 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Introduction
SBRT is an increasingly popular treatment for localized prostate cancer, though considerable variation in technical approach is common and optimal dose constraints are uncertain. In this study, we sought to identify dosimetric and patient-related predictors of acute rectal toxicity.
Methods
Patients included in this study were treated with prostate SBRT on a prospective institutional protocol. Physician-graded toxicity and patient-reported outcomes were captured at one week, one month, and three months following SBRT. DVH data were extracted and converted into relative volume differential DVHs for NTCP modeling. Patient- and disease-related covariates along with NTCP model predictions were independently tested for significant association with physician-graded toxicity or a decline in bowel-related QoL. A multivariate model was constructed using forward selection, and significant parameter cutoff values were obtained with Fischer’s exact test to group patients by risk of developing physician-graded toxicity or detriments in patient-reported QoL.
Results
One hundred and three patients treated for localized prostate cancer with SBRT were included in our analysis. 52% of patients experienced a clinically significant decline in bowel-related QOL within 1 week of completion of treatment, while only 27.5% of patients developed grade 2+ physician-graded rectal toxicity. Sequential feature selection multivariate logistic regression identified rectal V22.5 Gy (p = 0.001) and D19% (p = 0.001) as independent predictors of clinically significant toxicity, while rectal V20Gy (p = 0.004) and D25.3% (p = 0.007) were independently correlated with physician-graded toxicity. Global multivariate step-wise logistic regression identified only D19% (p = 0.001) and V20Gy (p = 0.004) as independent predictors of acute bowel bother or physician-graded rectal toxicity respectively.
Conclusions
Moderate doses to large rectal volumes, D19% and V20Gy, were associated with an increased incidence of a clinically significant decrease in patient-reported bowel QOL and physician-scored grade 2+ rectal toxicity, respectively. These dosimetric parameters may help practitioners mitigate acute toxicity in patients treated with prostate SBRT.
Introduction
Prostate cancer is the most common noncutaneous cancer in American males; approximately one in eight men will receive this diagnosis in their lifetime [Citation1]. Particularly since the advent of prostate-specific antigen (PSA) screening, a substantial proportion of these patients will present with clinically localized disease [Citation2]. Multiple standard-of-care treatment options are available for these patients including active surveillance (AS), radical prostatectomy (RP), and radiotherapy, as well as other investigational approaches including cryotherapy and high-frequency ultrasounds (US).
Radiotherapy can be safely and effectively delivered using a variety of approaches, including both external beam radiotherapy (EBRT) and brachytherapy. Over the past two decades, stereotactic body radiotherapy (SBRT), has emerged as an attractive treatment technique given both the patient convenience and unique radiobiology of prostate cancer [Citation3]. In addition to several institutional and prospective series, multiple randomized studies have now been conducted that support its use in the management of localized prostate cancer [Citation4–6]. Consequently, it has been incorporated into the most recent National Comprehensive Cancer Network (NCCN) Consensus Guidelines as an acceptable treatment for patients with low, intermediate, and high-risk prostate cancers [Citation7].
However, there is considerable variation across institutions in the technical approach to prostate SBRT [Citation8,Citation9]. Most early prostate SBRT treatments were delivered using robotic radiosurgery, but many other platforms have been subsequently employed, including conventional and MRI-guided linear accelerators [Citation8,Citation10]. While the five-fraction regimen (700 –800 cGy per fraction for a total of 3500 − 4000 cGy is most widely used, schema as short as two fractions (1300 cGy per fraction for a total of 2600 cGy) have been implemented with success as well [Citation11]. Even a patient who receives a nominally identical prescription dose may receive drastically different treatment between two institutions; some providers have attempted to mimic high-dose-rate (HDR) brachytherapy dosimetry as others have strived for a more homogeneous dose distribution [Citation12]. As a result of this heterogeneity, there is no clear consensus on optimal dose constraints that may mitigate genitourinary and gastrointestinal toxicity. Nonetheless, multiple studies have demonstrated that a subset of patients experiences a meaningful, acute decline in bowel-related quality of life following prostate SBRT [Citation6,Citation13,Citation14]. In this study, we sought to identify dosimetric and patient-related predictors of acute rectal toxicity.
Materials & methods
Patients & data collection
Patients included in this study had clinically localized prostate cancer and were treated with prostate SBRT on a prospective single institutional protocol. The details of radiation treatment planning and delivery have been previously described [Citation15]. All patients had intraprostatic fiducials placed prior to the CT simulation. Organs at risk (OARs) were contoured following the RTOG/NRG prostate contouring atlas [Citation16]. The planning target volume (PTV) consisted of the prostate and proximal seminal vesicles as defined on non-contrast CT and fused T2 MRI with a 3 mm posterior margin and a 5 mm margin in all other directions. Inverse plans were generated with a prescription dose (PD) of 3500 to 3625 cGy in five fractions to the PTV using 6 MV photons. Patients were instructed to perform an enema one hour prior to the simulation and before each delivered treatment fraction. SBRT treatment was delivered using the CyberKnife robotic radiosurgical platform (Accuray Inc., Sunnyvale, CA) on alternating weekdays over 11 total days. No patient received a hydrogel rectal spacer prior to simulation or treatment. Plan objectives and dose constraints utilized are summarized in .
Table 1. Dose constraints.
Physician-graded toxicity was scored prospectively using the Radiation Therapy Oncology Group (RTOG) and European Organization for Research and Treatment of Cancer (EORTC) scoring system. Clinically significant physician-graded toxicity was defined as grade 2+ rectal toxicity. Patients completed multiple QOL surveys including the Expanded Prostate Index Composite-26 (EPIC) prior to treatment as well as at one week, one month and three months following completion of SBRT. The EPIC-26 bowel domain includes five questions related to individual symptoms (questions 6a–e: urgency, frequency, pain, bloody stool, incontinence) and one question (question 7) related to overall bother (degree of annoyance caused by bowel symptoms). A clinically significant decline in acute bowel-related QOL was defined as a decline in EPIC bowel bother score (EPIC-26 question 7) of a one-half standard deviation below baseline, using maximum likelihood estimation.
Normal tissue complication probability (NTCP) modeling
Cumulative absolute volume rectal dose volume histograms (DVHs) were extracted from the treatment planning system and converted into relative volume differential DVHs using custom software written in Matlab (MathWorks, Inc., MA). The probability of developing acute bowel toxicity was modeled by fitting rectal DVH data to our QOL and CTCAE data using a probit function [Citation17]:
with summary parameter,
derived from a generalized Lyman model. This model has been defined previously and will only be described briefly.
For the Lyman model, is related to the effective uniform dose (EUD), or the homogenous whole organ dose equivalent that would be expected to result in the same complication probability as the actually delivered heterogeneous dose [Citation18]
and [Citation19]
where
is related to the slope of the sigmoidal NTCP dose-response curve,
is the homogeneous dose to the whole organ that would be expected to produce a 50% incidence of toxicity, and
describes the volume dependence of dose to the organ of interest. The sum is over the
physical dose bins (
) of the differential DVH with fractional volume,
All model parameters were estimated using maximum likelihood estimation (MLE) with the estimator [Citation20]:
Out of computational convenience, MLE is performed by maximizing the log-likelihood (LL) function. This is made possible by the fact that the likelihood function and its natural log are monotonically related so that they each share the same MLE. The subscripts p and q represent patients with and without acute bowel toxicity, respectively. The profile likelihood and Wald methods were used to generate estimates of the parameter 95% confidence intervals.
Statistical analysis
Patient- and disease-related covariates along with NTCP model predictions were independently tested for significant association with the development physician-graded toxicity or a clinically significant decline in acute bowel-related QOL. A multivariate model was then constructed using forward selection with a cutoff p-value of 0.05. Significant parameter cutoff values were obtained with Fischer’s exact test and then used to group patients by risk of developing either physician-graded or patient-reported toxicity.
Results
One hundred and three patients treated definitively for clinically localized prostate cancer were included. Briefly, the median age was 69 years old (range 48 – 85) and the majority of patients (n = 65, 63.1%) were diagnosed via PSA screening. The median gland size was 36 cc (13 to 125) and a majority of patients had intermediate-risk disease (n = 66, 64.1%). Roughly half of the patients received 3625 cGy in 5 fractions (n = 54, 52.4%) while the remainder received 3500 cGy in 5 fractions. Full baseline patient characteristics are available in . Complete QoL data for this cohort have previously been reported [Citation13].
Table 2. Baseline patient characteristics.
Fifty-two per cent of patients (n = 54) experienced a clinically significant decline in bowel-related QOL within 1 week of completion of prostate SBRT, while only 27.5% (n = 28) of patients developed grade 2+ physician-graded rectal toxicity. Univariate analysis of patient and disease-related factors demonstrated no significant predictors of a clinically significant decline in QoL or physician-graded toxicity (). Group-averaged cumulative and differential rectal DVH data are demonstrated in for patients with and without acute bowel bother, with a distinct rightward shift of the average curves in the moderate to high dose region. Fitting of rectal DVH data to a Lyman NTCP model for acute QoL bowel bother resulted in parameter estimates of m, TD50, and n of 0.15 (0 − 0.30), 20.8 (15.1 − 26.5), and 0.27 (0.01 − 0.53), respectively. For the acute physician-graded toxicity model, these values were 0.19 (0 − 0.38), 21.6 (15.9 − 27.3), and 0.39 (-0.11 − 0.89) respectively.
Figure 1. Group-averaged cumulative (A) and differential (B) DVH data, stratified by presence or absence of acute QoL bowel bother.
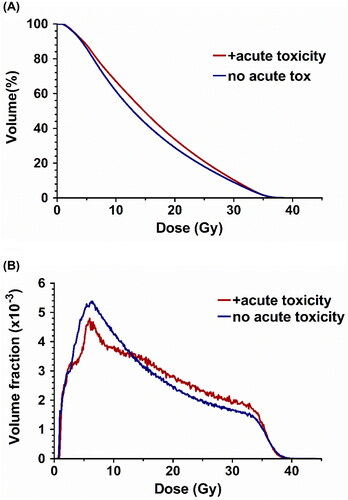
Table 3. Univariate analysis of the impact of patient and disease-related factors on acute rectal toxicity.
Univariate regression for predictors of physician-graded toxicity and acute bowel bother are shown in . Sequential feature selection multivariate logistic regression was then performed to identify independent dose and volume predictors of both acute bowel bother and physician-graded rectal toxicity. For acute bowel bother, rectal V22.5 Gy (OR 1.13, 95% CI 1.05 − 1.33, p = 0.001) and D19% (OR 1.37, 95% CI 1.14 − 1.66, p = 0.001) were identified as independent predictors of clinically significant toxicity, while rectal V20Gy (OR 1.11, 95% CI 1.03 − 1.19, p = 0.004) and D25.3% (OR 1.30, 1.07 − 1.58, p = 0.007) independently correlated with physician-graded toxicity.
Table 4. Univariate analysis of predictors of acute bowel bothers and physician-graded toxicity.
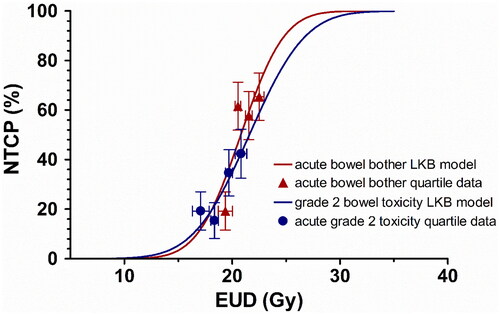
A global multivariate step-wise logistic regression was then performed including the independent logistic indices as well as generalized Lyman model predictions to identify predictors of acute toxicity. Only D19% (OR 1.37, 1.14 − 1.66, p = 0.001) and V20Gy (OR 1.11, 1.03 − 1.19, p = 0.004) were independent predictors of acute bowel bother or physician-graded rectal toxicity respectively. No other significant independent predictors of acute toxicity were identified. The median D19% for this cohort was 24.8 Gy (delivered over 5 fractions), with a median split incidence of acute bowel bother of 39% vs 65%. The median V20Gy for this cohort was 30.2%, with a median split incidence of grade 2+ rectal toxicity of 15% vs. 39% ().
Figure 2. Results of the LKB model fit differential rectal DVH data versus EUD (solid lines) together with incidence of clinically significant bowel bother and physician-graded toxicity among EUD quartiles (triangles, circles). Vertical error bars indicate the standard deviation of the binomial distribution and horizontal error bars represent the standard deviation of the EUD in each quartile.
Discussion
Acute gastrointestinal toxicity, particularly acute proctitis manifesting as a diarrhoea, loose stools, tenesmus, and cramping may occur following prostate radiotherapy, regardless of fractionation schema [Citation21]. Recent randomized data suggest that patients undergoing SBRT for localized prostate cancer experience similar acute gastrointestinal toxicity at similar rates to patients receiving conventional or moderately hypofractionated radiotherapy, although these symptoms typically peak and resolve more rapidly given the condensed time course of treatment [Citation6]. Nonetheless, rectal dose objectives for prostate SBRT are inconsistent and have largely been generated on a theoretical rather than empirical basis.
The current study demonstrates that moderate doses to the rectum were most predictive of both patient-reported detriments in QoL (D19%, median value 24.8 Gy in this cohort) and physician-graded toxicity (V20Gy, median value 30.2% in this cohort). While the small volumes of the rectum receiving ultra-high doses to the rectum are typical of most concern to the practicing radiation oncologist, no clear effect could be identified in this region. Multiple explanations are possible for the lack of impact in this dose range. First, patients in this study were treated with very strict and highly consistent high-dose rectal objectives. In , both the cumulative and differential rectal DVH curves are nearly identical for patients with and without toxicity, suggesting similar high-dose dosimetry across the entire patient cohort. Furthermore, it is reasonable to assume that while high doses may be associated with severe late complications (e.g. rectal ulcer) [Citation22], moderate doses to a larger volume may better predict acute toxicity. This finding is intuitive and aligns with acute toxicity profiles observed during the treatment of other malignancies such as testicular seminoma, where larger volumes of the bowel are exposed to modest doses of radiation [Citation23]. However, the impact of these findings and their correlation with late rectal toxicity in patients receiving prostate SBRT remain unresolved [Citation24].
Additionally, the advent of hydrogel spacers has further enhanced the ability of the radiation oncologist to minimize rectal dose and mitigate toxicity [Citation25,Citation26]. Much of the focus on the dosimetric advantages of a rectal spacer has highlighted the ability of the spacer to eliminate the highest doses to the rectum by eliminating overlap with the PTV [Citation27]. However, the seminal randomized trial which demonstrated a clinical benefit to spacer placement in patients undergoing conventionally fractionated EBRT also showed substantial dosimetric benefit in the moderate dose delivered to the rectum as well [Citation25]. Extrapolating from these findings, it is reasonable to assume that significant reductions in moderate doses to the rectum (e.g. the D19% and the V20Gy) may lead to mitigation of acute toxicity with a rectal spacer in prostate SBRT patients as well. Indeed, early data suggests a meaningful dosimetric and clinical benefit in patients who have a rectal spacer placed prior to SBRT [Citation28,Citation29].
Strengths of this study include the prospective nature of the data collection, the comprehensive physician-graded and patient-reported toxicity data, the consistency of treatment delivery, and the analysis of detailed dosimetry data. Weaknesses of the study include the retrospective nature of the analysis, the relatively small number of patients included, and the lack of long-term follow-up data. Overall, this study demonstrates the importance of mindfulness to moderate doses of radiation during prostate SBRT which may be easily overlooked in the treatment planning process.
Conclusions
Moderate doses to large rectal volumes, D19% and V20 Gy, were associated with an increased incidence of a clinically significant decrease in patient-reported bowel QoL and physician-scored grade 2+ rectal toxicity respectively. These dosimetric parameters may help guide practitioners to mitigate acute toxicity in patients treated with prostate SBRT. The impact of acute rectal toxicity after ultra-hypofractionated prostate radiotherapy on long-term bowel function remains unknown and additional research is warranted to better characterize this relationship.
Author contributions
TPK and SPC conceived the study. All authors participated in the collection of data. TPK performed the data analysis. MCR wrote the initial draft of the manuscript. TPK created the figures. All authors participated in editing and review of the manuscript.
Acknowledgements
The authors would like to thank the Dosimetry and Therapy staff at MedStar Georgetown University Hospital.
Declaration statement
Sean P. Collins serves as a consultant for Accuray (Sunnyvale, CA) and Boston Scientific (Marlborough, MA). The remaining authors have no relevant conflicts of interest to declare.
Meeting presentation
This project was presented as an oral presentation at the 12th International Stereotactic Radiosurgery Society Congress (Yokohama, Japan, 7–11 June 2015).
Data availability statement
The data that support the findings of this study are available from the corresponding author, [MCR], upon reasonable request.
References
- Miller KD, Nogueira L, Devasia T, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J Clin. 2022;72(5):409–436.
- Hung AY, Levy L, Kuban DA. Stage T1c prostate cancer: a heterogeneous category with widely varying prognosis. Cancer J Sudbury Mass. 2002;8(6):440–444.
- Royce TJ, Mavroidis P, Wang K, et al. Tumor control probability modeling and systematic review of the literature of stereotactic body radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2021; 110(1):227–236.
- Widmark A, Gunnlaugsson A, Beckman L, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet Lond Engl. 2019; 394(10196):385–395.
- Fransson P, Nilsson P, Gunnlaugsson A, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer (HYPO-RT-PC): patient-reported quality-of-life outcomes of a randomised, controlled, non-inferiority, phase 3 trial. Lancet Oncol. 2021; 22(2):235–245.
- Brand DH, Tree AC, Ostler P, et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2019; 20(11):1531–1543.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology. Prostate Cancer [Internet]. Version 4; 2022. Available from: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.
- Alongi F, Cozzi L, Arcangeli S, et al. Linac based SBRT for prostate cancer in 5 fractions with VMAT and flattening filter free beams: preliminary report of a phase II study. Radiat Oncol. 2013; 8(1):171.
- Fuller DB, Naitoh J, Lee C, et al. Virtual HDR CyberKnife treatment for localized prostatic carcinoma: dosimetry comparison with HDR brachytherapy and preliminary clinical observations. Int J Radiat Oncol Biol Phys. 2008; 70(5):1588–1597.
- Leeman JE, Cagney DN, Mak RH, et al. Magnetic resonance–guided prostate stereotactic body radiation therapy with daily online plan adaptation: results of a prospective phase 1 trial and supplemental cohort. Adv Radiat Oncol. 2022;7(5):100934.
- Alayed Y, Quon H, Cheung P, et al. Two versus five stereotactic ablative radiotherapy treatments for localized prostate cancer: a quality of life analysis of two prospective clinical trials. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2019; 140:105–109.
- Fuller DB, Crabtree T, Kane BL, et al. High dose “HDR-Like” prostate SBRT: PSA 10-year results from a mature, multi-institutional clinical trial. Front Oncol. 2022;12:935310.
- Paydar I, Cyr RA, Yung TM, et al. Proctitis 1 week after stereotactic body radiation therapy for prostate cancer: implications for clinical trial design. Front Oncol. 2016;6:167.
- Bhattasali O, Chen LN, Woo J, et al. Patient-reported outcomes following stereotactic body radiation therapy for clinically localized prostate cancer. Radiat Oncol Lond Engl. 2014;9:52.
- Chen LN, Suy S, Uhm S, et al. Stereotactic body radiation therapy (SBRT) for clinically localized prostate cancer: the Georgetown university experience. Radiat Oncol Lond Engl. 2013;8:58.
- Hall WA, Paulson E, Davis BJ, et al. NRG oncology updated international consensus atlas on pelvic lymph node volumes for intact and postoperative prostate cancer. Int J Radiat Oncol Biol Phys. 2021;109(1):174–185.
- Lyman JT. Complication probability as assessed from dose-volume histograms. Radiat Res Suppl. 1985;8: s 13–19.
- Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: the effective volume method. Int J Radiat Oncol Biol Phys. 1989; 16(6):1623–1630.
- Burman C, Kutcher GJ, Emami B, et al. Fitting of normal tissue tolerance data to an analytic function. Int J Radiat Oncol Biol Phys. 1991; 21(1):123–135.
- Kutcher GJ, Burman C, Brewster L, et al. Histogram reduction method for calculating complication probabilities for three-dimensional treatment planning evaluations. Int J Radiat Oncol Biol Phys. 1991; 21(1):137–146.
- Vernia P, Fracasso PL, Casale V, et al. Topical butyrate for acute radiation proctitis: randomised, crossover trial. Lancet Lond Engl. 2000; 356(9237):1232–1235.
- Kim DWN, Cho LC, Straka C, et al. Predictors of rectal tolerance observed in a dose-escalated phase 1-2 trial of stereotactic body radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2014; 89(3):509–517.
- Pasalic D, Prajapati S, Ludmir EB, et al. Outcomes and toxicities of proton and photon radiation therapy for testicular seminoma. Int J Part Ther. 2020; 7(2):11–20.
- Leufgens F, Gharib A, Schlenter M, et al. Consequential late effects up to >10 years following primary and postoperative radiotherapy for prostate cancer. Radiother Oncol J Eur Soc Ther Radiol Oncol. 2021; 156:188–192.
- Mariados N, Sylvester J, Shah D, et al. Hydrogel spacer prospective multicenter randomized controlled pivotal trial: dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy. Int J Radiat Oncol. 2015;92(5):971–977.
- Hamstra DA, Mariados N, Sylvester J, et al. Continued benefit to rectal separation for prostate radiation therapy: final results of a phase III trial. Int J Radiat Oncol. 2017; 97(5):976–985.
- Fischer-Valuck BW, Chundury A, Gay H, et al. Hydrogel spacer distribution within the perirectal space in patients undergoing radiotherapy for prostate cancer: impact of spacer symmetry on rectal dose reduction and the clinical consequences of hydrogel infiltration into the rectal wall. Pract Radiat Oncol. 2017; 7(3):195–202.
- Kataria S, Hong RL, McRae D, et al. The rectal dosimetric effects of perirectal hydrogel spacers in men undergoing prostate stereotactic body radiation therapy (SBRT). Int J Radiat Oncol. 2017; 99(2, Supplement):E676.
- Payne HA, Pinkawa M, Peedell C, et al. SpaceOAR hydrogel spacer injection prior to stereotactic body radiation therapy for men with localized prostate cancer: a systematic review. Medicine (Baltimore). 2021; 100(49):e28111.
