Introduction
The World Health Organization (WHO) first declared the severe acute respiratory syndrome coronavirus-2 (SAR-CoV-2) a pandemic on 11 March 2020 [Citation1]. Although children with COVID-19 infection generally present with mild form of the disease [Citation2], oncology patients are considered at increased risk of hospitalization and death from COVID-19 [Citation3,Citation4]. They are also known to have prolonged shedding of SARS-CoV-2, which may have clinical and public health implications [Citation5].
The PINETREE trial demonstrated that a short three-day course of remdesivir in high-risk adults with mild COVID-19 infection resulted in a lower risk of hospitalization or death [Citation6]. However, use in high-risk pediatric patients with mild infection has not been definitively established. The aim of this study was to describe the clinical manifestation of COVID-19 infection in pediatric oncology patients and to evaluate the real-world utility and safety of remdesivir for the treatment of COVID-19 infection in this cohort.
Methods
Study Population
This retrospective cohort study was conducted in KK Women’s and Children’s Hospital in Singapore. We included all pediatric oncology patients ≤18 years of age, who were admitted from 1 November 2021 (first pediatric oncology COVID-19 case) to 31 March 2022. Patients who were fully vaccinated with COVID-19 vaccines at the point of SARS-CoV2-2 diagnosis were excluded. All cases were confirmed via SARS-CoV-2 real-time reverse transcriptase-polymerase chain reaction (PCR) and were undergoing immunosuppressive treatment. Disease severity was classified according to the National Institute of Health (NIH) COVID-19 guidelines [Citation7].
Data Collection
Patient-specific data were obtained from the electronic hospital records. Degree of immunosuppression was adapted from Madhusoodhan et al. [Citation8] The primary outcome studied was the time to viral clearance, defined as the time interval between the 1st positive PCR and the 1st of two consecutive negative PCRs. Other outcomes studied included time to PCR cycle threshold (Ct) ≥25 (defined as the time interval between the 1st positive PCR and the 1st PCR with Ct ≥25), days to defervescence (defined as attainment and sustenance of body temperature of <38 °C), progression to severe COVID-19 infection (based on the NIH severity criteriaCitation7], length of stay, and 30-day mortality. A surrogate marker of viral load with SARS-CoV-2 PCR is the Ct value, and a cutoff Ct value of ≥25 was selected as the standardized criteria for de-isolation of patients in our institution, given that infectivity is reduced when Ct values are ≥25 [Citation9]. The study was approved by the Singhealth Centralized Institutional Review Board (CIRB 2020/2094). Written informed consent was waived in light of public health pandemic research.
Remdesivir treatment
Decision to initiate treatment with remdesivir was based on individual case’s clinical condition. Remdesivir was initiated at a loading dose of 5 mg/kg/dose (maximum dose of 200 mg) on day one, and 2.5 mg/kg/dose Q24H (maximum dose of 100 mg) on days two and three, together with daily monitoring of hepatic transaminases, baseline electrocardiogram, and continuous cardiac monitoring.
Statistical and data analysis
Independent t-test was used for comparison involving continuous variables. A p-value of p < 0.05 was considered statistically significant. SPSS statistical software (version 28.0, IBM, Illinois, USA) was used for data analyses. Cumulative frequency curves of the time to viral clearance and time to PCR Ct ≥25 were plotted.
Results
Clinical presentation
A total of 18 pediatric oncology patients were identified (). The distribution of immunosuppressive potential of the cohort was: Severe (n = 4, 22.2%), Moderate (n = 9, 50.0%) and Low (n = 5, 27.8%). The background diagnoses included lymphoma, acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), neuroblastoma, post-haematopoietic stem cell transplant recipients, juvenile myelomonocytic leukemia, optic glioma, atypical teratoid/rhabdoid tumor (ATRT) and pilomyxoid astrocytoma. All patients had mild COVID-19 infection, based on the NIH disease severity classification [Citation7]. The median PCR Ct value was 18.44 (IQR: 16.40 − 22.28) at time of diagnosis. Median time to viral clearance of the cohort was 31 days (IQR: 27 – 57) and time to PCR Ct value ≥25 was 7.0 days (IQR: 5.5 − 9.0).
Table 1. Demographics, clinical presentation, and clinical outcomes.
Utility and safety of remdesivir
Remdesivir was initiated in four patients (lymphoma, AML and ALL) at a median of 3.5 days (IQR: 2.75 − 4) from symptom onset. The documented clinical indications for remdesivir initiation included: recent receipt of chemotherapy; to prevent delay of planned 2nd cycle high-dose chemotherapy with autologous stem cell rescue; persistent symptoms and repeat SARS-COV-2 PCR Ct <25. Baseline immunosuppressive potential was similar in the treated and non-treated groups (p = 0.778). There were no statistically significant differences in baseline laboratory parameters although ANC was lower in the remdesivir group (0.55 × 109 cells/L vs 0.76 × 109 cells/L, p = 0.055) ().
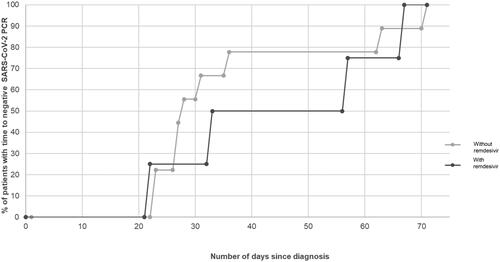
shows the cumulative frequency curves of the time to viral clearance, illustrating the proportion of patients with a negative SARS-CoV-2 PCR plotted against time. In terms of the point estimates of the median time to viral clearance, the median time was numerically longer in the remdesivir group but this was not statistically significant (45 days, vs 28 days, p = 0.136) with overlapping IQR.
Secondary outcomes such as time to PCR Ct ≥25 (7.5 days vs 6 days, p = 0.694), and days to defervescence (3.5 days vs 2 days, p = 0.597) were also similar between both groups. Length of stay was longer in the remdesivir group, but this difference was not statistically significant (7 days, vs 4.5 days, p = 0.306). This was due to one patient in the remdesivir group having inpatient chemotherapy after recovering from COVID-19. There were no reported remdesivir-related adverse events.
Discussion
Our cohort of pediatric oncology COVID-19 cases had mild disease at diagnosis and none progressed to severe disease or COVID-19 attributable deaths. We did not detect any clear benefit in terms of time to viral clearance or PCR Ct ≥25 between the treated versus non-treated groups, but our study findings are limited by its small sample size. Remdesivir was well tolerated in four of our pediatric oncology patients with no safety concerns detected.
Data on COVID-19 infection in pediatric oncology patients is currently limited to multi-national case series conducted in various waves of the pandemic [Citation8,Citation10–14]. Although severe cases have also been described in some immunocompromised children, the majority had mild infection [Citation15–18]. In our study, all patients had a mild course of COVID-19 which could be attributed to most of our cases being diagnosed during the Omicron wave where the Omicron variant had been reported to cause less severe disease compared to earlier variants.
Thus far, there have only been four cohort studies of remdesivir use for the treatment of mild COVID-19 in adult immunocompromised patients [Citation19–22]. At the time of writing, our study provides the only direct clinical experience on the use of remdesivir for the treatment of COVID-19 in pediatric oncology patients. No patients in either group reported viral clearance in the first 21 days of infection (). Persistent viral shedding has been reported in both immunocompromised children and adults [Citation23–25], as compared to a shorter duration of 16 to 17 days in healthy children [Citation26–28]. This is in keeping with our current cohort with viral shedding of up to 71 days, with impaired B-cell and T-cell function being postulated to be the main reason for their inability to clear the virus [Citation5,Citation24,Citation25].
The public health implications of variant evolution in these immunocompromised patients with prolonged viral shedding is a major cause for concern. Rapid viral evolution has also been described, with such patients having more transmissible or pathogenic SARS-CoV-2 escape variants over the entire course of their infection [Citation29]. Unfortunately, the evidence for remdesivir in accelerating viral clearance remains inconclusive in our cohort. There is hence an urgent need for larger multi-institution studies in pediatric oncology children to evaluate the impact of other treatment modalities on the time to viral clearance.
Limited data suggested that remdesivir was generally well-tolerated [Citation30–33], although severe adverse effects such as sinus bradycardia have been reported previously [Citation34–36]. Our small study provided real-world data that remdesivir use was well-tolerated in immunosuppressed pediatric oncology patients. More safety data in such high risk cohorts are needed.
Our study has several limitations. It was a small retrospective, observational study and most patients were admitted during the Omicron wave of the pandemic. We could not adjust for baselines differences in disease status or immunosuppression due to the small sample size. However, the immunosuppressive potential distribution was similar between both groups. SARS-CoV-2 PCR testing was performed at the clinical discretion of the medical team, which may result in ascertainment bias. More testing in the non-treated group could potentially increase the likelihood of detection of clearance or SARS-CoV-2 PCR Ct ≥25. Time to viral clearance was assessed via SARS-CoV-2 PCR which detects only viral RNA and may have poor correlation to viral infectivity [Citation37].
Conclusion
In our observational cohort of immunocompromised pediatric oncology children with mild COVID-19 disease, we found the median time to viral clearance was 31 days which was much higher than observed in healthy children. Treatment with remdesivir in our cohort was safe but did not lead to early clearance of SARS-CoV-2 or shorter time to PCR Ct ≥25. There is an urgent need for larger clinical studies of SARS-CoV-2 infection and treatment efficacy in immunocompromised pediatric patients with mild COVID-19 infections, in view of the high risk of emergence of variants from the cohort.
Ethical Approval
The study was approved by the Singhealth Centralized Institutional Review Board (CIRB 2020/2094). Written informed consent was waived in light of public health pandemic research, as the study involved was a non-interventional retrospective medical records review and the clinical management of patients was not affected. The research was conducted in compliance with the International Council for Harmonization of Technical Requirements for Pharmaceuticals for Human Use (ICH) Guideline for Good Clinical Practice. Precaution was taken to protect the privacy of research subjects and the confidentiality of their personal information. Any articles or data including information which could potentially identify an individual are excluded from the publication of this manuscript.
Acknowledgements
The authors thank Mr. Qing Lin TAN and Ms. Tzu-Tien THIO for their administrative support.
Disclosure statement
The authors of this study declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data Availability statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to restrictions (their containing information that could compromise the privacy of research participants).
Additional information
Funding
References
- Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72 314 cases from the chinese center for disease control and prevention. JAMA. 2020;323(13):1239–12 41.
- Ludvigsson JF. Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr. 2020;109(6):1088–1095.
- Graff K, Smith C, Silveira L, et al. Risk factors for severe COVID-19 in children. Pediatr Infect Dis J. 2021;40(4):e137–e145.
- Götzinger F, Santiago-García B, Noguera-Julián A, ptbnet COVID-19 Study Group, et al. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4(9):653–661.
- Aydillo T, Gonzalez-Reiche AS, Aslam S, et al. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383(26):2586–2588.
- Gottlieb RL, Vaca CE, Paredes R, GS-US-540-9012 (PINETREE) Investigators, et al. Early remdesivir to prevent progression to severe covid-19 in outpatients. N Engl J Med. 2022;386(4):305–315.
- National Institute of Health (NIH). NIH COVID-19 Treatment Guidelines: Clinical Spectrum of SARS-CoV-2 Infection. 2022. https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum
- Madhusoodhan PP, Pierro J, Musante J, et al. Characterization of COVID-19 disease in pediatric oncology patients: the New York-New Jersey regional experience. Pediatr Blood Cancer. 2021;68(3):e28843.
- Bullard J, Dust K, Funk D, et al. Predicting infectious severe acute respiratory syndrome coronavirus 2 from diagnostic samples. Clin Infect Dis. 2020;71(10):2663–2666.
- Mukkada S, Bhakta N, Chantada GL, Global Registry of COVID-19 in Childhood Cancer, et al. Global characteristics and outcomes of SARS-CoV-2 infection in children and adolescents with cancer (GRCCC): a cohort study. Lancet Oncol. 2021;22(10):1416–1426.
- Müller J, et al. Clinical Course of COVID-19 disease in children treated with neoplastic diseases in Hungary. Pathol Oncol Res. 2022;28:1610261.
- Mercolini F, Cesaro S. COVID-19 in children and adolescents: characteristics and specificities in immunocompetent and oncohematological patients. Mediterr J Hematol Infect Dis. 2022;14(1):e2022009.
- Węcławek-Tompol J, Zakrzewska Z, Gryniewicz-Kwiatkowska O, et al. COVID-19 in pediatric cancer patients is associated with treatment interruptions but not with short-term mortality: a polish national study. J Hematol Oncol. 2021;14(1):163.
- Hammad M, Shalaby L, Sidhom I, et al. Management and outcome of coronavirus disease 2019 (COVID-19) in pediatric cancer patients: a single Centre experience from a developing country. Clin Lymphoma Myeloma Leuk. 2021;21(11):e853–e864.
- Stokes CL, Patel PA, Sabnis HS, et al. Severe COVID-19 disease in two pediatric oncology patients. Pediatr Blood Cancer. 2020;67(9):e28432.
- Vicent MG, Martinez AP, Trabazo Del Castillo M, et al. COVID-19 in paediatric hematopoietic stem cell transplantation: the experience of spanish group of transplant (GETMON/GETH). Pediatr Blood Cancer. 2020;67(9):e28514.
- Andre N, Rouger-Gaudichon J, Brethon B, et al. COVID-19 in pediatric oncology from french pediatric oncology and hematology centers: high risk of severe forms? Pediatr Blood Cancer. 2020;67(7):e28392.
- Smith VR, Whittle SB, Coleman RD, et al. Severe COVID-19 infection in a child receiving immunotherapy for cancer. Pediatr Blood Cancer. 2021;68(3):e28710.
- Biscarini S, Villa S, Tomasello M, et al. Safety profile and outcomes of early COVID-19 treatments in immunocompromised patients: a single Centre cohort study. Biomedicines. 2022;10(8):2002.
- Lafont E, Pere H, Lebeaux D, et al. Targeted SARS-CoV2 treatment is associated with decreased mortality in immunocompromised patients with COVID-19. J Antimicrob Chemother. 2022;77(10):2688–2692.
- Jaroszewicz J, Kowalska J, Pawłowska M, et al. Remdesivir decreases mortality in COVID-19 patients with active malignancy. Cancers. 2022;14(19):4720.
- Aksak-Wąs BJ, Chober D, Serwin K, et al. Remdesivir reduces mortality in hemato-oncology patients with COVID-19. J Inflamm Res. 2022;15:4907–4920.
- Dolan SA, Mulcahy Levy J, Moss A, et al. SARS-CoV-2 persistence in immunocompromised children. Pediatr Blood Cancer. 2021;68(12):e29277.
- Haidar G, Mellors JW. Improving the outcomes of immunocompromised patients with coronavirus disease 2019. Clin Infect Dis. 2021;73(6):e1397-1401–e1401.
- Sepulcri C, Dentone C, Mikulska M, et al. The longest persistence of viable SARS-CoV-2 with recurrence of viremia and relapsing symptomatic COVID-19 in an immunocompromised patient – a case study. Open Forum Infect Dis. 2021;8(11):ofab217.
- Han MS, Choi EH, Chang SH, et al. Clinical characteristics and viral RNA detection in children with coronavirus disease 2019 in the Republic Of Korea. JAMA Pediatr. 2021;175(1):73–80.
- Kam KQ, Thoon KC, Maiwald M, et al. SARS-CoV2 viral RNA load dynamics in the nasopharynx of infected children. Epidemiol Infect. 2021;149:e18.
- Xu CLH, Raval M, Schnall JA, et al. Duration of respiratory and gastrointestinal viral shedding in children with SARS-CoV-2: a systematic review and synthesis of data. Pediatr Infect Dis J. 2020;39(9):e249-56–e256.
- Corey L, Beyrer C, Cohen MS, et al. SARS-CoV2 variants in immunosuppressed individuals. N Engl J Med. 2021;385(6):562–566.
- Goldman DL, Aldrich ML, Hagmann SHF, et al. Compassionate use of remdesivir in children with severe COVID-19. Pediatrics. 2021;147(5):e2020047803.
- Méndez-Echevarría A, Pérez-Martínez A, Gonzalez del Valle L, et al. Compassionate use of remdesivir in children with COVID-19. Eur J Pediatr. 2021;180(4):1317–1322.
- U.S. National Library of Medicine. Study to evaluate the safety, tolerability, pharmacokinetics, and efficacy of remdesivir (GS-5734TM) in participants from birth to <18 years of age with Coronavirus Disease 2019 (COVID-19) (CARAVAN). ClinicalTrials.gov identifier: NCT04431453. 2020. http://clinicaltrials.gov/ct2/show/NCT04431453
- Ahmed A, Rojo P, Agwu A, et al. Remdesivir treatment for COVID-19 in hospitalized children: CARAVAN interim results. In Conference on Retroviruses and Opportunistic Infections (CROI) 2022 Annual Meeting; 2022. Denver, CO. Abstract 744.
- Eleftheriou I, Liaska M, Krepis P, et al. Sinus bradycardia in children with remdesivir for COVID-19. Pediatr Infect Dis J. 2021;40(9):e356.
- Chow EJ, Maust B, Kazmier KM, et al. Sinus Bradycardia in a pediatric patient treated with remdesivir for acute coronavirus disease 2019: a case report and a review of the literature. J Ped Infect Dis Soc. 2021;10(9):926–929.
- Sanchez-Codez MI, Rodriguez-Gonzalez M, Gutierrez-Rosa I. Severe sinus bradycardia associated with remdesivir in children with severe SARS-CoV-2 infection. Eur J Pediatr. 2021;180(5):1627.
- Zapor M. Persistent detection and infectious potential of SARS-CoV-2 virus in clinical specimens from COVID-19 patients. Viruses. 2020;12(12):1384.