Abstract
Background
Insulin resistance is a critical cause of metabolic dysfunctions. Metabolic dysfunction is common in patients with cancer and is associated with higher cancer recurrence rates and reduced overall survival. Yet, insulin resistance is rarely considered in the clinic and thus it is uncertain how frequently this condition occurs in patients with cancer.
Methods
To address this knowledge gap, we performed a systematic review and a meta-analysis guided by the Preferred Items for Systematic Review and Meta-Analyses (PRISMA) statement. We included studies assessing insulin resistance in patients with various cancer diagnoses, using the gold-standard hyperinsulinemic–euglycemic clamp method. Studies eligible for inclusion were as follows: (1) included cancer patients older than 18 years of age; (2) included an age-matched control group consisting of individuals without cancer or other types of neoplasms; (3) measured insulin sensitivity using the hyperinsulinemic–euglycemic clamp method. We searched the databases MEDLINE, Embase, and Cochrane Central Register of Controlled Trials for articles published from database inception through March 2023 with no language restriction, supplemented by backward and forward citation searching. Bias was assessed using funnel plot.
Findings
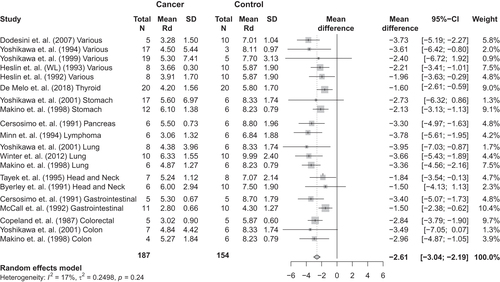
Fifteen studies satisfied the criteria. The mean insulin-stimulated rate of glucose disposal (Rd) was 7.5 mg/kg/min in control subjects (n = 154), and 4.7 mg/kg/min in patients with a cancer diagnosis (n = 187). Thus, the Rd mean difference was −2.61 mg/kg/min [95% confidence interval, −3.04; −2.19], p<.01). Heterogeneity among the included studies was insignificant (p=.24).
Interpretation
These findings suggest that patients with a cancer diagnosis are markedly insulin resistant. As metabolic dysfunction in patients with cancer associates with increased recurrence and reduced overall survival, future studies should address if ameliorating insulin resistance in this population can improve these outcomes thereby improving patient care.
Metabolic dysfunction increases cancer recurrence rates and reduces survival for patients with cancer.
Insulin resistance is a critical cause of metabolic dysfunctions.
To date, no comprehensive compilation of research investigating insulin resistance in cancer patients has been produced.
In this meta-analysis, we found that patients with various cancers were markedly insulin-resistant.
Key points
Introduction
Metabolic dysfunction is a common condition in patients with cancer. It represents a cluster of metabolic abnormalities including obesity, hypertension, insulin resistance, and dyslipidemia. Epidemiological studies have confirmed an association between metabolic dysfunction, including obesity, and the risk of various cancers, encompassing colorectal, breast, liver, pancreatic, ovarian, and endometrial cancer [Citation1,Citation2]. More strikingly, once diagnosed with cancer, metabolic dysfunction associates with poor cancer outcomes, such as increased cancer recurrence mortality rates [Citation3,Citation4]. Insulin resistance, defined as reduced responsiveness to insulin, is a primary driver of metabolic dysfunction in obesity and type 2 diabetes. In insulin-resistant states, more insulin is needed to obtain a given physiological outcome, such as the lowering of blood glucose. The resulting hyperinsulinemia can be detrimental to patients with cancer, as insulin is proposed to be an oncogenic factor [Citation5–7]. Potentially managing insulin resistance could be a major leap forward in the treatment of patients with cancer due to the prognostic impact of metabolic dysfunctions associated with insulin resistance. Yet, there is limited evidence regarding whether insulin resistance occurs in patients with cancer, and it is unknown if insulin resistance could be a driver of cancer-associated metabolic dysfunction.
The gold standard for assessing insulin sensitivity in humans is the hyperinsulinemic–euglycemic clamp method [Citation8]. This method has been used to identify insulin resistance in various metabolic conditions such as type 2 diabetes [Citation8,Citation9], polycystic ovary syndrome [Citation10], non-alcoholic fatty liver disease [Citation11], and cardiovascular disease [Citation12]. In addition, this method has been used to assess insulin sensitivity in patients with various cancers in prior studies. However, to our knowledge, no comprehensive collection of available data exists; therefore, there is a lack of consensus about whether patients with cancer suffer from insulin resistance. To address this knowledge gap, the objective of the present investigation was to determine insulin resistance in patients with cancer. To achieve that, we undertook a systematic review and a meta-analysis of studies assessing insulin sensitivity in patients with cancer using the gold-standard hyperinsulinemic–euglycemic clamp method.
Methods
This systematic review and meta-analysis was guided by the Preferred Items for Systematic Review and Meta-Analyses (PRISMA) statement [Citation13](see Appendix 1 for the PRISMA checklist).
Search strategy
The databases MEDLINE (via Ovid), Embase (via Ovid), and Cochrane Central Register of Controlled Trials (CENTRAL) were searched from inception until 5 March 2023. The search was developed around three concepts: (1) cancers in humans, (2) hyperinsulinemic–euglycemic clamp method, and (3) insulin sensitivity or resistance, using a combination of subject terms from the available controlled vocabularies (Medical Subject Headings and Emtree) as well as free-text terms. No restrictions were applied to publication type or language. The final search string was constructed for MEDLINE and subsequently translated to Embase and CENTRAL (see Appendix 2) by an information specialist (O.N.). In addition, the reference lists of the included studies were screened (backward citation) as well as studies citing the included studies (forward citation) using the online tool Citation Chaser [Citation14].
Eligibility criteria
Studies eligible for inclusion were as follows: (1) included cancer patients older than 18 years of age, (2) included an age-matched control group consisting of individuals without cancer or other types of neoplasms, and (3) measured insulin sensitivity using the hyperinsulinemic–euglycemic clamp method.
Studies were excluded if: (1) participants were taking anti-diabetic medications within 24 h prior to the study inclusion or undergoing interventions or treatments that affect insulin sensitivity; (2) participants were diagnosed with a confounding medical condition, such as diabetes; (3) insulin sensitivity data were not numerically provided, (4) studies were reusing data from the same patient, or (5) study subjects were animals.
Study selection
To determine the literature to be assessed further, three authors (M.C., J.M.M.P., and L.S) independently double-screened the titles and abstracts of all identified records for eligibility. Full-text reports of the remaining studies were retrieved for double-screening by three authors (J.M.M.P., M.C., and L.S). Discrepancies were resolved through discussions and consensus. The screening process was carried out in EPPI-Reviewer Web [Citation15].
Data extraction
A standardized form (initially piloted on 10 included studies) was used for data extraction of characteristics of all included studies. Three study authors (J.M.M.P., S.H.R., and L.S.) extracted the following data from included studies: study type, methods, participants, number of subjects, rate of glucose disposal (Rd) numerical values (mg/kg/min), insulin dose used, etc.
Risk of bias assessment
Two review authors independently assessed the quality of the included studies using risk of bias assessment via the Newcastle-Ottawa Scale. All eligible studies were included regardless of quality and risk of bias. A funnel plot was used to assess heterogeneity between the studies.
Data analysis
As the studies provided the means of continuous outcomes, the mean difference (MD) in insulin sensitivity between cancer cases and controls were used as summary statistics. Insulin sensitivity was reported as the Rd (mg/kg/min), which is the rate of whole-body glucose disposal and reflects the amount of exogenous glucose necessary to fully compensate for hyperinsulinemia while maintaining euglycemia [Citation16]. Under steady-state plasma glucose conditions, Rd matches the amount of glucose metabolized. It was assumed that hepatic glucose production is fully inhibited by the high-physiological insulin concentrations obtained during the clamp procedure, which was indeed confirmed in several [Citation17–22], of the included studies.
We used random effects models with inverse variance weighting to pool the estimates from the different studies since heterogeneity between the studies was anticipated. The results of the two meta-analyses are presented as forest plots. Analyses were conductedin R (4.1.1) using the meta package (R Foundation for Statistical Computing, Vienna, Austria).
Results
Eligible studies
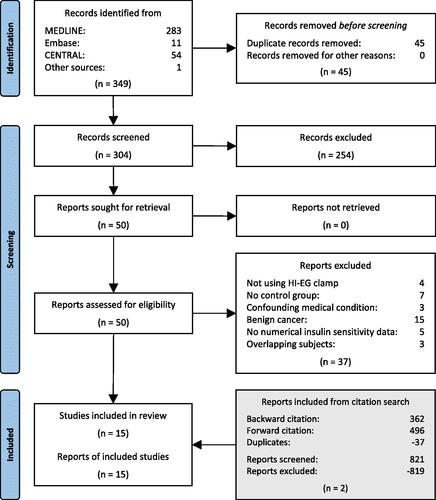
Our search yielded 304 records after the removal of 45 duplicates . After a screening of titles and abstracts, 50 records remained for full-text screening. One report was written in a language (Japanese) that the authors do not understand and was subsequently screened in full-text by a native-speaking colleague. It was possible to retrieve all full-text articles. Thirty-seven reports were excluded for not meeting our eligibility criteria (see reasons for exclusion in Appendix 3). Additionally, two reports were identified through citation searching. In total, 15 studies covering 20 cancer patient cohorts reporting measures of insulin sensitivity using the hyperinsulinemic–euglycemic clamp and the association with cancer were included in the meta-analysis.
Study characteristics
summarizes the main characteristics of the included studies. Of the 15 studies included (n = 187 cancer patients, n = 154 controls), one cohort included pancreatic cancer [Citation19], two cohorts included stomach cancer [Citation28,Citation30], two cohorts included various gastrointestinal cancers [Citation23,Citation29], three cohorts included colorectal cancer [Citation24,Citation28,Citation30], one cohort included thyroid cancer [Citation25], three cohorts included lung cancer [Citation22,Citation28,Citation30], two cohorts included head and neck cancer [Citation17,Citation21], and one cohort included lymphoma [Citation20]. For five studies, various cancer sites were mixed [Citation26–28,Citation31,Citation32]. For two studies of the last category [Citation28,Citation30], data were provided separately for each cancer site and therefore were included in the analysis treated as separate cohorts. Thus, 15 studies with 20 different patient cohorts were included in the analysis. The insulin doses used in the included studies ranged from 0.9 mU/kg/min to 1.34 mU/kg LBM/min, and from 20 mU/m2/min to 40 mU/m2/min and resulted in insulin concentrations within the high physiological range (). When glucose disposal measurements at different concentrations of insulin were reported, only values at an insulin dose of 1 mU/kg/min were included.
Table 1. Basic characteristics of the included studies.
The sample size in each cohort ranged from 4 to 20 patients. Cancer cases were identified by histopathology or cytology in two of the studies, whereas no information regarding this was provided in the remaining 13 studies.
Quality assessment and publication bias
The results of the quality and risk of bias assessment of the included studies using the Newcastle-Ottawa Scale are shown in Appendix 4. The funnel plot did not suggest the presence of publication bias (Supplementary Figure 1) since it was reasonably symmetric. We observed insignificant heterogeneity between the studies (I2 =17%, t2=0.2498, p = .24) [Citation33,Citation34].
Meta-analyses
The mean insulin-stimulated glucose disposal was 7.5 mg/kg/min in control subjects and 4.7 mg/kg/min in patients with a cancer diagnosis when exposed to the same insulin rate. Glucose disposal (n = 347) was significantly lower in patients with cancer than control subjects without cancer (MD −2.61 mg/kg/min [95% confidence interval (CI), −3.07; −2.13], p < .01; ).
Discussion
Our meta-analysis demonstrated marked insulin resistance in patients with cancer. This observation has an important impact because insulin resistance could be a primary driver of cancer-associated metabolic dysfunction, which in turn elevates the risk of cancer recurrence and risk of cancer death. To our knowledge, this is the first systematic review and meta-analysis to assess insulin resistance in patients with cancer. Thus, by compiling data obtained using the gold-standard hyperinsulinemic–euglycemic clamp to assess insulin sensitivity, we identified insulin resistance in patients with cancer. The mean insulin-stimulated glucose disposal was 7.5 mg/kg/min in control subjects, whereas glucose disposal was 4.7 mg/kg/min in patients with a cancer diagnosis at the same insulin infusion rate. Thus, on average, glucose disposal was 2.61 mg/kg/min lower in patients with cancer compared to control subjects. Remarkably, the mean level of insulin resistance in patients with cancer was similar to or even higher than insulin-resistant patients diagnosed with type 2 diabetes [Citation8].
Insulin resistance increases the risk of developing type 2 diabetes [Citation9] because insulin is the major hormone responsible for maintaining glycemic control by increasing glucose uptake into peripheral tissues while inhibiting hepatic glucose production [Citation35]. Thus, our results are in line with recent epidemiological data showing that patients with a cancer diagnosis have an increased risk of developing new-onset diabetes [Citation36,Citation37]. Our data corroborate the growing evidence that impaired glucose homeostasis is a hitherto overlooked co-morbidity in patients with cancer. The importance of addressing insulin resistance, which is the leading cause of hyperinsulinemia, is illustrated in a recent 28-year follow-up study, showing that a high daily insulin dose in patients with type 1 diabetes is associated with a fourfold increased risk of developing cancer compared to patients on a low daily insulin dose [Citation6]. This is perhaps why type 2 diabetes [Citation36] and metabolic dysfunction are proven negative prognostic factors for outcomes including survival in various types of cancers [Citation3,Citation4,Citation38]. Insulin resistance is a well-recognized contributing factor to the development of many diseases/conditions including type 2 diabetes [Citation9], dyslipidemia [Citation39], cardio-vascular disease [Citation40], cancer-associated muscle wasting (cachexia) [Citation41], and sarcopenia [Citation42]. Our present study emphasizes the need for a better understanding of how cancer and insulin resistance are intertwined, and to what degree insulin resistance contributes to metabolic dysfunction in patients with cancer.
The mechanisms underlying insulin resistance in patients with cancer could be due to the direct effects of cancer per se, the oncological treatment, and co-occurring risk factors. Tumors might directly influence the host metabolism, as they are known to secrete various factors, including tumor necrosis factor alpha, interleukin 6, activin A, and HIF-1, which have all been implicated in metabolic regulation and/or insulin resistance [Citation41,Citation43]. Furthermore, cancer causes metabolic rewiring of fatty acid metabolism, which also contributes to the development of insulin resistance in preclinical models of cancer [Citation44,Citation45]. These direct cancer effects might contribute to the insulin resistance observed in the studies included in the current systemic review and meta-analysis.
Moreover, treatment with chemotherapy agents may induce insulin resistance [Citation46], and anti-estrogen treatment, mTOR-inhibitors, 5-fluorouracil, and l-asparaginase may cause hyperglycemia [Citation47]. Patients with cancer are often treated with glucocorticoids to alleviate side effects of chemotherapy, such as emesis, anorexia, or as pain management or to treat cerebral edema [Citation48]. Glucocorticoids are known to result in hyperglycemia in at least 30% of patients with cancer receiving ≥100 mg prednisolone/day for 12 days [Citation49]. Unfortunately, the papers included in our study offered limited information regarding treatment with chemotherapy and glucocorticoids and, therefore, we cannot shed light on whether these treatments may have contributed to the development of insulin resistance.
Finally, insulin resistance in patients with cancer could be due to common risk factors for cancer and insulin resistance, such as obesity, physical inactivity, unbalanced diet, smoking, and excess alcohol consumption. If obesity was driving the insulin resistance in patients diagnosed with cancer, we would expect insulin resistance to be particularly pronounced for obesity-related cancers, such as colorectal, breast, uterus, ovary, and kidney cancer [Citation50].
Our study highlights an important aspect regarding insulin resistance in patients with cancer that warrants further investigation. Potentially managing insulin resistance could be a major leap forward in the treatment of patients with cancer due to the prognostic impact of metabolic dysfunctions associated with insulin resistance [Citation3,Citation4,Citation38]. Yet, assessing insulin resistance is challenging in the clinic. Still, some simple methods, from which insulin resistance indices can be derived, have been validated, e.g., homeostasis model assessment (HOMA), quantitative insulin sensitivity check index (QUICKI), and Matsuda that could be used in the clinic to inform oncologists. Since the risk of cancer recurrence and new-onset cancers are also increased in patients with metabolic dysfunction [Citation3,Citation4], managing insulin resistance could also be advantageous for cancer patients following treatment.
The significance of insulin resistance in cancer patients may have been overlooked in the past, particularly in relation to the management of the disease. A safe non-pharmacological intervention may already exist in the form of exercise [Citation51], which is a well-described intervention to prevent insulin resistance [Citation52]. Concurrently, while the efficacy of exercise still needs to be investigated across different cancer diagnoses, multiple international organizations have already implemented recommendations regarding exercise interventions during and after cancer treatment [Citation53].
Limitations of the present study include the low number of subjects in the studies that met the inclusion criteria. Moreover, some heterogeneity was observed between the study populations and patients within the studies. The major contributors to this heterogeneity include various treatment regimens, doses, and durations. Unfortunately, the potential use of glucocorticoids, the use and/or type and duration of chemotherapy, or when during the cancer treatment the hyperinsulinemic–euglycemic clamp was performed, was not reported in the majority of the included studies. It should also be considered that the majority of included studies originated from Western countries, and extrapolation of our results to other populations warrants further investigation. Moreover, our study also did not identify the underlying cause of insulin resistance in patients with cancer, which is an area ripe for further investigation. A critical factor for insulin resistance is low activity levels and low fitness level [Citation54]. Patients with cancer are typically 30% less physically active than the general population [Citation55], which could partially explain the observed insulin resistance as physical activity levels were not controlled in the included studies.
Experimentally, insulin increases glucose disposal in a dose-dependent manner. Thus, glucose disposal depends on the amount of circulating insulin, which in turn depends on the infusion dose and hepatic clearance of insulin. The insulin dose used in the included studies was similar between studies, resulting in plasma insulin concentrations within the high physiological range. However, two of the studies found elevated insulin clearance in patients with lymphoma [Citation20] and head/neck cancer [Citation17], resulting in lower circulating insulin levels in patients with cancer. Another experimental limitation is that during the hyperinsulinemic–euglycemic clamp, it was assumed that hepatic glucose production was fully suppressed. However, insulin suppression of hepatic glucose production was confirmed only in some of the included studies [Citation17–22]. These limitations of this study should be considered when interpreting these results. Despite these limitations, our study shows that patients with a cancer diagnosis are highly insulin resistant. Projecting forward, an important extension of this work will be to determine the underlying biological mechanisms and establish whether ameliorating insulin resistance in this population improves patient outcomes.
Conclusion
Our data revealed severe insulin resistance in patients with cancer compared with healthy control subjects. To our knowledge, this is the first systematic review and meta-analysis to assess insulin resistance, measured using the gold standard hyperinsulinemic–euglycemic clamp in patients with cancer. Future work is needed to identify the underlying factors and establish whether managing insulin resistance will lower morbidity and reduce mortality in cancer survivors.
Author contributions
Conceptualization – J.M.M.P. and L.S. Data curation – J.M.M.P., M.C., S.H.R., E.A.R., and L.S. Formal analysis – J.M.M.P. and M.K.G. Methodology – O.N., J.M.M.P., and L.S. Supervision – L.S. Validation – all authors. Writing: review and editing – all authors.
Supplemental Material
Download PDF (678.6 KB)Supplemental Material
Download PDF (55.3 KB)Supplemental Material
Download PDF (85.5 KB)Supplemental Material
Download PDF (193.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, LS, upon reasonable request.
Additional information
Funding
References
- Renehan AG, Tyson M, Egger M, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578.
- Stattin P, Björ O, Ferrari P, et al. Prospective study of hyperglycemia and cancer risk. Diabetes Care. 2007;30(3):561–567.
- Bhaskaran K, Douglas I, Forbes H, et al. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5·24 million UK adults. Lancet. 2014;384(9945):755–765.
- Campbell PT, Jacobs EJ, Newton CC, et al. Diabetes and cause-specific mortality in a prospective cohort of one million U.S. adults. Diabetes Care. 2012;35(9):1835–1844.
- Zhang AMY, Magrill J, de Winter TJJ, et al. Endogenous hyperinsulinemia contributes to pancreatic cancer development. Cell Metab. 2019;30(3):403–404.
- Zhong W, Mao Y. Daily insulin dose and cancer risk among patients with type 1 diabetes. JAMA Oncol. 2022;8(9):1356.
- Goodwin PJ, Ennis M, Pritchard KI, et al. Fasting insulin and outcome in early-stage breast cancer: results of a prospective cohort study. J Clin Oncol. 2002;20(1):42–51.
- Tam CS, Xie W, Johnson WD, et al. Defining insulin resistance from hyperinsulinemic–euglycemic clamps. Diabetes Care. 2012;35(7):1605–1610.
- DeFronzo RA, Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl. 2):S157–S163.
- Hansen SL, Svendsen PF, Jeppesen JF, et al. Molecular mechanisms in skeletal muscle underlying insulin resistance in women who are lean with polycystic ovary syndrome. J Clin Endocrinol Metab. 2019;104(5):1841–1854.
- Tilg H, Moschen AR, Roden M. NAFLD and diabetes mellitus. Nat Rev Gastroenterol Hepatol. 2017;14(1):32–42.
- Bressler P, Bailey SR, Matsuda M, et al. Insulin resistance and coronary artery disease. Diabetologia. 1996;39(11):1345–1350.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
- Haddaway NR, Grainger MJ, Gray CT. Citationchaser: a tool for transparent and efficient forward and backward citation chasing in systematic searching. Res Synth Methods. 2022;13(4):533–545.
- Thomas J, Graziosi S, Brunton J, et al. EPPI-Reviewer: advanced software for systematic reviews, maps and evidence synthesis. https://eppi.ioe.ac.uk/EPPIReviewer-Web. 2022.
- DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214–E223.
- Byerley LO, Heber D, Bergman RN, et al. Insulin action and metabolism in patients with head and neck cancer. Cancer. 1991;67(11):2900–2906.
- Pisters PWT, Cersosimo E, Rogatko A, et al. Insulin action on glucose and branched-chain amino acid metabolism in cancer cachexia: differential effects of insulin. Surgery. 1992;111(3):301–310.
- Cersosimo E, Pisters PWT, Pesola G, et al. Insulin secretion and action in patients with pancreatic cancer. Cancer. 1991;67(2):486–493.
- Minn H, Lindholm P, Nuutila P, et al. In vivo effects of insulin on tumor and skeletal muscle glucose metabolism in patients with lymphoma. Cancer. 1994;73(5):1490–1498.
- Tayek JA. Reduced non-oxidative glucose utilization in cancer patients is associated with a low triiodothyronine concentration. J Am Coll Nutr. 1995;14(4):341–348.
- Winter A, MacAdams J, Chevalier S. Normal protein anabolic response to hyperaminoacidemia in insulin-resistant patients with lung cancer cachexia. Clin Nutr. 2012;31(5):765–773.
- Cersosimo E, Pisters PW, Pesola G, et al. The effect of graded doses of insulin on peripheral glucose uptake and lactate release in cancer cachexia. Surgery. 1991;109:459–467.
- Copeland GP, Leinster SJ, Davis JC, et al. Insulin resistance in patients with colorectal cancer. Br J Surg. 1987;74(11):1031–1035.
- de Melo TG, Souza AL, Ficher E, et al. Reduced insulin sensitivity in differentiated thyroid cancer patients with suppressed TSH. Endocr Res. 2018;43(2):73–79.
- Dodesini AR, Benedini S, Terruzzi I, et al. Protein, glucose and lipid metabolism in the cancer cachexia: a preliminary report. Acta Oncol. 2007;46(1):118–120.
- Heslin MJ, Newman E, Wolf RF, et al. Effect of systemic hyperinsulinemia in cancer patients. Cancer Res. 1992;52:3845–3850.
- Makino T, Noguchi Y, Yoshikawa T, et al. Circulating interleukin 6 concentrations and insulin resistance in patients with cancer. Br J Surg. 1998;85(12):1658–1662.
- McCall JL, Tuckey JA, Parry BR. Serum tumour necrosis factor alpha and insulin resistance in gastrointestinal cancer. Br J Surg. 1992;79(12):1361–1363.
- Yoshikawa T, Noguchi Y, Doi C, et al. Insulin resistance in patients with cancer: relationships with tumor site, tumor stage, body-weight loss, acute-phase response, and energy expenditure. Nutrition. 2001;17(7–8):590–593.
- Yoshikawa T, Noguchi Y, Doi C, et al. Insulin resistance was connected with the alterations of substrate utilization in patients with cancer. Cancer Lett. 1999;141(1–2):93–98.
- Yoshikawa T, Noguchi Y, Matsumoto A. Effects of tumor removal and body weight loss on insulin resistance in patients with cancer. Surgery. 1994;116(1):62–66.
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions. Chichester, UK: Wiley; 2019. p. 1–694.
- Deeks JJ, Higgins JPT, Altman DG. Analysing data and undertaking meta-analyses. In: Cochrane handbook for systematic reviews of interventions [Internet]; 2019 [cited 2022 Jul 16]. p. 241–284. Available from: 10.1002/9781119536604.ch10
- Sylow L, Tokarz VL, Richter EA, et al. The many actions of insulin in skeletal muscle, the paramount tissue determining glycemia. Cell Metab. 2021;33(4):758–780.
- Sylow L, Grand MK, von Heymann A, et al. Incidence of new-onset type 2 diabetes after cancer: a Danish Cohort Study. Diabetes Care. 2022;45(6):e105–e106.
- Hwangbo Y, Kang D, Kang M, et al. Incidence of diabetes after cancer development: a Korean National Cohort Study. JAMA Oncol. 2018;4(8):1099–1105.
- Park J, Morley TS, Kim M, et al. Obesity and cancer—mechanisms underlying tumour progression and recurrence. Nat Rev Endocrinol. 2014;10(8):455–465.
- Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. CA Cancer J Clin. 2010;60(4):207–221.
- Boudoulas KD, Triposkiadis F, Gumina R, et al. Cardiovascular disease, cancer, and multimorbidity interactions: clinical implications. Cardiology. 2022;147(2):196–206.
- Fearon KCH, Glass DJ, Guttridge DC. Cancer cachexia: mediators, signaling, and metabolic pathways. Cell Metab. 2012;16(2):153–166.
- Honors MA, Kinzig KP. The role of insulin resistance in the development of muscle wasting during cancer cachexia. J Cachexia Sarcopenia Muscle. 2012;3(1):5–11.
- Sylow L, Vind BF, Kruse R, et al. Circulating follistatin and activin A and their regulation by insulin in obesity and type 2 diabetes. J Clin Endocrinol Metab. 2020;105(5):dgaa090.
- Han X, Raun SH, Carlsson M, et al. Cancer causes metabolic perturbations associated with reduced insulin-stimulated glucose uptake in peripheral tissues and impaired muscle microvascular perfusion. Metabolism. 2020;105:154169.
- Raun SH, Knudsen JR, Han X, et al. Cancer causes dysfunctional insulin signaling and glucose transport in a muscle-type-specific manner. FASEB J. 2022;36(3):e22211.
- Ariaans G, de Jong S, Gietema JA, et al. Cancer-drug induced insulin resistance: innocent bystander or unusual suspect. Cancer Treat Rev. 2015;41(4):376–384.
- Hwangbo Y, Lee EK. Acute hyperglycemia associated with anti-cancer medication. Endocrinol Metab. 2017;32(1):23–29.
- Perez A, Jansen-Chaparro S, Saigi I, et al. Glucocorticoid-induced hyperglycemia. J Diabetes. 2014;6(1):9–20.
- Schultz H, Pedersen-Bjergaard U, Jensen AK, et al. The influence on survival of glucocorticoid induced diabetes in cancer patients with metastatic spinal cord compression. Clin Transl Radiat Oncol. 2018;11:19–25.
- Renehan AG, Zwahlen M, Egger M. Adiposity and cancer risk: new mechanistic insights from epidemiology. Nat Rev Cancer. 2015;15(8):484–498.
- Raun SH, Buch-Larsen K, Schwarz P, et al. Exercise—a panacea of metabolic dysregulation in cancer: physiological and molecular insights. Int J Mol Sci. 2021;22(7):3469.
- Sylow L, Richter EA. Current advances in our understanding of exercise as medicine in metabolic disease. Curr Opin Physiol. 2019;12:12–19.
- Schmitz KH, Campbell AM, Stuiver MM, et al. Exercise is medicine in oncology: engaging clinicians to help patients move through cancer. CA Cancer J Clin. 2019;69(6):468–484.
- Laaksonen DE, Lindström J, Lakka TA, et al. Physical activity in the prevention of type 2 diabetes: the Finnish Diabetes Prevention Study. Diabetes. 2005;54(1):158–165.
- Granger CL, McDonald CF, Irving L, et al. Low physical activity levels and functional decline in individuals with lung cancer. Lung Cancer. 2014;83(2):292–299.