Abstract
Background
Lung cancer (LC) is the leading cause of cancer deaths worldwide. Several new treatments have become available in recent decades, but little research exists on the impact of these on productivity, early retirement and survival for LC patients and their spouses. This study evaluates the effect of new medicines on productivity, early retirement and survival for LC patients and their spouses.
Methods
Data from the period 1 January 2004–31 December 2018 were collected from complete Danish registers. LC cases diagnosed before approval of first targeted therapy (19 June 2006, Before patients) were compared with those who received at least one new cancer treatment, diagnosed after this date (After patients). Subgroup analyses based on cancer stage, and epidermal growth factor receptor (EGFR) or anaplastic lymphoma kinase (ALK) mutation were conducted. Linear regression and cox regression were used to estimate the outcomes including productivity, unemployment, early retirement, and mortality. Spouses of Before and After patients were compared on earnings, sick leave, early retirement, and healthcare utilisation.
Results
The study population comprised of 4,350 patient (2,175 After/2,175 Before). Patients who received new treatments had a significantly reduced risk of death (Hazard ratio = 0.76, Confidence interval: 0.71 − 0.82) and reduced risk of early retirement (Hazard ratio: 0.54, Confidence interval: 0.38 − 0.79). No significant differences in earnings, unemployment, or sick leave were found. Spouses of Before patients had a higher cost of healthcare services after diagnosis compared to spouses of After patients. For productivity, early retirement and sick leave, no significant differences were found between the spouse groups.
Conclusion
Patients who received innovative new treatments had reduced risk of death and reduced risk of early retirement. Spouses of LC patients who received new treatments had lower healthcare costs in the years following diagnosis. All findings indicate that recipients of new treatments had reduced burden of illness.
Introduction
Lung cancer (LC) is the leading cause of cancer deaths worldwide, with tobacco smoking being the primary cause of illness. The illness and treatment of cancer leads to disrupted function of the primary organs, leading to severe reduction in health-related quality of life (HRQoL) [Citation1]. For patients diagnosed 1997–2007, the five-year survival rate in Europe was only 13% [Citation2]. Subsequently, new innovative targeted cancer treatments, for example, inhibitors of epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), and immune checkpoint inhibitors (ICIs), designed to block the action of immune checkpoints have improved treatment efficacy, reduced toxicity and improved clinical outcomes [Citation3].
Cancer is considered a chronic disease in the workforce [Citation4]. LC survivors have 2–3 times lower probability of being employed after treatment, compared to the general population [Citation5]. Moreover, less than half of LC survivors employed at time of diagnosis return to work after treatment [Citation6], with ‘Fatigue’ being reported as the primary reason [Citation7].
Returning to work or maintaining normal physical functioning after treatment is essential for self-esteem and quality of life. From a healthcare sector and societal perspective, there is a need for updated information on the real-world impact that these new treatments have on patients’ work productivity, life expectancy and the possible impact on close relatives.
In this study, we analyse productivity, risk of early retirement, unemployment and overall mortality for LC patients who received new innovative LC treatments in Denmark between 19 June 2006 and 31 December 2018. We matched and compared these LC patients with those diagnosed before the introduction of new targeted treatments – that is, patients diagnosed with LC between 1 January 2004 and 18 June 2006. Moreover, we identified spouses of LC patients as informal caregivers. We considered the productivity, sick leave, risk of early retirement and health service utilisation following their partner’s LC diagnosis.
Material and methods
The study was designed as a retrospective cohort study using the national Danish registers, covering the entire Danish population (5.7 million). LC patients were identified as individuals registered with International Statistical Classification of Diseases and Related Health Problems (ICD-10) code DC34 (including all subgroups) as primary or secondary diagnosis in the Danish National Patient Register (NPR) [Citation8]. All Danish residents have a unique 10-digit personal identification number, making it possible to merge person-level data from all Danish registers. The population was validated by cross-checking registrations in the Danish Cancer Register (DCR) [Citation9]. All patients diagnosed after 1 January 2004 and before 31 December 2018 were included.
Lung cancer patients
LC cases who received at least one of the new medications in the year of or in the year following date of diagnosis was identified in the NPR. Characteristics of tumour size, lymph node involvement and metastatic spread (TNM classification of malignant tumours) from DCR was used to determine LC stage at time of diagnosis. Date of birth, marital status, region of residence and date of death (if applicable) were collected from the Central Person Register (CPR) [Citation10]. Highest achieved education of the patients before diagnosis was collected from the Population Education Register (PER) [Citation11]. Patients income and labour market affiliation were gathered from the Income Register [Citation12] and the DREAM database (The Danish Register of sickness absence, compensation benefits and social transfer payments) [Citation13], respectively. Tumour pathology was defined using the Systemised Nomenclature of Medicine (SNOMED) codes from The Danish National Pathology Register and Databank [Citation14].
Spouses of lung cancer patients
Spouses, defined as a person married to or in a domestic relationship with LC patients at the time of diagnosis were identified in the CPR. For spouses, date of birth, region of residence from the CPR [Citation10], highest achieved education from the PER [Citation11], income from the Income Register [Citation12] and labour market affiliation from the DREAM database [Citation13] was also collected.
Healthcare utilisation for spouses was measured as contacts and cost from the NPR for hospital care, from the National Health Insurance Register [Citation15] for primary care, and the Register of Medicinal Product Statistics for costs of prescription drugs.
Study populations
LC patients diagnosed between 1 January 2004 and 31 December 2018 were included. On 19 June 2006, the new targeted medication erlotinib became available for Danish LC patients. Patients diagnosed after this date who were treated with any of the predefined new targeted cancer medicines were defined as the study’s After population. New targeted medications were identified by treatment codes in the NPR. These treatments were (treatment code in parenthesis) erlotinib (BWHA404), bevacizumab (BOHJ19B1), gefitinib (ML01EB01), crizotinib (BWHA413), afatinib (BWHA417), ceritinib (ML01ED02), Osimertinib (BWHA434), alectinib (BWHA440), brigatinib (ML01ED04), dacomitinib (ML01EB07), lorlatinib (BWHA448), pembrolizumab (BOHJ19J3) and nivolumab (BOHJ19H2). Patients diagnosed before 19 June 2006, were regarded as the Before population (control).
In addition, six subgroup analyses (SG 1–6) were performed. The first two subgroup analyses used later dates for introduction of new targeted therapies (SG 1 and 2). SG 3 only considered Stage I–III LC patients, while SG 4 considered Stage IV LC patients. SG 5 compared ALK-positive LC patients in the After group with Stage IV LC patients in the Before group. Lastly, LC patients with EGFR-mutations in the After group were compared with Stage IV LC patients in the Before group (SG 6).
SG 1 focussed on the introduction of crizotinib on 26 November 2012, using this date to separate between Before and After populations. SG 2 focussed on the additional new ICIs pembrolizumab and nivolumab used to treat locally advanced or metastatic non-small cell LC (NSCLC). In this subgroup analysis, 1 January 2017 was used to distinguish between Before and After populations.
In SG 3 and 4, Stages I–III and Stage IV patients were considered separately. Cancer stage (I–IV) were determined by TNM codes from the DCR. During the study period, there were three versions of the TNM classifications. We applied the staging algorithm used by Norwegian Cancer Register to determine stage. There is extensive cooperation between the Nordic Cancer registers, thus the algorithm should be applicable to Danish data.
In SG 5, ALK-positive LC patients were analysed. ALK-positive patients were identified if observed with SNOMED code T08 or T25-T29 (including sublevels) in combination with F2911 within 30 days of diagnosis. The first ALK-positive LC patient was identified in 2007, but ALK-specific treatment was not available before 2012. ALK-positive LC patients was compared with Stage IV LC patients in the Before group.
Similarly, in SG 6, patients observed with SNOMED code T08 or T25-T29 (including sublevels) in combination with one of the following codes: FE13CA, FE13CB, FE13CC, FE13CD, FE13CK, FE13CP, F29616, FE13C3, FE13C5 within 30 days of diagnosis were classified as EGFR-positive patients. EGFR patients diagnosed during the After period was compared with Stage IV LC patients in the Before period.
Two analyses focussed on the effect on Spouses of LC patients. One analysis where spouses of Before LC patients are matched and compared with spouses of After LC patients (Matched 1:1). And one analysis of spouses of Before LC patients are matched and compared with Spouses of After LC patients and individuals not associated with cancer (Matched 1:1:3). The reason for conducting two analyses, as opposed to one, was to retain as many spouses as reasonably possible in the analysis.
Outcomes for lung cancer patients
For LC patients, the following outcomes were analysed; productivity (proxied by earnings), unemployment, risk of early retirement, and risk of death.
Productivity was measured as average salary (active income) of Before/After LC patients in the year before diagnosis (t-1) and five years after diagnosis (t5). Earnings were adjusted to a 2018 price level according to the consumer price index as calculated by Statistics Denmark. Transfer payments was not included.
Unemployment was measured as average number of weeks with unemployment each year from t-1 to t5 in the Before and After groups.
Early retirement is granted on a municipality level if a physician evaluates a person as being permanently unfit to work. Due to a policy change for early retirement in 2013, we censored cases on 1 January 2013 for this analysis.
The follow-up time for overall mortality ranged from date of diagnosis until 31 December 2019.
Outcomes for spouses of lung cancer patients
For spouses of LC patients, the following outcomes were analysed; productivity (proxied by earnings), risk of early retirement, incidence of long-term sick leave and health service utilisation (proxied by cost).
Productivity and risk of early retirement was evaluated same way as with the LC patients.
Use of health services was measured as the sum of the costs of healthcare services consumed in primary and hospital sector as well as cost of prescription drugs. The costs were adjusted to 2018 price level according to the Consumer Price Index. Fees and Diagnosis Related Group (DRG)-tariffs were applied as unit costs for primary healthcare and hospital sector resource use. Market prices were applied as unit costs for prescription drugs.
Long-term sick leave was defined as sick leave lasting longer than four weeks. As all shorter sick leave periods were discarded, we consider the fifth week of sick leave as the first week of long-term sick leave.
Balancing the samples
Because the After sample only comprised those LC patients who received one of the innovative LC medicines in focus, we matched these patients with Before patients to ensure comparable populations. Matching was done with optimal pairwise propensity score matching, minimising the distance between each patient in the After sample with a suitable match in the Before sample. Distance was evaluated by Mahalanobis distances. In the case of Stage I–III and EGFR subgroup analysis, GLM (generalized linear model) distances was used to achieve successful matching. Matching covariates was age, sex, region of residency, cancer stage, education, and income in t-1.
Spouses of Before patients was matched with spouses of After patients using the same method but evaluated with GLM distances. Covariates were age, sex, region of residence, income in t-1 and education. Additionally, an analysis was performed where spouses of Before and After patients and persons not associated with cancer was matched on the same criteria, using exact matching (1:1:3, Before spouses: After spouses: No Cancer)
Successful matching was achieved if the following three criteria were fulfilled: (1) <15% deviance in variance ratio, (2) <±0.1 difference in standardised mean, and (3) at least 80% of cases in the smallest group was retained.
Statistical analysis
Earnings, unemployment, sick leave and utilisation of healthcare services, was analysed using used ordinary least square (OLS) regression with the treatment variable (Before/After) interacted with index period (t-1 to t5) to estimate the average value of the respective outcomes by period.
Early retirement and all-cause mortality was analysed using Cox proportional hazard regression (Cox model) to estimate the difference in risk of event between the groups. The hazard ratios produced from the Cox model was adjusted for age, sex, cancer stage (where applicable), education and region of residence. In analysis of early retirement, cases were censored at death, emigration or when turning 65 years old. The latter corresponding to criteria for age related retirement until 2008 in the study period.
To avoid immortal time, survival was analysed from day of first treatment onwards. First treatment was defined as treatment with antibodies or immunomodulatory treatment (procedure code BOHJ, including subgroups), or treatments conditional to special medical treatment principles, for example cytostatic agents (procedure codes BWH, including subgroups).
Statistical significance was set to 5% in all models and was not adjusted for multiple testing and should be interpreted as such.
All analyses were performed in the statistical software R, version 4.1.0 (www.r-project.org).
Results
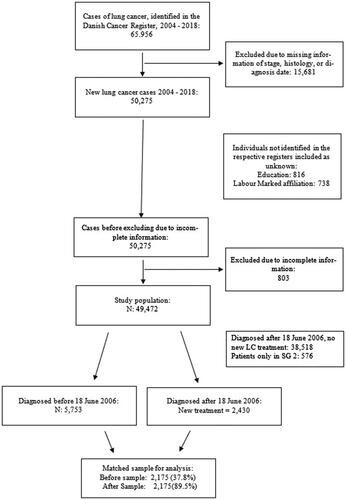
presents a flow chart of the merging of data sets. A total of 50,275 eligible LC cases were identified during the study period.
A total of 803 cases were not identified in the CPR. Table 1A in the Supplementary appendix presents summary statistics on the remaining 49,472 patients and their spouses. The patients had a median age of 69 years, with 51% being males. In the Before period, 5,753 cases were to be matched with 2,430 After cases. A total of 38,518 patients were classified as ‘Other’, as they were diagnosed in the After period, but did not receive any of the new innovative treatments.
For spouses, 25,541 spouses were identified, with median age of 67 (Supplementary Table 1A). Of these spouses, 1,643 were spouses of LC patients in the After population. A total of 3,414 were spouses of Before LC patients (3,272 spouses of LC Patients diagnosed between 1 January 2004 and 18 June 2006). For LC patients in SG 2, 397 spouses were identified. The remaining 20,087 spouses were spouses of ‘Other’ LC patients. Summary statistics for all matched subsets are provided in Supplementary Appendix A.
display LC incidence by stage and histologic subtype during the study period. There was a 26.5% increase in cancer incidence between 2004 and 2018. Males had an 18% increase in incidence, while females had a 54% increase. More than half of the cases (51%) had Stage IV cancer at time of diagnosis. ALK-positive and EGFR-positive mutations were not registered before 2006 but had a steady increase in cases until 2012, when the incidence became stable.
Table 1. Annual incidence by gender, stage, pathology and total.
Results main analysis
present summary characteristics of the matched sample used in the main analysis. The matched sample was on average 1 year younger than the overall population (presented in Supplementary Table 1A) with a higher proportion of females, but otherwise comparable.
Table 2. Summary statistics, matched lung cancer sample, main analysis.
Out of 2175 Before patients 2130 died during the study period, while the equivalent numbers for After patients was 1953 out of 2175. Six Before patients died in the period between test and diagnosis. All the After patients received any of the new innovative LC medicine, while 1213 Before patient received standard medical treatment. In adjusted analysis, After patients had significantly reduced risk of death (HR = 0.76, 0.71−0.82) compared to the matched Before group.
For productivity, no significant differences were found between Before and After populations. Average income in the year before diagnosis was $9600 (DKK 72,000) and decreased to about $4000 (DKK 30,000) five years after diagnosis. No significant differences were found in unemployment (LC patients) or long-term sick leave (spouses) between Before and After groups (data not shown).
Out of 649 (20,5%) at risk Before cases, 133 (20,5%) entered early retirement before 1 January 2013 versus 40 out of 334 (12%) at-risk After cases. Patients in the After sample had significantly reduced risk of early retirement (HR: 0.54, 0.38−0.79). For spouses, we found no significant differences (HR 1.06, 0.23−4.94). However, this result should be seen in context of fewer than five spouses of After LC patients entered early retirement.
Spouses of After patients had significantly lower healthcare costs in the years following diagnosis (t-1–t5). In , the costs are displayed. Results of analysis with spouses of Before and After patients are to the left, and results of analysis with spouses of Before and After patients as well as persons without cancer to the right.
Figure 2. Healthcare costs (Eur) generated by spouses of LC patients. A: Analysis of spouses of Before and After patients (NSpouseBefore = NSpouseAfter= 1292). B: Analysis of spouses of Before and After patients, as well as persons not associated with cancer (NSpouseBefore = NSpouseAfter= 1139, NNoCancer = 3415). For panel (A) difference was tested between Before and After patients, for panel (B) difference was tested between After – Control, and Before-Control.
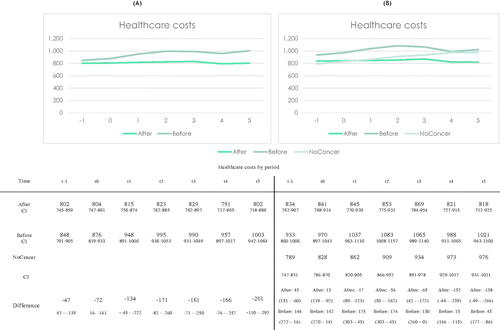
Healthcare costs generated by spouses of After patients seems unaffected by the LC diagnosis. For spouses of Before patients, however, the annual healthcare costs increased slightly about $95 (700 DKK) from t0 to t1 and plateaued around this new level. In the analysis including individuals not associated with cancer, a similar pattern was seen. There were no significant differences in healthcare costs between persons without cancer and spouses of After LC patients, until t4 and t5 ().
Subgroup analyses
Results from risk of death and early retirement for subgroup analyses are presented in . All After subgroups had reduced risk of death compared with their respective Before populations (see ). The largest reduction in risk of death was found in ALK-positive patients (SG 5) (HR = 0.37, 0.25−0.55) corresponding to a 63% reduction in hazard of death, while the smallest reduction was found in the analysis of Stages I–III patients (SG 3) (HR = 0.82, 0.73−0.92).
Table 3. Results from subgroup analyses.
For SG 1 and 2, analysis of early retirement was not applicable due to the reform in early retirement requirements in 2013. In analysis of SG 5 and 6, there were fewer than five events. As a result, there is high uncertainty associated with these estimates.
In SG4, there was a significant reduced risk of early retirement (HR = 0.57, 0.37−0.87) corresponding to a 43% reduction in hazard. For SG 3, HR indicated a reduced hazard of early retirement in the After group however, not a statistically significant reduction.
Patients diagnosed after 26 November 2012 who received new LC medicines (SG2) and patients with ALK-positive cancer (SG5) both earned more in the year before diagnosis, compared with their matched Before samples. In SG2, After patients had significantly higher unemployment rate two years after diagnosis compared with their matched Before group. No other significant differences were found in unemployment or sick leave for any subgroup (results available from corresponding author).
Discussion
When comparing LC patients before and after introduction of new innovative medicine, it was found that LC patients receiving new innovative medicines had reduced hazard of death overall, and in all subgroups. This is in line with expectations as new treatments are most often approved based on a proven survival benefit compared to former standard treatment in clinical randomised trials. Note, no new systemic treatments targeting early-stage LC and locally advanced LC (stage I–III) were introduced during the study. The reduced hazard of death is therefore likely due to newer operation techniques and radiotherapy deliveries which have increased survival in this subgroup. The reduced hazard in advanced stages is therefore probable due to advancements in systemic treatment for subgroups of patients. In early and locally advanced stages (I–III), LC patients had a 18% reduction in hazard of death, while ALK patients who were treated with new targeted medicine during this time reduced their hazard with 63%.
LC patients who received the new innovative LC medicines had a significantly reduced hazard of early retirement. The subgroup analyses showed a significant hazard reduction for Stage IV LC patients. This finding coincides well with the reduced toxicity from new innovative medicines.
For the other outcomes, there were sporadic significant differences in subgroups. However, without a consistent pattern of differences these results might be a result of natural variation, rather than an effect of the treatment. It should be noted that higher survival rate in the After population likely causes survival bias. In other words, when more patients survive into retirement age, they are registered with zero income, thus reducing the average earnings in the After population.
The combination of reduced risk for both early retirement and death is interesting. A previous study found that LC patients did not live long enough to retire early [Citation16]. However, LC patients in advanced settings who received new innovative treatments both lived longer and had reduced risk of early retirement. With fatigue being the primary cause of exit from the labour market [Citation7], it could suggest that reduced toxicity through personalised medicine reduces fatigue symptoms in the patients.
Spouses of After LC patients had significantly lower healthcare costs in the years following their partners LC diagnosis, compared to spouses of Before LC patients. Severity of diagnosis has been shown to have a positive correlation with the burden on informal caregivers [Citation17].
Unobserved variables such as identification of biomarkers, new surgical techniques, improved radiotherapeutic treatment and methods for earlier diagnosis [Citation18] as well as changes in the patient population could be responsible for some of the reduced risk of mortality or early retirement. Particularly, incidence of LC for women is increasing [Citation19]. During the study period, the ratio of female patients changed from 45.5% in 2004 to 51% in 2018. Such changes in the patient population underline the importance of real-world studies, due to tendency of selection of specific patient populations used in randomised controlled trials, that might not be representative of the real-world LC population.
Strength and weaknesses
The analysis is based on national registers, spanning 17 years with reliable, routinely collected data before and after date of diagnosis.
Misclassification of data might occur. The authors could not validate the coding practice used in the inclusion/exclusion criteria. However, the Danish registers are of high quality and are routinely used in population-based inquiries such as the present. Furthermore, the sample size reduces the impact of random variation on the effect estimates.
For some matches there are several years between dates of diagnosis. Other developments in LC treatment, Small Cell LC vs Non-Small Cell LC, performance status and other clinically covariates were not accounted for when matching. Therefore, there could be differences in patients’ health at baseline not captured by the stage indicator or distribution of Non-Small/Small Cell LC can vary in the Before and After population. This can have affected the results.
The matching criteria were met for all sets, except for the ALK-positive group. Due to the relatively small sample of ALK-positive cases, we chose to breach the variance ratio criterion for age to retain all but two ALK-positive cases.
As there are multiple outcomes and populations and subpopulations there is a risk of Type 1 error. The reader should be aware of this and consider any result in context of all results.
Conclusion
The new innovative LC medicines introduced in Denmark during the study period have had a positive effect on the patients’ life. This is evident in survival, risk of early retirement and reduced healthcare utilisation for spouses of LC patients. The reduced risk of early retirement demonstrates an improvement of the low labour market participation in LC patients following diagnosis and treatment. Spouses of those who received the new treatments generated lower healthcare costs, possible due to a reduced burden of illness for the LC patients.
While significant improvements in treatment have been achieved in the last 20 years, the prognosis for LC patients is still not satisfactory, and continued effort to improve clinical outcomes are necessary.
Ethics approval and consent to participate
The study was register-based and complied with the regulations and instructions set up by Statistics Denmark. No additional ethics committee approval is required for register-based research according to Danish law.
Supplemental Material
Download MS Word (29.6 KB)Disclosure statement
The study was financed by Pfizer Denmark. Jan Håkon Rudolfsen and Mikkel H. Pedersen are employees at Incentive, which is a paid vendor of Pfizer Denmark. Mads D. Hjortsø and Trine Pilgaard are employee of Pfizer Denmark. Mette Pøhl was paid by Pfizer Denmark for her work as an oncology specialist in the project group.
Data availability statement
The data that support the findings of this study are available from Statistics Denmark’s Research Service, but restrictions apply to the availability of these data, which were used under licence for the current study, and so are not publicly available. Additional analyses are however available from the authors upon reasonable request and with permission of Statistics Denmark’s Research Service.
Additional information
Funding
References
- Novello S, Kaiser R, Mellemgaard A, et al. Analysis of patient-reported outcomes from the LUME-Lung 1 trial: a randomised, double-blind, placebo-controlled, phase III study of second-line nintedanib in patients with advanced non-small cell lung cancer. Eur J Cancer. 2015;51(3):317–326.
- Francisci S, Minicozzi P, Pierannunzio D, EUROCARE-5 Working Group, et al. Survival patterns in lung and pleural cancer in Europe 1999–2007: results from the EUROCARE-5 study. Eur J Cancer. 2015;51(15):2242–2253.
- The global challenge of cancer. Nat Cancer. 2020;1(1):1–2.
- Lawless GD. The working patient with cancer: implications for payers and employers. Am Health Drug Benefits. 2009;2(4):168–173.
- Vayr F, Savall F, Bigay-Game L, et al. Lung cancer survivors and employment: a systematic review. Lung Cancer. 2019;131:31–39.
- Rashid H, Eichler M, Hechtner M, et al. Returning to work in lung cancer survivors—a multi-center cross-sectional study in Germany. Support Care Cancer. 2021;29(7):3753–3765.
- Kim YA, Yun YH, Chang YJ, et al. Employment status and work-related difficulties in lung cancer survivors compared with the general population. Ann Surg. 2014;259(3):569–575.
- Lynge E, Sandegaard JL, Rebolj M. The Danish national patient register. Scand J Public Health. 2011;39(7_suppl):30–33.
- Gjerstorff ML. The Danish cancer registry. Scand J Public Health. 2011;39(7_suppl):42–45.
- Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39(7_suppl):22–25.
- Jensen VM, Rasmussen AW. Danish education registers. Scand J Public Health. 2011;39(7_suppl):91–94.
- Baadsgaard M, Quitzau J. Danish registers on personal income and transfer payments. Scand J Public Health. 2011;39(7_suppl):103–105.
- Hjollund NH, Larsen FB, Andersen JH. Register-based follow-up of social benefits and other transfer payments: accuracy and degree of completeness in a Danish interdepartmental administrative database compared with a population-based survey. Scand J Public Health. 2007;35(5):497–502.
- Erichsen R, Lash TL, Hamilton-Dutoit SJ, et al. Existing data sources for clinical epidemiology: the Danish national pathology registry and data bank. Clin Epidemiol. 2010;2:51–56.
- Andersen JS, Olivarius NDF, Krasnik A. The Danish national health service register. Scand J Public Health. 2011; Jul39(7_suppl):34–37.
- Taskila-Åbrandt T, Pukkala E, Martikainen R, et al. Employment status of Finnish cancer patients in 1997. Psychooncology. 2005;14(3):221–226.
- Alshammari B, Noble H, McAneney H, et al. Factors associated with burden in caregivers of patients with end-stage kidney disease (A systematic review). Healthcare. 2021;9(9):1212.
- Lemjabbar-Alaoui H, Hassan OU, Yang YW, et al. Lung cancer: biology and treatment options. Biochim Biophys Acta BBA - Rev Cancer. 2015;1856(2):189–210.
- Egleston BL, Meireles SI, Flieder DB, et al. Population-based trends in lung cancer incidence in women. Semin Oncol. 2009;36(6):506–515.