Abstract
Background
Historically, endocrine therapy was used in a range of scenarios in patients with rising PSA, both as a treatment for locally advanced non-metastatic prostate cancer and PSA recurrence following curative intended therapy. In the present study the objective was to investigate if chemotherapy added to endocrine therapy could improve progression-free survival (PFS).
Materials and Methods
Patients with hormone-naïve, non-metastatic prostate cancer and rising prostate-specific antigen (PSA), enrolled from Sweden, Denmark, the Netherlands, and Finland, were randomized to long-term bicalutamide (150 mg daily) or plus docetaxel (75 mg/m2, q3w, 8–10 cycles) without prednisone, after stratification for the site, prior local therapy or not, and PSA doubling time. The primary endpoint was 5-year PFS analyzed with a stratified Cox proportional hazards regression model on intention to treat basis.
Results
Between 2009 and 2018, a total of 348 patients were randomized; 315 patients had PSA relapse after radical treatment, 33 patients had no prior local therapy. Median follow-up was 4.9 years (IQR 4.0–5.1). Adding docetaxel improved PFS (HR 0.68, 95% CI 0.50–0.93; p = 0.015). Docetaxel showed an advantage for patients with PSA relapse after prior local therapy (HR 0.67, 95% CI 0.49–0.94; p = 0.019). One event of neutropenic infection/fever occurred in 27% of the patients receiving docetaxel. Limitations were slow recruitment, lack of enrolling patients without radical local treatment, and too short follow-up for evaluation of overall survival in patients with PSA relapse.
Conclusion
Docetaxel improved PFS in patients starting bicalutamide due to PSA relapse after local therapy or localized disease without local therapy. Confirmatory studies of the efficacy of docetaxel in the setting of PSA-only relapse in addition to endocrine therapies may be justified if longer follow-up will show increased metastatic-free survival.
Introduction
Androgen-signaling inhibition is the mainstay systemic treatment for patients with recurrent prostate cancer after local treatment and for patients with progressive disease unsuitable for local therapy. In northern European countries, oral anti-androgen bicalutamide has been adopted as the first intervention in these settings for non-metastatic patients. Systemic side effects are lower with androgen-receptor blockade compared with androgen-deprivation therapy (ADT), and effects on prostate cancer mortality are similar [Citation1–4].
International guidelines recommend restricting ADT for prostate-specific antigen (PSA)-only relapse to patients with adverse pathology or PSA doubling times (PSADTs) <12 months [Citation5–8]. For PSADTs <6 months, immediate ADT is associated with significantly better metastasis-free survival and cancer-specific survival compared with delayed ADT [Citation9–11].
Nowadays, improved overall survival from adding docetaxel to ADT is established in both castration-resistant and hormone-naïve metastatic prostate cancer [Citation12–15]. On the contrary, in the setting of PSA-only relapse after primary local treatment, docetaxel as an add-on to a gonadotropin-releasing hormone (GnRH) analogue had no effect on PSA progression-free survival [Citation16] or progression-free survival [Citation17].
This study investigated if there is a beneficial effect on progression-free survival of adding docetaxel to first-line long-term bicalutamide therapy in non-metastatic, hormone-naive prostate cancer patients with rising PSA after curative treatment or local progressive disease not suitable for radical therapy; the latter group consisted of only 33 patients due to a change in the clinical management for this category, mainly based on the outcome of the SPCG-7 trial [Citation18].
Patients and methods
Study design
SPCG-14, an investigator-initiated phase 3 trial, randomized patients from Sweden, Denmark, Finland, and The Netherlands (Supplementary Table 1) after stratification by site, prior local therapy (yes/no) and PSADT (<6 months or ≥6 months). Patients were randomly assigned in a 1:1 ratio to either anti-androgen alone (AA) or anti-androgen in combination with docetaxel (AA + Doc). The investigators randomized patients with a centralized web-based procedure, described in the Supplement and assigned the participants to the randomized intervention. The study was not blinded to the investigator or the participants.
A power calculation was performed based on the hypothesis that the addition of docetaxel would increase progression-free survival from 50% to 65%, at a follow-up of 60 months. With a power of 80% and the expectation of 15% censored observations (lost to follow-up or withdrawal of consent) within 5 years, we required a total of 430 patients.
The trial was designed and conducted by Scandinavian Prostate Cancer Foundation. Good clinical practice, the Declaration of Helsinki, and national laws/regulations were followed. Ethical approval was obtained in each country. All patients provided informed consent. The trial was registered at EudraCT (2008-003138-33) and ClinicalTrials.gov (NCT03119857). The protocol and the statistical analysis plan as well as trial support are available in the Supplement.
The external Trial Safety Committee was advised due to the extended inclusion period. The slower-than-expected recruitment was mainly caused by the very few patients enrolled with the locally advanced disease without prior local therapy. For this category, the clinical management was changed already after the publication of the SPCG-7 trial in 2009 [Citation18] showing an advantage in overall survival by giving radiotherapy to the prostate. The Trial Safety Committee recommended stopping inclusion based on a favorable outcome for adding docetaxel to bicalutamide versus bicalutamide alone (p = 0.012) based on 95 events for 291 included patients. Based on the recommendation a decision was made to stop inclusion but continue the follow-up of the included patients according to protocol. In February 2021 validated data from the database was retrieved for this first analysis; then almost 50% of the patients had reached the primary endpoint.
Patients, intervention, and follow-up
Eligible patients (ages 18–80 years) had histological adenocarcinoma of the prostate and rising PSA after local treatment or localized prostate cancer not suitable for curative therapy; detailed inclusion criteria are presented in . PSADT was calculated by the investigator according to the Memorial Sloan Kettering Cancer Center method [Citation19]. A negative bone scan within 3 months before the start of therapy was mandatory; CT of the abdomen was optional. Further details are presented in the protocol, see the Supplement.
All patients received, after prophylactic irradiation of the mammary glands, bicalutamide (150 mg) once daily until disease progression. At the start of bicalutamide intervention, patients in the AA + Doc group were prescribed 8 to 10 cycles of docetaxel (75 mg/m2 body surface area infused over 60 min per cycle) initiated every third week, without daily prednisone. The number of cycles within this range was decided by the investigator. Granulocyte colony-stimulating factor (G-CSF) was not prescribed initially. Guidelines for use of G-CSF, dose prescriptions and modifications are presented in the Supplement.
Patients in the AA + Doc group had laboratory tests between each cycle of docetaxel and underwent a physical examination every second cycle. Laboratory tests for bicalutamide toxicity were performed at the discretion of the investigator. Patients with hepatic toxicity from bicalutamide discontinued treatment and were managed according to the prevailing clinical practice: either the bicalutamide dose was reduced, or they were switched to a GnRH analogue.
All patients had PSA tests every three months after randomization for five years or until disease progression and were clinically examined every six months. At PSA progression or clinical symptoms, a bone scan examination was mandatory, which thereafter should be performed yearly. Side effects were assessed, according to the Common Terminology for Adverse Events (CTCAE) v3.0.
End points
The primary endpoint was progression-free survival (PFS), defined as the time from randomization to the date of the first event. Events included an increase in PSA by ≥2.0 ng/mL above the nadir value, if confirmed after three to six weeks, or image-based progression, or any death, whichever occurred first within five years from randomization. In addition, the number of deaths within 5-years will be presented in this first report.
Statistical analysis
The full statistical analysis plan is presented as supplementary. Progression-free survival was analyzed with a stratified Cox proportional hazards regression model. Of the 19 participating sites, 10 included fewer than seven patients, so we did not stratify by the site in the analyses. Censoring events were either withdrawal of consent, loss to follow-up, or last follow-up. Progression-free survival was estimated by the Kaplan–Meier method, with treatment groups compared using the log-rank test. Efficacy analyses were performed according to intention-to-treat. Median follow-up time was determined through the reverse-censoring on progression, in which survival is treated as the event and progression (PSA-progression, metastasis or death of any cause) as censoring events, using the reverse Kaplan–Meier estimator [Citation20]. All tests were two-sided; p-values <0.05 were considered statistically significant. The effect of treatment on subgroups was evaluated with a forest plot, performed with the macro “ipdover,” written for Stata by David Fisher. All statistical analyses were carried out with Stata/IC 16.1 for Mac-10.
Results
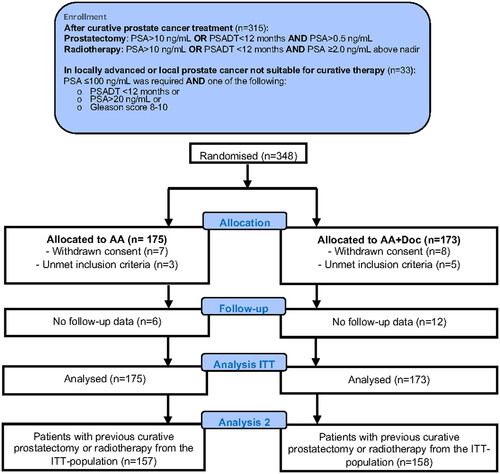
Between 3 March 2009, and 15 February 2018, a total of 348 patients were randomized, and the number by site presented (Supplementary Table 1); 175 patients were allocated to AA and 173 to AA + Doc. Fifteen patients withdrew consent, and eight patients were incorrectly randomized ( and Supplementary Table 2). The two treatment groups were well-balanced for baseline patient characteristics ().
Table 1. Baseline characteristic of included patients.
Of the patients in the AA + Doc group, 22% received ten cycles of docetaxel, 52% at least eight cycles, 71% at least six cycles, and 86% received four cycles or more. Seven patients did not receive any docetaxel because they withdrew consent (n = 5) or did not meet the inclusion criteria (n = 2). G-CSF support was given to 70% of patients in cycle two or later. The docetaxel dose was reduced once for 55% of patients, and twice for 9.7% of patients (Supplementary Table 3). Serum testosterone, assessed at each docetaxel cycle, increased over time (Supplementary Figure 1 and Supplementary results).
Analysis of the entire cohort
At the data cutoff, 172 study patients had experienced a first event within five years: 75 in the AA + Doc group and 97 in the AA group. One patient had biopsy-verified liver metastasis (AA + Doc) and seven patients had died, without preceding detectable rise in PSA ().
Table 2. Primary events within 5 years.
The median follow-up time was 4.9 years (interquartile range 4.0–5.1). Of note, 25% of the patients still alive had less than 3.8 years of follow-up. Adding docetaxel to bicalutamide provided an advantage over bicalutamide alone for progression-free survival (HR 0.68, 95% CI 0.50–0.93; p = 0.015; ).
Figure 2. Kaplan–Meier estimates of the probability of progression-free survival (A) all 348 included patients; (B) patients who had a prior curative treatment or not.
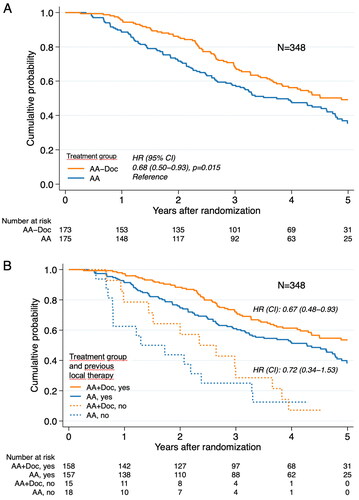
The five-year progression-free survival was 49% (95% CI 41–57) for the AA + Doc group, and 36% (95% CI 27–44) for the AA group. The median times to progression were 4.9 years (95% CI 3.8 to undetermined) for the AA + Doc patients and 3.7 years (95% CI 2.9–4.6) for the AA group. There were 37 deaths within five years, 24 in the AA group and 13 in the AA + Doc group (HR 0.47, CI 0.24-0.94). For restricted mean survival time see Supplementary material.
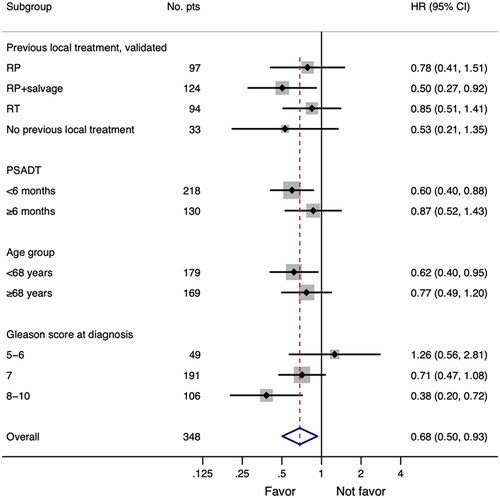
A forest plot analysis showed that adding docetaxel yielded the most pronounced benefit in younger patients, in patients with prior prostatectomy followed by salvage radiotherapy, and in those with Gleason score 8–10 and PSADT< 6 months ().
Figure 3. Forest plot of hazard ratios (HRs) for progression-free survival (PFS) for included patients according to baseline characteristics. The vertical solid line indicates HR = 1, and HR. <1 indicates longer PFS, in favor of the AA + Doc treatment. The red dashed line shows HR for all 348 patients. #: number of patients
Safety
Adverse events (AE) and severe adverse events (SAEs) were reported throughout the trial (). In the 173 patients receiving docetaxel, a total of 46 episodes of neutropenic fever/infection grade 3 or 4 were reported, and affected once 45 patients (27%) without G-CSF and most often after the first cycle; one patient experienced a second event in spite of support with G-CSF.
Table 3. Reported numbers of serious adverse events.
Sixty-one patients terminated the docetaxel treatment after one to seven cycles because of AEs (n = 51) or SAEs (n = 10) (Supplementary Table 3). Twenty-five patients of those receiving one to seven cycles (33%) interrupted the docetaxel treatment due to neuropathy of any grade; another eight patients terminated the treatment after eight to nine cycles (15%) for the same reason.Post-hoc analysis of the cohort of patients with PSA-only relapse after local therapy.
Of the 315 patients included in the trial, 97 had undergone a prostatectomy, another 124 had also salvage radiotherapy, and 94 had received curative dose-escalated radiotherapy. At a median follow-up time of 4.9 years (interquartile range 4.0-5.1), progression-free survival was improved with docetaxel added to bicalutamide compared with bicalutamide alone (HR 0.67, 95% CI 0.48–0.94; p = 0.019) (). The 5-year progression-free survival was 54% (95% CI 44–62) for the AA + Doc group and 38% (95% CI 29–47) for the AA group, with respective median times to progression of 6.1 years (95% CI 4.3 to undetermined) and 4.3 years (95% CI 3.2–4.8).
A forest plot analysis for the 315 patients with PSA-only relapse after prior local therapy was presented (Supplementary Figure 2). Of the 315 patients, 59% had Gleason score 7 and 26% had Gleason score 8–10; the distribution in Gleason scores was similar within the subgroups receiving different primary treatments (Supplementary Table 4).
In the sub-group of patients in the AA + Doc group offered and accepted/tolerated 8-10 cycles there was a risk reduction of 42% of progression compared to patients in the AA group (Supplementary data and Supplementary figure 3). PSA response rate is included in supplementary data.
In the patient cohort with previous local treatment, 11 patients died in the AA + Doc group and 17 in the AA group within five years. The five-year overall survival was 92% (95% CI 85–95) for the AA + Doc group and 87% (80–92) for the AA group (HR 0.67, 95% CI 0.31–1.42). Deaths of any cause as the first event occurred in three patients in the AA + Doc group and three patients in the AA group (); the other deaths were preceded by PSA progress in eight and 14 patients, respectively.
Discussion
We provide evidence that adding docetaxel when starting long-term bicalutamide 150 mg improves progression-free survival in patients with non-metastatic prostate cancer of whom 91% (315 patients) had rising PSAs after prostatectomy plus/minus salvage radiotherapy or dose-escalated local radiotherapy, only 9% (33 patients) had no prior curative therapy. The novel finding is that adding docetaxel to bicalutamide improved progression-free survival for patients with PSA-only relapse with a risk reduction of 33%.
Previous studies investigating docetaxel in addition to endocrine therapy for PSA-relapse after curative treatment include Oudard et al. [Citation16] and TAX3503 trial [Citation17]. Neither of them did show evidence of the benefit of combination therapy. Oudard et al. randomly assigned 254 patients to 12 months of GNRH analogue ± 6 cycles of docetaxel (70 mg/m2 q3w), no benefit was observed for neither PSA progression-free survival, radiographic-progression free survival nor overall survival at a median follow-up-of-more-than ten years. The authors conclude that despite an approximately 15% lower risk of progression in the combination of docetaxel and endocrine therapy the results were not statistically significant. The TAX3503 trial randomly assigned 413 patients to 18 months of GnRH analogue ± ten cycles of docetaxel (75 mg/m2 q3w); there was a trend to improvements in progression-free and overall survival with the addition of docetaxel at a median follow-up of 33 months.
The different androgen signalling inhibition interventions could have affected the outcome of docetaxel. The complete PSA response, nadir ≤0.1 ng/mL, reported by Oudard et al. with GnRH analogue was similar to our finding with bicalutamide, implying no obvious difference in efficacy between the GnRH analogue and bicalutamide.
Serum testosterone assessment indicated that docetaxel had no obvious influence on testosterone production (Supplementary Figure 1).
Preclinical in vitro and in vivo studies have shown that the sensitivity of prostate cancer cells to taxanes can be increased by adding bicalutamide, interpreted as an androgen receptor–independent effect [Citation21–23]. In line with this, a recent study on a xenograft prostate cancer model showed that enzalutamide increased the efficacy of cabazitaxel, independent of the effect of enzalutamide alone on the tumor growth [Citation24]. Furthermore, at clinically relevant concentrations, docetaxel had no anti-hormonal effect [Citation25]. Most probably, the microtubule-stabilizing and anti-mitotic characteristics of docetaxel result in an additive hormone-independent eradication of prostate cancer cells [Citation26].
Docetaxel treatment showed an advantage in the present SPCG-14 trial but not in the SPCG-12 [Citation27] and VA#553 [Citation28] trials as an adjuvant to prostatectomy or in SPCG-13 trial [Citation29] as an adjuvant to radiotherapy. One reason may be the difference in the bioavailability of docetaxel. In the adjuvant setting to radical treatment with no rise in PSA, docetaxel reaches tumor cells by diffusion. In contrast, in the PSA-only relapse setting, docetaxel reaches the manifested tumor directly through new tumor vascularization, adding a higher docetaxel concentration to tumor cells in this case. Tumor vascularization also improves the nutrient and oxygen supply compared with tumor cells that must rely on diffusion. Well-oxygenated tumor cells in the PSA-recurrence setting proliferate to a greater extent and therefore have a higher sensitivity to docetaxel than quiescent tumor cells targeted in the adjuvant setting. These considerations have been reviewed in detail by Tannock [Citation30].
The study was not designed to investigate any difference in the number of docetaxel cycles, but a per protocol analysis was stipulated in the protocol and this was addressed by a sensitivity analysis investigating the benefit of docetaxel in patients receiving 8–10 cycles (52% of those in the AA + Doc group). Ten cycles were prescribed in the TAX327 [Citation12] and also in the subsequent FIRSTANA trial [Citation31], investigating the efficacy in castration-resistant prostate cancer. In contrast, 6 cycles were usually prescribed to hormone-naïve patients, first in trials on metastatic disease and later in adjuvant settings [Citation14–16,Citation27–29]. Whether six cycles of docetaxel to subclinical/minimal disease underdosed patients with higher tolerance of docetaxel in previous negative trials in these settings cannot be excluded [Citation16,Citation17,Citation27–29].
The trial was powered for progression-free survival, including any event of disease progress and any death, whichever occurred first. For patients with PSA-only relapse the first sign of disease progression after the intervention is expected to be PSA progress, albeit bone, visceral, and lymph node metastasis may be detected concomitantly. At the time point of this report, no metastasis and only 6 deaths were registered as the first event, i.e. the primary endpoint PFS became almost the same as PSA-progression-free survival.
PSA progression-free survival has not yet been accepted as a surrogate endpoint for overall survival, while metastasis-free survival and progression-free survival both are robust surrogates for overall survival in non-metastatic prostate cancer [Citation32,Citation33]. A much longer follow-up is necessary to reach enough events for accurate analyses of metastasis-free survival and overall survival in the setting of PSA-only relapse.
Concerning the toxicity of docetaxel, neutropenic fevers/infections grade 3 or 4 affected 27% of the patients before support with G-CSF was initiated and occurred usually already after the first cycle. G-CSF prevented further episodes in all but one patient who experienced a second event. Therefore, prophylactic G-CSF from docetaxel start should be considered to minimize haematological toxicity.
Neuropathy can cause long-term discomfort after docetaxel and occurs in 25–30%, of all grades, and grade 3 in 2–3%, after ten cycles according to the TAX327 [Citation12] and FIRSTANA trials [Citation31]. In our study, 20% of the patients interrupted the treatment due to neuropathy of any degree which was the main reason for docetaxel termination. We found no support for a correlation between neuropathy and accumulated docetaxel dosage up to ten cycles; thus, the risk and severity of neuropathy are probably determined by patient characteristics to a great extent.
The choice to use bicalutamide 150 mg in this trial instead of ADT is because it was standard of care for PSA-relapse after curative treatment in the Nordic countries with or without prior salvage strategies. The guidelines still recommend this after salvage in M0 disease based on lower toxicity with similar efficacy. All patients with PSA progression on bicalutamide in the trial were recommended a switch to ADT. Previous studies show that bicalutamide has a similar effect as ADT in locally advanced PC and only 6 weeks shorter median survival time M1 disease, but with significantly longer time without treatment-related side effects when compared to ADT [Citation3,Citation4].
PSMA-PET was not available when the study started in 2009 and CT-scan was not always performed in these patients according to practice. Thus, patients were at randomizationcharacterized as non-metastatic based only on bone scans. It is probable that PSMA-PET would reveal metastases in a greater portion of these patients based on the median PSA level at inclusion (3 ng/ml) [Citation34,Citation35]. However, since this is a randomized study, we believe any difference in M1-stage at baseline will be evenly distributed between the two groups in this trial. The knowledge that this study may include patients with metastatic disease does not exclude the possibility that docetaxel may have a place in the early relapse situation in node and/or oligometastatic settings. PSMA-PET is now more readily available, unlike at the time of the present study, and if PSMA-PET positive patients in the relapse situation may benefit more from intensification of the systemic therapy such as the addition of docetaxel or if metastasis-targeted therapy would be an option is for future studies to resolve.
The long recruitment time was a limitation of the SPCG-14 trial. Lack of compliance with the protocol resulted in only half of the cohort receiving 8–10 cycles, probably influenced by the CHAARTED [Citation14] and STAMPEDE trials [Citation15], and SPCG-12 and SPCG-13 [Citation27–29], which all prescribed 6 cycles of docetaxel. Furthermore, the follow-up is fairly short considering the lifeexpectancy for patients with PSA-only relapse, making the current overall survival data immature. Another limitation is that the protocol-based follow-up will end at 5 years and all endpoints after that timepoint will be based on follow-up according to clinical routine. However, both metastasis-free survival and overall survival will be collected and reported when data is mature.
Conclusion
This is the first study showing the advantages of adding docetaxel to endocrine therapy at first PSA relapse after local therapy. If longer follow-up will show increased metastasis-free survival, these results encourage to further study of early docetaxel combined with endocrine therapy in selected patients with early PSA relapse after surgery or radiotherapy.
Author Contributions
All authors were part of the investigation, data curation, resources, validation, and writing review and editing. AJ, ÅJ, and JED oversaw all aspects of the project. JED and AJ were grant holders. AJ, KB, PM, SA, ML, PV, RdW, and JED administered the study. AJ, ÅJ, EH, and JED were responsible for writing the original draft and for the formal analyses, methodology, and visualization. AW and JED designed the trial. EH was responsible for the statistical analyses. All authors had access to any data on request in the study and had final responsibility for the decision to submit for publication.
Supplemental Material
Download MS Word (37 KB)Supplemental Material
Download PDF (46.2 KB)Supplemental Material
Download PDF (1.3 MB)Supplemental Material
Download PDF (3.6 MB)Supplemental Material
Download PDF (1 MB)Supplemental Material
Download PDF (24.1 KB)Acknowledgments
The authors gratefully acknowledge all patients and the trial personnel at each clinical trial unit. Additionally, we thank Birgitta Olsson and Agnetha Sundberg for administrative assistance with the database, and we thank Sandra Grandell for administrative support. We also thank the Trial Safety Committee: Erik Holmberg (statistician), Prof Ola Bratt (consultant urologist), and Prof Sten Nilsson (a consultant oncologist). We also thank Esa Kähkönen for their contributions to the study.Special thanks to all the study nurses at the sites for all their work in this trial.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author, JED. The data are not publicly available due to restrictions in content containing information that could compromise the privacy of research participants.
Additional information
Funding
References
- Beckmann K, Garmo H, Adolfsson J, et al. Androgen deprivation therapies and changes in comorbidity: a comparison of gonadotropin-releasing hormone agonists and antiandrogen monotherapy as primary therapy in men with high-risk prostate cancer. Eur Urol. 2019;75(4):676–683.
- Thomsen FB, Bosco C, Garmo H, et al. Anti-androgen monotherapy versus gonadotropin-releasing hormone agonists in men with advanced, non-metastatic prostate cancer: a register-based, observational study. Acta Oncol. 2019;58(1):110–118.
- Tyrrell CJ, Kaisary AV, Iversen P, et al. A randomised comparison of 'casodex’ (bicalutamide) 150 mg monotherapy versus castration in the treatment of metastatic and locally advanced prostate cancer. Eur Urol. 1998;33(5):447–456.
- Iversen P, McLeod DG, See WA, et al. Antiandrogen monotherapy in patients with localized or locally advanced prostate cancer: final results from the bicalutamide early prostate cancer programme at a median follow-up of 9.7 years. BJU Int. 2010;105(8):1074–1081.
- Mohler JL, Antonarakis ES, Armstrong AJ, et al. Prostate cancer, version 2.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2019;17(5):479–505.
- Cornford P, van den Bergh RCN, Briers E, et al. EAU-EANM-ESTRO-ESUR-SIOG guidelines on prostate cancer. Part II-2020 update: treatment of relapsing and metastatic prostate cancer. Eur Urol. 2021;79:263–282.
- Virgo KS, Rumble RB, de Wit R, et al. Initial management of noncastrate advanced, recurrent, or metastatic prostate cancer: ASCO guideline update. J Clin Oncol. 2021;39(11):1274–1305.
- Van den Broeck T, van den Bergh RCN, Arfi N, et al. Prognostic value of biochemical recurrence following treatment with curative intent for prostate cancer: a systematic review. Eur Urol. 2019;75(6):967–987.
- Pinover WH, Horwitz EM, Hanlon AL, et al. Validation of a treatment policy for patients with prostate specific antigen failure after three-dimensional conformal prostate radiation therapy. Cancer. 2003;97(4):1127–1133.
- Klayton TL, Ruth K, Buyyounouski MK, et al. PSA doubling time predicts for the development of distant metastases for patients who fail 3DCRT Or IMRT using the phoenix definition. Pract Radiat Oncol. 2011;1(4):235–242.
- van den Bergh RC, van Casteren NJ, van den Broeck T, et al. Role of hormonal treatment in prostate cancer patients with nonmetastatic disease recurrence After local curative treatment: a systematic review. Eur Urol. 2016;69(5):802–820.
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502–1512.
- Petrylak DP, Tangen CM, Hussain MH, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N Engl J Med. 2004;351(15):1513–1520.
- Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373(8):737–746.
- James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163–1177.
- Oudard S, Latorzeff I, Caty A, et al. Effect of adding docetaxel to androgen-deprivation therapy in patients with high-risk prostate cancer with rising prostate-specific antigen levels after primary local therapy: a randomized clinical trial. JAMA Oncol. 2019;5(5):623–632.
- Morris MJ, Mota JM, Lacuna K, et al. Phase 3 randomized controlled trial of androgen deprivation therapy with or without docetaxel in high-risk biochemically recurrent prostate cancer after surgery (TAX3503). Eur Urol Oncol. 2021;4(4):543–552.
- Widmark A, Klepp O, Solberg A, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373(9660):301–308.
- Arlen PM, Bianco F, Dahut WL, et al. Prostate specific antigen working group guidelines on prostate specific antigen doubling time. J Urol. 2008;179(6):2181–2185. discussion 2185–2186.
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–346.
- Ylitalo EB, Thysell E, Thellenberg-Karlsson C, et al. Marked response to cabazitaxel in prostate cancer xenografts expressing androgen receptor variant 7 and reversion of acquired resistance by anti-androgens. Prostate. 2020;80(2):214–224.
- Zhu Y, Liu C, Armstrong C, et al. Antiandrogens inhibit ABCB1 efflux and ATPase activity and reverse docetaxel resistance in advanced prostate cancer. Clin Cancer Res. 2015;21(18):4133–4142.
- Lombard AP, Liu C, Armstrong CM, et al. ABCB1 mediates cabazitaxel-docetaxel cross-resistance in advanced prostate cancer. Mol Cancer Ther. 2017;16(10):2257–2266.
- Mout L, van Royen ME, de Ridder C, et al. Continued androgen signalling inhibition improves cabazitaxel efficacy in prostate cancer. EBioMedicine. 2021;73:103681.
- Mang J, Merkle K, Heller M, et al. Molecular complexity of taxane-induced cytotoxicity in prostate cancer cells. Urol Oncol. 2017;35(1):32.e39–32.e16.
- Dumontet C, Jordan MA. Microtubule-binding agents: a dynamic field of cancer therapeutics. Nat Rev Drug Discov. 2010;9(10):790–803.
- Ahlgren GM, Flodgren P, Tammela TLJ, et al. Docetaxel versus surveillance after radical prostatectomy for high-risk prostate cancer: results from the prospective randomised, open-label phase 3 scandinavian prostate cancer group 12 trial. Eur Urol. 2018;73(6):870–876.
- Lin DW, Shih MC, Aronson W, et al. Veterans affairs cooperative studies program study #553: chemotherapy after prostatectomy for high-risk prostate carcinoma: a phase III randomized study. Eur Urol. 2020;77(5):563–572.
- Kellokumpu-Lehtinen PL, Hjälm-Eriksson M, Thellenberg-Karlsson C, et al. Docetaxel versus surveillance after radical radiotherapy for intermediate- or high-risk prostate cancer-results from the prospective, randomised, open-label phase III SPCG-13 trial. Eur Urol. 2019;76(6):823–830.
- Tannock IF. The five rs of chemotherapy. Lancet Oncol. 2016;17(6):703–705.
- Oudard S, Fizazi K, Sengelov L, et al. Cabazitaxel versus docetaxel as first-line therapy for patients with metastatic castration-resistant prostate cancer: a randomized phase III Trial-FIRSTANA. J Clin Oncol. 2017;35(28):3189–3197.
- Maeda H, Takeda K, Urushihara H, et al. Searching for potential surrogate endpoints of overall survival in clinical trials for patients with prostate cancer. Cancer Rep. 2021;4(3):e1334.
- Xie W, Regan MM, Buyse M, et al. Metastasis-free survival is a strong surrogate of overall survival in localized prostate cancer. J Clin Oncol. 2017;35(27):3097–3104.
- Caglar M, Tuncel M, Yildiz E, et al. Bone scintigraphy as a gatekeeper for the detection of bone metastases in patients with prostate cancer: comparison with ga-68 PSMA PET/CT. Ann Nucl Med. 2020;34(12):932–941.
- Crocerossa F, Marchioni M, Novara G, et al. Detection rate of prostate-specific membrane antigen tracers for positron emission tomography/computed tomography in prostate cancer biochemical recurrence: a systematic review and network meta-analysis. J Urol. 2021;205:356–369.