Abstract
Background: ‘Trials-within-Cohorts’ (TwiCs), previously known as ‘cohort multiple randomized controlled trials’ is a pragmatic trial design, supporting an efficient and representative recruitment of patients for (future) trials. To our knowledge, the ‘COhort for Lung cancer Outcome Reporting and trial inclusion’ (COLOR) is the first TwiCs in lung cancer patients. In this study we aimed to assess the feasibility and first year results of COLOR.
Material and Methods: All patients diagnosed with lung cancer referred to the Radiotherapy department were eligible to participate in the ongoing prospective COLOR study. At inclusion, written informed consent was requested for use of patient data, participation in patient-reported outcomes (PROs), and willingness to participate in (future) trials. Feasibility was studied by assessing participation and comparing baseline PROs to EORTC reference values. First-year results of PROs at baseline and 3 months after inclusion were evaluated separately for stereotactic body radiotherapy (SBRT) and conventional radiotherapy patients.
Results: Of the 338 eligible patients between July 2020 and July 2021, 169 (50%) participated. Among these, 127 (75%) gave informed consent to PROs participation and 110 (65%) were willing to participate in (future) trials. The inclusion percentage dropped from 77% to 33% when the information procedure was switched from in-person to by phone (due to COVID-19 pandemic measures). Baseline PROs for physical and cognitive functioning were comparable in COLOR patients compared to the EORTC reference values. No significant changes in PROs were observed 3 months after inclusion, except for a slight increase in pain scores in the SBRT group (n = 97).
Conclusions: The TwiCs-design appears feasible in lung cancer patients with fair participation rates (although negatively impacted by the COVID-19 pandemic). With a planned expansion to other centers, the COLOR-study is expected to enable multiple (randomized) evaluations of experimental interventions with important advantages for recruitment, generalizability, and long-term outcome data collection.
Introduction
Lung cancer is responsible for 11.4% of all new cancer cases worldwide [Citation1]. In The Netherlands, annually 14,000 new patients are diagnosed and approximately 11,000 patients die of lung cancer [Citation2]. New techniques and treatment strategies in radiotherapy, surgery, and systemic therapy (i.e., chemotherapy, immunotherapy, and targeted therapy) are continuously being developed. To implement these new approaches in daily practice, multiple clinical trials are needed to assess safety and efficacy and optimize protocols. The cornerstone of evidence-based medicine before considering the implementation of novel treatments in clinical practice is the randomized controlled trial (RCT). However, one frequently observed issue of oncologic RCTs is the recruitment of patients of a highly selective (generally more fit) study population, leading to low generalizability of the results. Secondly, RCT inclusion target rates, also in lung cancer, are often not reached due to slow accrual and limited willingness in both patients and clinicians [Citation3,Citation4]. This delays the assessment and implementation of innovative treatment strategies in daily clinical practice.
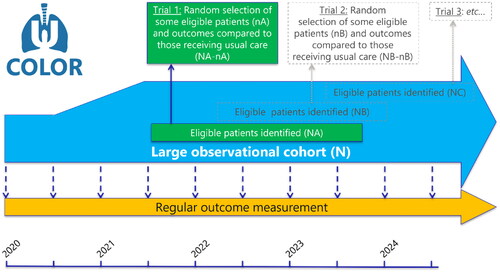
To overcome these issues of RCTs, the ‘Trials-within-Cohorts’ (TwiCs) design, formerly known as ‘cohort multiple RCTs’ was developed [Citation5]. This is a pragmatic trial design that allows for more efficient and representative recruitment of patients for trials [Citation5,Citation6]. TwiCs starts as an observational prospective cohort containing patients with similar patient and disease characteristics who undergo standard treatment. During the study period, the outcomes of patients undergoing standard-of-care can be observed using predetermined repeated measurements of outcomes, with generally a focus on patient-reported outcomes (PROs). Furthermore, when researchers want to investigate a novel intervention, eligible patients can be randomized within the TwiCs cohort, with logistics for data collection already in place. Only patients randomly allocated to the intervention arm are offered the experimental intervention (which they can accept or refuse). Patients who are randomly allocated to the control arm receive standard care, and are not informed about serving in the control arm. It has been demonstrated previously that the majority of patients (93%) responds positively or neutrally on having served as a control without a notification at the time of randomization [Citation7]. After randomization, collected outcomes can be compared with the outcomes of the representative pool of patients in the control group receiving the standard-of-care within the cohort. Multiple randomized trials can be conducted simultaneously within one cohort, without the need of asking for consent again before randomization, as they gave broad consent to be randomly allocated to experimental interventions. Naturally, patients allocated to the intervention arm, will always be informed and asked for their consent to participate in the next trial.
The described advantages of TwiCs in comparison to the classic RCT have the potential to result in more efficient (i.e., faster) trial inclusion and less selection bias (i.e., better representation of real-world practice). Another major advantage is the reduction of disappointment bias. Patients and doctors often have a strong preference for the experimental treatment and expect it to be superior, although it has not been proven yet. Patients allocated to the conventional arm therefore often show disappointment when reporting outcomes. This is of particular concern in studies with a subjective outcome (e.g., PROs) as a primary endpoint. By choosing the TwiCs trial design, as control patients are unaware of being allocated to the control arm, this disappointment bias is overcome. In addition, the standard of care is likely to be unaffected by treatment allocation, and will therefore better resemble routine practice. This has convinced many investigators to adopt the TwiCs design in a wide variety of fields (e.g., breast cancer, rectal cancer, mental health, care of the elderly, rare diseases, COVID-19) [Citation9,Citation10].
To the best of our knowledge, the first application of the TwiCs design in lung cancer was initiated in The Netherlands as the ‘Cohort for Lung cancer Outcome Reporting and trial inclusion’ (COLOR) study. The primary aim of the current analysis was to assess the feasibility of the TwiCs design in patients with lung cancer as determined by participation rates and by comparing baseline PROs to European Organization for Research and Treatment of Cancer (EORTC) reference values. The secondary aim was to describe the first results of COLOR in terms of the short-term quality of life (QoL) of patients undergoing radiotherapy.
Material and methods
Study design and population
COLOR is an ongoing observational prospective cohort study, following the TwiCs design (), and opened to enroll patients in July 2020. The ethical considerations of this design were extensively discussed previously [Citation11]. The study was approved by the institutional review board of the UMC Utrecht and conducted in accordance with the principles of the Declaration of Helsinki and the Good Clinical Practice Guidelines. The study was registered with clinicaltrials.gov (NCT05069792).
Study population
All patients ≥18 years diagnosed with lung cancer referred to the Department of Radiotherapy of the University Medical Center Utrecht were eligible to participate. In case no histological or cytological confirmation of lung cancer was available, radiologic evidence with a lung cancer suspicion as determined by a multidisciplinary tumor board was sufficient for inclusion. All patients with disease stages I-IV, either with a first diagnosis of lung cancer or with disease recurrence (who were not previously approached for study inclusion) were eligible. For the purpose of analyzing the PROs, patients who did not receive radiotherapy were excluded. Subsequently, patients were divided into a group who received stereotactic body radiation therapy (SBRT) and a group who received conventional (fractionated) radiotherapy. Patients eligible for inclusion in our center’s preexisting cohorts (–. patients with bone metastases in PRESENT [Citation12], brain metastases in COIMBRA [NCT05267158] and oligometastases in OLYMPOS [Citation13]) were excluded from COLOR and asked for inclusion in these specific cohorts.
Procedures
At the first presentation of eligible patients in the department, written informed consent was requested for the use of patient data (as a minimum requirement for inclusion), and they could opt-in for participation in QoL questionnaires for the collection of PROs (optional). In a separate question, we asked patients for their broad consent to be randomly allocated to experimental interventions in the (near) future (optional). The request for study participation was performed by trained independent college-educated research assistants and took place in-person at the outpatient clinic by preference, but by phone from December 2020 to July 2021 due to distancing measures related to the COVID-19 pandemic. After inclusion, patient-, tumor-, and treatment-related characteristics, as well as toxicity, follow-up, survival and QoL data were registered [Citation14]. Patients were followed-up regularly by their own physician, according to national guidelines, which includes imaging with computed tomography (CT), as well as history and physical examination and scoring of toxicity (according to CTCAE version 5) every 3 months. Regarding PROs, internationally validated questionnaires were sent out to participants at predetermined time points to assess QoL (EORTC QLQ-C30[Citation15], EQ5D-5L[Citation16]), disease-specific symptoms of lung cancer and adverse effects of treatment (EORTC QLQ-LC13[Citation17]), cancer-related outcomes such as fatigue (MFI-20[Citation18]), depression and anxiety (HADS[Citation19]) and -for patients aged ≤67 years- work-ability (WAI[Citation20]). Patients were given the choice to complete questionnaires either online or on paper. For the online questionnaires, the digital patient tracking system Patient Reported Outcomes Following Initial treatment and Long term Evaluation of Survivorship (PROFILES) was used [Citation21]. Reminders were sent when required both online and on paper.
Outcomes
Regarding the primary aim of studying the feasibility of TwiCs in lung cancer patients, studied outcomes were the patient participation rates for inclusion in the cohort (i.e., consenting to use patient data) and for participating in the QoL questionnaires, as well as the rate of patients confirming willingness to participate in (future) trials. To assess feasibility in terms of representativeness of the cohort, the EORTC QLQ-C30 functioning scales of participants were compared with the published EORTC reference values [Citation22].
With regard to the secondary aim, the short-term results (at baseline and 3 months after inclusion) of the PROs measured with QoL questionnaires were assessed. These results were analyzed in the SBRT and conventional radiotherapy groups separately. PRO scores extracted from EORTC questionnaires were calculated based on EORTC scoring manuals and ranged from 0 to 100. A higher score (0–100) on the EORTC QLQ-C30 global health score and functional scales signifies better health or functioning. Conversely, higher scores (0–100) on the specific symptom scales in the EORTC-QLQ-C30 and the EORTC-QLQ-LC13 signify a higher burden of symptoms. With the EQ5D-5L questionnaire, participants were asked to score 5 health dimensions on a Likert scale from ‘no problems/complaints’ to ‘extreme problems/complaints’ (1–5). MFI-20 yields a sum score from 5 to 20 with a higher score signifying more fatigue in the specific category. The sum scores of anxiety and depression of the HADS questionnaire were subdivided into 3 categories (0–7, normal; 8–10, borderline abnormal; 11–21, abnormal). A sum score of 7–27 on the WAI questionnaire indicates bad work-ability, whereas higher scores (28–36, medium; 37–43, good; 44–49, perfect) indicate better workability.
Statistical analysis
Participation rates were calculated as proportions with 95% confidence intervals (CIs). Baseline characteristics were presented separately for the SBRT and conventional radiotherapy groups, as numbers with percentages or means ± standard deviations (SDs). Baseline EORTC-QLQ-C30 scores were compared to an EORTC reference population of unselected patients with lung cancer using one-sample T-tests [Citation22].
All QoL scores at baseline were presented as means (±SD). Missing outcome data were observed at 3 months, which were considered to be ‘missing at random,’ meaning that the propensity to the outcome data point to be missing was related to – or could be explained by – (part of) the observed data (e.g., baseline characteristics or baseline PRO scores). Missing PRO data 3 months after inclusion was imputed using multiple imputations (20x) [Citation23]. The changes at 3 months compared to baseline were pooled across the imputed datasets and expressed as means with 95% confidence intervals (CIs). The mean changes in QoL from baseline to 3 months after inclusion were tested using paired T-tests and visualized as box plots. Analyses were performed using SPSS version 27.0 (IBM Corp, IBM SPSS Statistics for Windows, Armonk, NY). A p-value <0.05 was considered statistically significant.
Results
Of the 338 eligible patients with lung cancer who presented between July 2020 and July 2021, 169 (50%) signed informed consent for participating in the COLOR study. The inclusion percentage changed from 77% (up to December 2020) to 33% (from December 2020 to July 2021; p < 0.001) when the in-person information procedure was switched to an information procedure by phone due to distancing measures related to the COVID-19 pandemic. Among these 169 patients, 127 (75%) provided informed consent to participate in the QoL questionnaires and 110 (65%) expressed willingness to participate in (future) trials within the cohort. For subsequent PRO analyses, 10 patients were excluded because they did not receive radiotherapy. Of the remaining 159 patients, 97 (61%) were treated with SBRT and 62 (39%) received conventional radiotherapy.
Baseline characteristics stratified per treatment type are presented in . The SBRT group mainly consisted of patients with stage I disease (73%) whereas the majority of patients in the conventional group had locally advanced (stage III) disease (69%).
Table 1. Baseline characteristics.
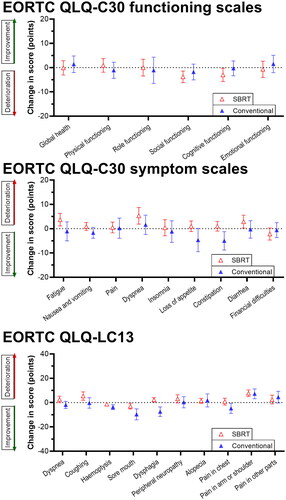
Patients’ physical and cognitive functioning scores at baseline (according to the EORTC QLQ-C30 questionnaire) were comparable to the EORTC reference values for patients with lung cancer [Citation22], whereas the mean scores for social, role, and emotional functioning were slightly higher in the COLOR cohort (). Among the 159 included patients who underwent radiotherapy, 120 (75%) were willing to participate in PRO questionnaires, of whom 97 (81%) completed the questionnaires at baseline and 64 (53%) after 3 months. The WAI questionnaire was completed by only 10 patients (6%) at baseline and 4 patients (3%) after 3 months, mostly due to the age restriction, and therefore WAI scores were not analyzed.
Table 2. One sample T-test of PRO mean compared to the mean of EORTC reference values.
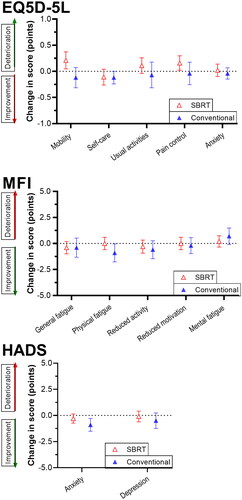
PROs at baseline and the mean changes after 3 months within the SBRT group and conventional group are presented in . Mean changes of PROs at 3 months in both groups are visualized in . In general, the SBRT group scored relatively high on the functioning scales (indicating better functioning), whereas the conventional group scored relatively high on the symptom scales (indicating more symptoms). The mean scores on the HADS questionnaire for depression and anxiety were categorized as ‘normal’ for both groups. In the SBRT group, a significant increase in pain in the arm and shoulder (mean change +7.7, p = 0.004) was found. In the conventional radiotherapy group, none of the PROs significantly changed from baseline to 3 months later. No other significant differences between baseline and 3 months were demonstrated.
Figure 2. Mean changes of PROs at 3 months in the SBRT and conventional group.
Table 3. Within-group changes in patient reported outcome from baseline to follow-up at 3 months among respondents in the lung cancer cohort.
Discussion
TwiCs is a pragmatic trial design with specific benefits in comparison to the classical RCT and here we report the results on the feasibility of the first TwiCs study (COLOR) in patients with lung cancer. The response rate of all lung cancer patients referred to our Radiotherapy department in the first year of COLOR was 50% (n = 169). However, we found that this participation rate was 77% when patients were informed in person, compared to 33% when informed by phone (which was imposed by distance measures imposed by the COVID-19 pandemic). The observed participation rate in QoL questionnaires of 75% is comparable to that found in other TwiCs designs like PLCRC (colorectal cancer, 73%) [Citation24] and UMBRELLA (breast cancer, 88%) [Citation25]. Patients’ willingness to be contacted for participation in (future) trials within COLOR (65%) was slightly lower compared with these earlier mentioned cohorts (PLCRC >85%, UMBRELLA 87%). A possible explanation could be the higher age of patients included in our cohort compared to PLCRC (66 years) and UMBRELLA (59 years) or a potentially higher disease burden in lung cancer patients.
Several RCTs conducted in lung cancer research failed due to a lack of patients that could be recruited. For example, the trials that aimed to compare the effects of SBRT versus lobectomy in early-stage operable non-small cell lung cancer (NSCLC) had difficulties with recruiting patients. The ROSEL trial (which had an estimated enrollment of 960 participants in 60 months), included 22 patients in 26 months, and the STARS trial included 36 patients in 49 months [Citation3,Citation4]. Furthermore, the ACOSOG trial (NCT01336894), a randomized phase III study of surgery with or without brachytherapy versus SBRT in high-risk patients with stage I NSCLC, recruited 13 patients in 23 months and was terminated early because of the disappointing accrual. The TwiCs design can have a beneficial effect on the recruitment of patients for similar research because the TwiCs approach avoids disappointment bias in patients allocated to the control arm (i.e., the phenomenon observed in a classical RCT among patients randomized to the control arm, while hoping to be randomized to the intervention arm) [Citation6]. In a trial within the cohort, only patients randomized to the intervention arm receive information about the new intervention. As such, patients in the control arm are not prone to disappointment and associated reporting of more negative outcomes, which makes the TwiCs especially attractive in trials assessing a subjective outcome such as QoL.
The vision of the European Society Radiation Oncology (ESTRO) is to create a holistic database including PROs in radiation oncology as fundamental information in defining and improving value-based health care [Citation26]. The TwiCs design can facilitate such a broad and real-world reflection of PROs for example in lung cancer patients, because patients in any stage of the disease and given treatment can be included. On the contrary, RCTs include patients with specific patient characteristics, who are fit enough to be randomized to either the control or intervention group. Therefore, PROs in specific RCTs are more difficult to extrapolate to other lung cancer patients.
The functioning scale data of the COLOR cohort showed good correspondence to the EORTC reference values, but slight differences were observed [Citation22]. However, these EORTC reference values were published more than a decade ago (2008), which could (in part) explain the differences. Over the last decade, there has been a huge development in both radiotherapy techniques and chemotherapy/immunotherapy treatments which could have had a positive effect on the functioning scale means of patients with lung cancer. The short-term results of the PROs in the COLOR cohort showed a slight increase in pain in patients who underwent SBRT. No significant changes in PROs were observed in patients in the conventional group.
A few limitations apply to our study. First, the sample size was limited to what could be achieved in the first year of the prospective study. A larger sample size could reduce the risk of type II errors, and therefore a continuation of patients in the study as well as expansion to other centers is planned. Second, for this analysis, we limited inclusion eligibility to lung cancer patients referred to the Radiotherapy department. This could limit the generalizability of our results to non-irradiated lung cancer patients. Our future efforts will focus on asking all non-irradiated lung cancer patients for participating in COLOR as well. Third, patients with bone or brain metastases were not included in COLOR and not analyzed in the current study. However, these patients were eligible for very similar TwiCs studies within our institution and when a full picture of all lung cancer patients is desired in the future, this could be realized by combining the studies. Fourth, PROs in the conventional group might have been subject to a certain degree of performance bias due to the variation in the start of chemotherapy (concurrent or sequential) in relation to PRO time points. Fifth, long-term outcomes, such as local control and survival rates, were not presented in the current analysis due to the immaturity of data. Strengths of this study include the prospective observational design, standardized outcome measurements, and representativeness for real-world clinical practice.
The introduction of future randomized trials within the TwiCs cohort could also come with a few limitations. Presumably, noncompliance in the intervention arm will be higher, as patients are asked to participate after randomization. However, this may (partly) be compensated for by the lower (or even absent) noncompliance in the control arm. Also, in case of substantial refusal in the interventional arm, this can be accounted for by instrumental variable analysis as previously described [Citation8]. In addition, because only the patients randomly allocated to the interventional arm are asked for consent to participate in the trial, there might be a risk of imbalance between comparative arms. The slightly healthier and less frail patients may be more likely to give consent to the interventional arm, whereas almost all patients allocated to the conventional arm are expected to be compliant. This could induce a bias that may diminish the effect size of the intervention arm, but this can be partly compensated by analyzing the data according to intention-to-treat per-protocol principles.
In conclusion, COLOR is the first cohort of patients with lung cancer that makes use of the TwiCs design. The TwiCs design appears feasible in patients with lung cancer with fair participation and response rates, although these were negatively impacted by distancing measures imposed by the COVID-19 pandemic. With continuous inclusion embedded in standard departmental logistics and planned expansion to other centers, the COLOR study is expected to enable multiple (randomized) evaluations of experimental interventions with important advantages for recruitment, generalizability, and long-term outcome data collection for patients with lung cancer.
Disclosure statement
All authors report no disclosures relevant to this work.
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article.
Additional information
Funding
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71(3):209–249.
- Integraal Kankercentrum Nederland. Nederlandse Kankerregistratie. www.cijfersoverkanker.nl. Updated 2021. Accessed 09/11, 2021. [Internet]
- Louie AV, Van Werkhoven E, Chen H, et al. Patient reported outcomes following stereotactic ablative radiotherapy or surgery for stage IA non-small-cell lung cancer: results from the ROSEL multicenter randomized trial. Radiother Oncol. 2015;117(1):44–48.
- Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. 2015;16(6):630–637.
- Relton C, Torgerson D, O'Cathain A, et al. Rethinking pragmatic randomised controlled trials: introducing the “cohort multiple randomised controlled trial” design. BMJ. 2010;340(1):c1066–c1066.
- van der Velden JM, Verkooijen HM, Ayoung-Afat D, et al. The cohort multiple randomized controlled trial design: a valid and efficient alternative to pragmatic trials? Int J Epidemiol. 2017;46:96–102.
- Verweij ME, Gal R, Burbach JPM, et al. Most patients reported positively or neutrally of having served as controls in the trials within cohorts design. J Clin Epidemiol. 2022;148:39–47.
- van der Velden JM, Verkooijen HM, Seravalli E, et al. Comparing conVEntional radiotherapy with stereotactIC body radiotherapy in patients with spinAL metastases: study protocol for an randomized controlled trial following the cohort multiple randomized controlled trial design. BMC Cancer. 2016;16(1):909.
- Relton C, Thomas K, Nicholl J, et al. Review of an innovative approach to practical trials: the ‘cohort multiple RCT’ design. Trials. 2015;16(S2). :1–1.
- Holt H, Talaei M, Greenig M, et al. Risk factors for developing COVID-19: a population-based longitudinal study (COVIDENCE UK). Thorax. 2022;77(9):900–912.
- Young-Afat DA, Verkooijen HAM, Van Gils CH, et al. Brief report: staged-informed consent in the cohort multiple randomized controlled trial design. Epidemiology. 2016;27(3):389–392.
- van de Ven S, van den Bongard D, Pielkenrood B, et al. Patient-reported outcomes of oligometastatic patients after conventional or stereotactic radiation therapy to bone metastases: an analysis of the PRESENT cohort. Int J Radiat Oncol Biol Phys. 2020;107(1):39–47.
- Werensteijn-Honingh AM, Wevers AFJ, Peters M, et al. Progression-free survival in patients with 68 Ga-PSMA-PET-directed SBRT for lymph node oligometastases. Acta Oncol. 2021;60(10):1342–1351.
- Castor Electronic Data Capture [Internet]. 2019, Aug 27. Available from: https://castoredc.com.
- Groenvold M, Klee MC, Sprangers MAG, et al. Validation of the EORTC QLQ. C30 quality of life questionnaire through combined qualitative and quantitative assessment of patient-observer agreement. J Clin Epidemiol. 1997;50(4):441–450.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–1736.
- Bergman B, Aaronson NK, Ahmedzai S, et al. The EORTC QLQ-LC13: a modular supplement to the EORTC core quality of life questionnaire (QLQ-C30) for use in lung cancer clinical trials. EORTC study group on quality of life. Eur J Cancer. 1994;30A:635–642.
- Smets EMA, Garssen B, Bonke B, et al. The multidimensional fatigue inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315–325.
- Spinhoven P, Ormel J, Sloekers PPA, et al. A validation study of the hospital anxiety and depression scale (HADS) in different groups of dutch subjects. Psychol Med. 1997;27(2):363–370.
- Tuomi K, Ilmarinen J, Jahkola A, et al. Work ability index. 2nd revised edition ed. Helsinki. Finnish Inst Occup Heal. 1998.
- Van De Poll-Franse LV, Horevoorts N, Eenbergen MV, et al. The patient reported outcomes following initial treatment and long term evaluation of survivorship registry: scope, rationale and design of an infrastructure for the study of physical and psychosocial outcomes in cancer survivorship cohorts. Eur J Cancer. 2011;47(14):2188–2194.
- Scott NW, Fayers PM, Aaronson NK, et al. EORTC QLQ-C30 Reference Values [Internet]Available from: https://www.eortc.org/app/uploads/sites/2/2018/02/reference_values_manual2008.pdf.
- Sterne JAC, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338(1):b2393–b2393.
- Coebergh van den Braak RRJ, van Rijssen LB, van Kleef JJ, et al. Nationwide comprehensive gastro-intestinal cancer cohorts: the 3P initiative. Acta Oncol. 2018;57(2):195–202.
- Young-Afat DA, van Gils CH, van den Bongard HJGD, et al. The Utrecht cohort for multiple BREast cancer intervention studies and long-term evaLuAtion (UMBRELLA): objectives, design, and baseline results. Breast Cancer Res Treat. 2017;164(2):445–450.
- Lievens Y, Ricardi U, Poortmans P, et al. Radiation oncology. Optimal health for all, together. ESTRO vision, 2030. Radiother Oncol. 2019;136:86–97.