‘worst case’). Our main conclusion that GTV D98 is similar across the three types of plans will therefore not change with the more advanced methodology of dose tracking based on deformable registration. For OARs, we were interested in the relative difference between the three plans. While, there may be differences between the accumulated dose on CBCTs (with deformably added doses) and planning CT (with DVH addition for CBCT contours), we assume that such differences are similar across plans. If deviations due to dose accumulation methodology are similar across plans, the relative differences between plans are expected to be well approximated through DVH addition. This means that while the estimated difference in OARs doses may not be completely precise, we still consider these as fair estimates of the overall tendency.
Since there is limited knowledge about dose-effect relations for normal tissue in the inguinal region, it is difficult to predict whether the dosimetric advantage we demonstrated, will also lead to a reduction of morbidity. This would need to be tested in a clinical trial.
Our study showed that a 5 mm margin was adequate for boosting the inguinal LN with the CovP technique. This can be the basis for proposing margins used for inguinal LN boosting.
Conclusions
Inguinal nodal boosting with 5 mm PTV-N margin and using the CovP planning method is an adequate technique, which results in acceptable target coverage during long-course RT and reduces the dose to normal tissues in the groin.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data available on request from the authors.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30.
- Choi S, Sherertz T. Vulvar cancer. In: Hansen E, Roach III M, editors. Handbook of evidence-based radiation oncology. Cham, Switzerland: Springer; 2018. p. 299–712.
- Maggino T, Landoni F, Sartori E, et al. Patterns of recurrence in patients with squamous cell carcinoma of the vulva. A multicenter CTF study. Cancer. 2000;89(1):116–122.
- Stehman FB, Bundy BN, Thomas G, et al. Groin dissection versus groin radiation in carcinoma of the vulva: a gynecologic oncology group study. Int J Radiat Oncol Biol Phys. 1992;24(2):389–396.
- Petereit DG, Mehta MP, Buchler DA, et al. Inguinofemoral radiation of N0,N1 vulvar cancer may be equivalent to lymphadenectomy if proper radiation technique is used. Int J Radiat Oncol Biol Phys. 1993;27(4):963–967.
- Dutta S, Coleman RL. Radiation therapy for vulvar carcinoma. 2019. Available from: https://www.glowm.com/section_view/heading/Radiation
- Oonk MHM, Slomovitz B, Peter JW1, et al. Radiotherapy versus inguinofemoral lymphadenectomy as treatment for vulvar cancer patients with micrometastases in the sentinel node: results of GROINSS-V II. J Clin Oncol. 2021;39(32):3623–3632.
- Stecklein SR, Frumovitz M, Klopp AH, et al. Effectiveness of definitive radiotherapy for squamous cell carcinoma of the vulva with gross inguinal lymphadenopathy. Gynecol Oncol. 2018;148(3):474–479.
- Beriwal S, Shukla G, Shinde A, et al. Preoperative intensity modulated radiation therapy and chemotherapy for locally advanced vulvar carcinoma: analysis of pattern of relapse. Int J Radiat Oncol Biol Phys. 2013;85(5):1269–1274.
- Moore DH, Ali S, Koh WJ, et al. A phase II trial of radiation therapy and weekly cisplatin chemotherapy for the treatment of locally-advanced squamous cell carcinoma of the vulva: a gynecologic oncology group study. Gynecol Oncol. 2012;124(3):529–533.
- Natesan D, Hong JC, Foote J, et al. Primary versus preoperative radiation for locally advanced vulvar cancer. Int J Gynecol Cancer. 2017;27(4):794–804.
- Gaffney DK, King B, Viswanathan AN, et al. Consensus recommendations for radiation therapy contouring and treatment of vulvar carcinoma. Int J Radiat Oncol Biol Phys. 2016;95(4):1191–1200.
- Rao YJ, Chundury A, Schwarz JK, et al. Intensity modulated radiation therapy for squamous cell carcinoma of the vulva: treatment technique and outcomes. Adv Radiat Oncol. 2017;2(2):148–158.
- Ramlov A, Assenholt M, Jensen M, et al. Clinical implementation of coverage probability planning for nodal boosting in locally advanced cervical cancer. Radiother Oncol. 2017;123(1):158–163.
- Lindegaard JC, Assenholt M, Ramlov A, et al. Early clinical outcome of coverage probability based treatment planning for simultaneous integrated boost of nodes in locally advanced cervical cancer. Acta Oncol. 2017;56(11):1479–1486.
- Pötter R, Tanderup K, Kirisits C, et al. The EMBRACE II study: the outcome and prospect of two decades of evolution within the GEC-ESTRO GYN working group and the EMBRACE studies. Clin Transl Radiat Oncol. 2018;9:48–60.
- Baum C, Alber M, Birkner M, et al. Robust treatment planning for intensity modulated radiotherapy of prostate cancer based on coverage probabilities. Radiother Oncol. 2006;78(1):27–35.
- Mohamed S, Fokdal L, et al. Dosimetric impact of edemal on inguinal lymph node boost in locally advanced vulvar cancer. J Appl Clin Med Phys. 2021;22:315–319.
- Berger T, Seppenwoolde Y, Pötter R, et al. Importance of technique, target selection, contouring, dose prescription, and dose-planning in external beam radiation therapy for cervical cancer: evolution of practice from EMBRACE-I to II. Int J Radiat Oncol Biol Phys. 2019;104(4):885–894.
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991;21(1):109–122.
- Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76(3):S10–S9.
- Evans JD, Gomez DR, Amini A, et al. Aortic dose constraints when reirradiating thoracic tumors. Radiother Oncol. 2013;106(3):327–332.
