Abstract
Background
Cost-effectiveness is important in the prioritisation between interventions in health care. Exercise is cost-effective compared to usual care during oncological treatment; however, the significance of exercise intensity to the cost-effectiveness is unclear. In the present study, we aimed to evaluate the long-term cost-effectiveness of the randomised controlled trial Phys-Can, a six-month exercise programme of high (HI) or low-to-moderate intensity (LMI) during (neo)adjuvant oncological treatment.
Methods
A cost-effectiveness analysis was performed, based on 189 participants with breast, colorectal, or prostate cancer (HI: n = 99 and LMI: n = 90) from the Phys-Can RCT in Sweden. Costs were estimated from a societal perspective, and included cost of the exercise intervention, health care utilisation and productivity loss. Health outcomes were assessed as quality-adjusted life-years (QALYs), using EQ-5D-5L at baseline, post intervention and 12 months after the completion of the intervention.
Results
At 12-month follow-up after the intervention, the total cost per participant did not differ significantly between HI (€27,314) and LMI exercise (€29,788). There was no significant difference in health outcome between the intensity groups. On average HI generated 1.190 QALYs and LMI 1.185 QALYs. The mean incremental cost-effectiveness ratio indicated that HI was cost effective compared with LMI, but the uncertainty was large.
Conclusions
We conclude that HI and LMI exercise have similar costs and effects during oncological treatment. Hence, based on cost-effectiveness, we suggest that decision makers and clinicians can consider implementing both HI and LMI exercise programmes and recommend either intensity to the patients with cancer during oncological treatment to facilitate improvement of health.
Keywords:
Introduction
As resources are limited in health care, information on cost-effectiveness of alternative interventions is needed. It is important to consider the opportunity costs of resources used, which is the value of the best alternative use of the same resources. A cost-effectiveness analysis, considering both costs and health outcomes of interventions, is an important tool in the decision-making process when prioritising between interventions or when implementing new interventions in health care. Further, the concept of quality-adjusted life-years (QALY), which combines values of the health state with the duration of time in that state, is an established health outcome that allow comparisons between different interventions in health care, such as exercise programmes in cancer care [Citation1].
There is strong evidence that exercise improves health-related quality of life (HRQoL) and physical function, and reduces fatigue, anxiety and depressive symptoms during and after oncological treatments [Citation2–5]. Exercise may also reduce hospital stays [Citation6,Citation7], decrease sick leave rates [Citation8], and improve survival [Citation9], although more studies are needed to confirm this. Supervised exercise seems to be more effective than unsupervised [Citation3], but entails additional costs. Interventions with combined endurance and resistance training, two to three sessions per week, for at least 12 weeks have demonstrated effect on HRQoL [Citation2]. However, there is insufficient evidence on which exercise intensity is most effective to improve the HRQoL during oncological treatment. While higher exercise intensity leads to additional physical health benefits in general [Citation10], one study showed that patients with cancer prefer low intensity exercise [Citation11]. However, from a public health perspective, the greatest health gains are achieved when reaching an impact in a larger population or with lasting effects [Citation10]. The health outcome of different exercise intensities during oncological treatment in relation to its costs in the long-term is thus important information for the decision makers.
The few cost-effectiveness analyses performed on exercise interventions during oncological treatment show benefits compared to usual care; however, the results are inconsistent due to heterogeneity with regard to health systems, payment structures, intervention characteristics, cancer populations and follow-up durations [Citation12–14]. Furthermore, cost-effectiveness analyses focussing on different intensities are limited. In a study by Van Waart et al. (2017) comparing usual care to high or low exercise intensity, high was considered cost effective, while low was not considered cost effective, compared to usual care [Citation15]. Though, no comparison of the cost-effectiveness between the different exercise intensities was made. In another study by Kampshoff et al. high intensity exercise was considered cost effective compared to low-to-moderate intensity. However, the exercise programme was performed after chemotherapy [Citation16]. Thus, more cost-effectiveness analyses with exercise interventions during oncological treatment comparing high versus low intensity is requested [Citation12].
The Phys-Can (Physical Training and Cancer) RCT evaluated six months of combined supervised resistance training and homebased endurance training of high-intensity (HI) or low-to-moderate intensity (LMI) [Citation17]. At post-intervention, HI was slightly more beneficial compared to LMI regarding muscle strength, cardiorespiratory fitness and physical fatigue (main outcome), although the differences were not considered clinically important for physical fatigue. There were no differences between groups regarding overall HRQoL, anxiety, depression, functioning in daily life or sleep. At 12-month follow-up after the intervention, there were no differences in total costs [Citation18], or in HRQoL [Citation19] between the exercise intensities. Still, it remains to explore QALYs and hence evaluate the cost-effectiveness between the exercise intensities. In the present study, the aim was to evaluate the long-term cost-effectiveness of an exercise programme of HI or LMI during (neo)adjuvant oncological treatment.
Method
Research design
A cost-effectiveness analysis was performed from a societal perspective with an 18-month time horizon (6 months intervention and 12 months follow-up), based on the data from the Phys-Can RCT. The design including sample size calculations is previously described in detail [Citation20,Citation21]. CHEERS 2022 statement checklist was applied to guide this report [Citation22].
Study sample
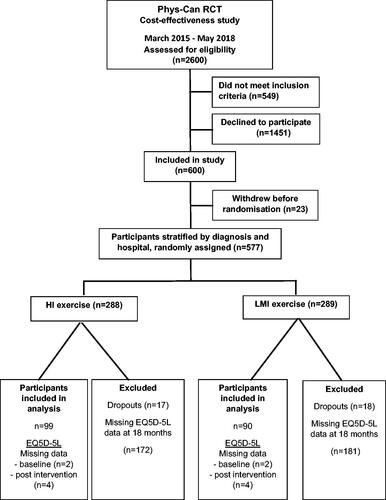
Briefly, participants were recruited at three university hospitals in Sweden from 2015 to 2018. Inclusion criteria were patients with breast, colorectal or prostate cancer, aged ≥18 years and scheduled for neoadjuvant and/or adjuvant oncology treatment. Exclusion criteria were health conditions that might contraindicate high-intensity exercise (e.g., heart failure, chronic obstructive pulmonary disease, orthopaedic conditions, or neurological disorders). After completion of baseline measurements, participants were stratified by cancer diagnosis and hospital, and randomly assigned to one of four conditions: LMI exercise, LMI exercise with behaviour change support (BCS), HI exercise, or HI exercise with BCS. The present study only included participants who had completed EQ-5D-5L (see Data Collection) at the 12-month follow-up (n = 189 of 577, 33%; ). Those who completed EQ-5D-5L were older, had larger proportions of retired participants, and men with prostate cancer, together with lower proportions of participants on sick leave and women with breast cancer receiving chemotherapy, compared to participants who did not complete EQ-5D-5L. This study was approved by the Swedish Ethical Review Authority in Uppsala, Sweden (Ref.no 2014/249) and was conducted in accordance with the Helsinki Declaration. Informed consent was obtained from all participants.
The exercise intervention
The six-month exercise intervention consisted of supervised resistance training and home-based endurance training and was initiated when the oncological treatment began, presented in detail elsewhere [Citation17,Citation20]. The resistance training was performed twice a week at a public gym and consisted of three exercises for the upper extremities and three for the lower extremities. Four additional exercises were advised for the trunk and pelvic floor. The HI group performed one weekly session of 3 × 6 repetition maximum (RM) (2 min rest between sets), and one weekly session of 3 × 10 RM (1 min rest between sets). The LMI group performed one weekly session 3 × 12 repetitions at 50% of 6 RM (2 min between sets), and one weekly session of 3 × 20 repetitions at 50% of 10 RM (1 min rest between sets). For the endurance training, the HI group performed interval sessions twice a week with 2 min of exercise (e.g., running, cycling, walking up-hill) at 80–90% heart rate reserved (HRR) followed by 2 min of active rest (e.g., walking). The number of intervals increased from 5 intervals, until max 10 during the intervention period. The LMI group performed 150 weekly minutes of endurance training (walking, cycling) in bouts of minimum 10 min at 40–50% of HRR. Half of the participants in the HI and in the LMI group received additional BCS. In the present study, we focussed on differences between the exercise intensities since additional BCS did not improve health outcomes at post-intervention [Citation17]. The exercise was performed according to a standardised protocol. Coaches (physiotherapists and personal trainers) were educated to provide the intervention. The exercise performed by the participants was monitored by the coaches to follow adherence and progression according to the protocol. Research staff monitored that the coaches followed the intervention protocol.
Data collection
Background characteristics
Participants reported sociodemographic data and comorbidities at baseline. Medical background data were retrieved from the medical records and the Swedish National Quality Register.
Cost measures
Societal costs included the costs of the exercise intervention, health care utilisation, and productivity loss [Citation18]. Data were collected for 6 months prior to baseline measurement and up to 12 months after completion of the exercise intervention. The costs for the exercise intervention were estimated from invoices and included labour costs for the coaches (including their education and exercise supervision); time worked + overheads, fitness centre membership fees, maximal oxygen uptake (V ̇O2 max) tests, and heart rate monitors. Travel costs were considered out-of-pocket money for the participants and were compensated by mileage according to the Swedish Tax Agency in 2019 [Citation23]. Health care costs included outpatient visits (except for primary care), hospitalisation and prescribed medication. Health care utilisation was retrieved from the Swedish National Board of Health and Welfare [Citation24] and each visit was applied with costs according to the Swedish NordDRG pricelists [Citation25]. Cost of the prescribed medications were estimated using market prices [Citation26]. Productivity loss included days absent from paid work (sick-leave and disability pension) and were obtained from the Swedish Social Insurance Agency [Citation27]. In Sweden, the first 14 days of sick leave are paid by the employer, which means we don’t have data on periods shorter than 15 calendar days, but we have added the first 14 days for periods longer than that. The human capital approach was used to value the average productivity costs of full-time employees including all taxes and social fees from 2019 (€4550) [Citation28] and recalculated as full-time equivalent days. We did not discount any costs since the time horizon was only 18 months. Cost was calculated in SEK converted to Euros using an exchange rate of €1 = SEK 9.963 (28 October 2021) [Citation29].
Health outcomes
The health outcome QALYs gained were estimated using EQ-5D-5L [Citation30]. EQ-5D-5L health state consists of five dimensions of health: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression, with a five-level severity scale of no, slight, moderate, severe, and extreme/unable to [Citation19]. The EQ-5D-5L health state index, which is based on these five dimensions, was converted to a health state value ranging from 0 (dead) to 1 (full health). Primarily, we mapped the EQ-5D-5L to EQ-5D-3L [Citation31] and used the UK value set by Dolan [Citation32]. Secondarily, we used the Swedish value set by Burström et al. [Citation33] and the English value set by Devlin et al. [Citation34] as comparisons. QALYs were calculated by combining the health state values with duration in time. The maximum number of QALYs that could be gained were 1.5 (since the time horizon was 18 months). EQ VAS was used to measure the overall health on a scale from 0 to 100, where endpoints were the worst health and the best health you can imagine.
Statistical analyses
Analyses were performed in IBM SPSS statistics 28 and conducted according to intention-to-treat. A statistical level of p ≤ .05 was considered significant. Descriptive statistics were used to compared background characteristics between HI and LMI, and between participants with complete or incomplete data. Missing values of the EQ-5D-5L value set (2% of the observations) were imputed using the last observation carried forward (LOCF) method. Differences between groups were compared using independent sample t-test for QALYs, costs and health state values. Mann-Whitney U-test was used to compare differences in distribution of EQ-5D-5L dimensions of health and ANCOVA for EQ VAS (adjusted for baseline measurement) between groups. Within group differences over time were analysed using paired samples t-test for the health state values and Wilcoxon matched-pairs test for the distribution of the EQ-5D-5L dimensions. The cost-effectiveness, as evaluated by the incremental cost-effectiveness ratios (ICERs), were calculated by dividing the difference in the total costs by the difference in health outcome (QALY) between the groups [Citation1]. The uncertainty around the ICERs was estimated both from a societal and a health care perspective in Microsoft Excel 2016 using bootstrap intervals (10,000 replications) with the use of probabilistic sensitivity analyses, and cost-effectiveness planes were constructed. In addition, deterministic sensitivity analyses were performed with all Phys-Can participants with complete cost measures in the RCT (HI: n = 269 and LMI: n = 265) [Citation18].
Results
The response rate was 98% on the EQ-5D-5L questionnaire of the participants included in this study (). Background characteristics of the participants were similar between HI and LMI (). The majority were women with breast cancer. More than half of the participants had employment and at least one comorbid condition at baseline. No mortality was reported in either HI or LMI group.
Table 1. Background characteristics at baseline in the HI and LMI exercise group.
Cost measures
The total cost was €27,314 (SD: €26,105) per participant in the HI group and €29,788 (SD: €27,517) in the LMI group at the 12-month follow-up and did not differ significantly between the groups (mean cost difference €−2474; 95% CI: (−10,170 to 5243). There were no significant differences in costs between the cost categories; exercise intervention, health care and productivity loss ().
Table 2. Costs and cost differences between HI vs LMI exercise at 12-month follow-up.
Health outcomes
At the 12-month follow-up, QALYs did not differ significantly between the intensity groups (95% CI: −0.058 to 0.067). On average, the HI group generated 1.190 (SD = 0.223) QALYs and the LMI group 1.185 (SD = 0.211) QALYs. There was no significant difference between the intensity groups over time in health state values for any of the value sets or in the five single dimensions of health in EQ-5D-5L. For EQ VAS, HI scored better overall health than LMI at baseline (mean diff 6; p=.026), no other significant differences were found ().
Table 3. Impact of exercise intensity on EQ-5D-5L health state and EQ-VAS.
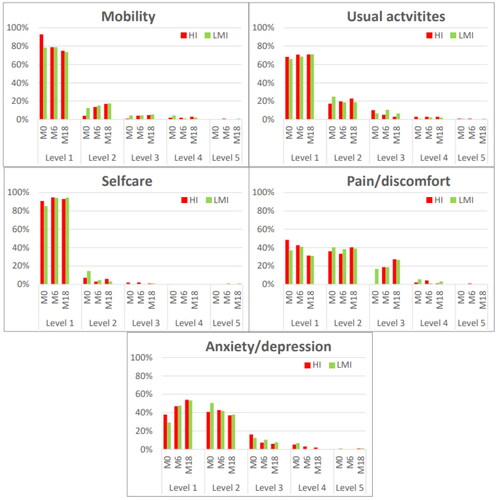
No significant changes over time in health state values were found within either HI or LMI. EQ VAS improved significantly from baseline to 12-month follow-up in both HI (mean diff 4.3; p=.011) and LMI (mean diff 7.5; p<.001). At 12-month follow-up, HI scored worse on mobility (p<.001) and pain/discomfort (p=.004), while both intensity groups scored reduced anxiety/depression (HI: p=.001 and LMI: p<.001) compared to baseline ().
Figure 2. Proportion of responses by level of severity for EQ-5D-5L dimensions at baseline (M0), post intervention (M6) and 12-month follow up (M18) in the high intensity (HI) and low-to-moderate (LMI) exercise group. Level 1: no problems, level 2: slight problems, level 3: moderate problems, level 4: severe problems, level 5: extreme problems or unable.
Cost-effectiveness
The ICER provides a ratio of additional cost per unit of health outcome in HI exercise vs LMI exercise. The mean ICER of HI compared with LMI was −516.698, but there was no significant difference in either total cost or in effects between the intensity groups.
Sensitivity analyses
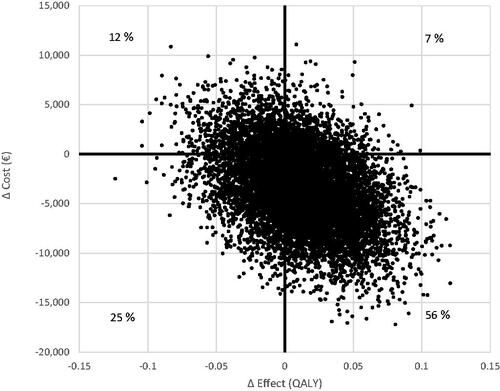
Bootstrap analysis showed that the ICER was dominant in 56% of the 10,000 replications, meaning that HI was more effective and cost less than LMI. The uncertainty around the ICER for QALYs gained from HI vs LMI exercise are large (). However, there was also a 12% risk that HI generated higher costs and less effects compared with LMI. From a health care perspective, the ICER was dominant in 54% of the 10,000 replications. Furthermore, there was no significant difference between the exercise intensities in total cost (mean HI: €35,519 and LMI: €33,387) or in QALYs (HI: 1.189 and LMI 1.190) after including all participants.
Discussion
HI exercise during oncological treatment appears to be cost-effective compared to LMI exercise at longer term, as HI showed a tendency of lower costs and similar health outcomes compared to LMI. However, there was a large uncertainty around the ICER and no significant difference in costs or in QALYs between the exercise intensities. As previous studies have shown that exercise is cost effective compared to usual care [Citation12–14], our findings support the implementation of exercise regardless of intensity during oncological treatment.
Our result indicates that HI may be cost-effective compared to LMI, in line with the findings of the Kampshoff et al. study [Citation16]. However, their study evaluated a shorter exercise intervention of 12 weeks after oncological treatment and is not directly comparable with our study. A previous systematic review that included seven studies of cost-effectiveness on exercise interventions in cancer survivors concluded that high-intensity exercise may be more cost-effective than low-intensity exercise when compared to usual care [Citation12]. Thus, our finding adds to the previous findings that high intensity exercise might be cost-effective compared to low-to-moderate intensity during oncological treatment. However, there was a high degree of uncertainty around the ICER in our study, thus our results must be interpreted with caution. Also, we included only 33% participants of Phys-Can, but sensitivity analyses showed no significant differences in costs or in effects between the exercise intensities after including all participants with complete cost data in the analysis [Citation18]. Hence, we suggest well-designed RCT to evaluate the cost-effectiveness between different exercise intensities to make these results more robust.
The exercise intensity does not seem to have an impact on the total costs during oncology treatment in our study, in line with the results in the Kampshoff et al. [Citation16]. While we found no differences in costs in either health care, productivity loss or intervention between the exercise intensities, the Kampshoff et al. study demonstrated lower health care costs and higher intervention costs in the high intensity exercise compared to the low-to-moderate intensity exercise. However, these studies are not directly comparable, and it is also difficult to compare studies with different health care systems and/or payment structures. Yet, it has been shown that no difference in total costs was found between exercise interventions and usual care in the van Waart et al. study [Citation15] and in a recent study anchored in Phys-Can [Citation18]. This means that implementing exercise programmes of either HI or LMI in cancer care does not seem to add nor save costs during treatment.
The health outcomes analysed in this study did not differ between the exercise intensities at post-intervention and in the long-term (12 months after the intervention), which is consistent to earlier findings from the Phys-Can which revealed no statistically significant difference between HI and LMI with regard to HRQoL [Citation19]. The lack of significant differences might be due to the exercise programme in Phys-Can being very comprehensive, and both exercise intensities consisting of combined endurance and resistance training. Thus, while HI exercise was more effective in improving strength and cardiorespiratory fitness and reducing physical fatigue compared to LMI at postintervention in Phys-Can [Citation17], exercise intensity may be important to some but not all outcomes in patients undergoing oncological treatment, but this needs to be further illuminated in forthcoming studies. Furthermore, no significant differences were found over time in health state values at either intensity. Both exercise intensities might be successful in preventing a decline in health state value, but the lack of a control group with no exercise intervention hindered us in drawing conclusions on the effect of exercise on health state over time. However, a previous study within the Phys-Can project showed improvements in aspects of HRQoL at both exercise intensities compared to usual care [Citation19]. Also, participants in the present study already had rather high health state values at baseline (HI = 0.80 and LMI = 0.76), leaving little room for improvement. In comparisons, a systematic review showed a mean range of health state value of 0.58 to 0.99 in early breast cancer [Citation35]. Since strong evidence shows that exercise is beneficial for several health outcomes during treatment compared to usual care [Citation2,Citation36,Citation37], our findings support that exercising with at least low-to-moderate intensity can be recommended to gain health benefits during treatment.
In this study we performed an intention-to-treat analysis. In a previous study based on the Phys-Can, the adherence to the prescribed volume of supervised resistance training was 50% in both HI and LMI, while the adherence to home-based endurance training volume was lower in HI (40%) compared to LMI (55%) [Citation17]. It is possible that adherence has implications for the cost-effectiveness, since it is likely that the effect on health would increase with greater adherence, but at the same costs. Thus, it is necessary to study the impact of adherence to the exercise prescription in future cost-effectiveness studies.
Some methodological reflections can be made from this study. There were no differences on the EQ-5D-5L health state index between the exercise intensities but using the Swedish value set by Burström generated higher values than the British ones by Dolan and Devlin. Although the values may differ due to national preferences, the Burström et al. [Citation33] value set was experience based, while the value sets of Dolan et al. [Citation32] and Devlin et al. [Citation34] were hypothetically based on health state. Hence, direct comparisons of health state between interventions can only be made when using the same value set.
Strengths within the present study were the use of long-term data from an RCT comparing a comprehensive exercise programme with well-defined differences between HI and LMI, which were closely monitored and followed a standardised protocol. In addition, we collected cost measures from national registers with little missing data. The present study also had limitations including that we excluded a large number of participants with missing EQ-5D-5L data at 12-month follow up which resulted in loss of power. Also, the LOCF imputation method might have introduced bias in the results as the missing values remained constant. Furthermore, Phys-Can RCT was designed to primarily study the effect of HI and LMI exercise on fatigue and secondarily cost-effectiveness. Thus, it is possible that the significance of exercise intensity to cost-effectiveness might be underestimated in the present study. Other limitations were a lack of data on visits to primary care, use of medication not prescribed and short-term sick leave. However, the participants in this study were scheduled for oncological treatment and therefore patients that were attached a hospital trajectory. Therefore, visits to primary care might account for a smaller share of the total health care consumption. The majority of participants were highly educated women with breast cancer; thus, our results might not be generalisable to other cancer populations. Also, our results might not be applicable to other countries with different health care systems and/or payment structures.
Conclusion
We concluded that HI and LMI exercise have similar costs and effects during oncological treatment. Hence, based on cost-effectiveness, we suggest that decision makers and clinicians can consider implementing both HI and LMI exercise programmes and recommend either intensity to the patients with cancer during oncological treatment according to their own preferences to facilitate improvement of health.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, [AA], upon reasonable request.
Additional information
Funding
References
- Drummond MF, Sculpher MJ, Claxton K, et al., editors. Methods for the economic evaluation of health care programmes. 4th ed. Oxford: Oxford University Press; 2015.
- Campbell KL, Winters-Stone KM, Wiskemann J, et al. Exercise guidelines for cancer survivors: consensus statement from international multidisciplinary roundtable. Med Sci Sports Exerc. 2019;51(11):2375–2390.
- Sweegers MG, Altenburg TM, Chinapaw MJ, et al. Which exercise prescriptions improve quality of life and physical function in patients with cancer during and following treatment? A systematic review and meta-analysis of randomised controlled trials. Br J Sports Med. 2018;52(8):505–513.
- Mishra SI, Scherer RW, Snyder C, et al. Are exercise programs effective for improving health-related quality of life among cancer survivors? A systematic review and meta-analysis. Oncol Nurs Forum. 2014;41(6):E326–E342.
- Stout NL, Baima J, Swisher AK, et al. A systematic review of exercise systematic reviews in the cancer literature (2005-2017). PM&R. 2017;9(9s2):S347–S384.
- Potiaumpai M, Doerksen SE, Chinchilli VM, et al. Cost evaluation of an exercise oncology intervention: the exercise in all chemotherapy trial. Cancer Rep. 2022;5(3):e1490.
- Edmunds K, Scuffham P, Reeves P, et al. Demonstrating the value of early economic evaluation alongside clinical trials: exercise medicine for men with metastatic prostate cancer. Eur J Cancer Care. 2021;30(5):e13479.
- Mijwel S, Jervaeus A, Bolam KA, et al. High-intensity exercise during chemotherapy induces beneficial effects 12 months into breast cancer survivorship. J Cancer Surviv. 2019;13(2):244–256.
- McTiernan A, Friedenreich CM, Katzmarzyk PT, et al. Physical activity in cancer prevention and survival: a systematic review. Med Sci Sports Exerc. 2019;51(6):1252–1261.
- Biddle SJH, Batterham AM. High-intensity interval exercise training for public health: a big HIT or shall we HIT it on the head? Int J Behav Nutr Phys Act. 2015;12(1):95.
- Wong JN, McAuley E, Trinh L. Physical activity programming and counseling preferences among cancer survivors: a systematic review. Int J Behav Nutr Phys Act. 2018;15(1):48.
- Gubler-Gut BE, Pöhlmann J, Flatz A, et al. Cost-effectiveness of physical activity interventions in cancer survivors of developed countries: a systematic review. J Cancer Surviv. 2021;15(6):961–975.
- Khan KA, Mazuquin B, Canaway A, et al. Systematic review of economic evaluations of exercise and physiotherapy for patients treated for breast cancer. Breast Cancer Res Treat. 2019;176(1):37–52.
- Mewes JC, Steuten LM, Ijzerman MJ, et al. Effectiveness of multidimensional cancer survivor rehabilitation and cost-effectiveness of cancer rehabilitation in general: a systematic review. Oncologist. 2012;17(12):1581–1593.
- van Waart H, van Dongen JM, van Harten WH, et al. Cost-utility and cost-effectiveness of physical exercise during adjuvant chemotherapy. Eur J Health Econ. 2018;19(6):893–904.
- Kampshoff CS, van Dongen JM, van Mechelen W, et al. Long-term effectiveness and cost-effectiveness of high versus low-to-moderate intensity resistance and endurance exercise interventions among cancer survivors. J Cancer Surviv. 2018;12(3):417–429.
- Demmelmaier I, Brooke HL, Henriksson A, et al. Does exercise intensity matter for fatigue during (neo-)adjuvant cancer treatment? The Phys-Can randomised clinical trial. Scand J Med Sci Sports. 2021;31(5):1144–1159.
- Ax AK, Husberg M, Johansson B, et al. Long-term resource utilisation and associated costs of exercise during (neo)adjuvant oncological treatment: the Phys-Can project. Acta Oncol. 2022;61(7):888–896.
- Ax AK, Johansson B, Lyth J, et al. Short- and long-term effect of high versus low-to-moderate intensity exercise to optimise health-related quality of life after oncological treatment-results from the Phys-Can project. Support Care Cancer. 2022;30(7):5949–5963.
- Berntsen S, Aaronson NK, Buffart L, et al. Design of a randomized controlled trial of physical training and cancer (Phys-Can) – the impact of exercise intensity on cancer related fatigue, quality of life and disease outcome. BMC Cancer. 2017;17(1):218.
- Mazzoni AS, Brooke HL, Berntsen S, et al. Exercise adherence and effect of self-regulatory behavior change techniques in patients undergoing curative cancer treatment: secondary analysis from the Phys-Can randomized controlled trial. Integr Cancer Ther. 2020;19:1534735420946834.
- Husereau D, Drummond M, Augustovski F, et al. Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BJOG. 2022;129(3):336–344.
- The Swedish Tax Agency. [cited 2021 Dec 7]. Available from: https://www.skatteverket.se/privat/skatter/beloppochprocent/2021.4.5b35a6251761e6914204479.html#h-Bilersattningmilersattning
- The National Board of Health and Welfare. Registers [cited 2022 June 1]. Available from: https://www.socialstyrelsen.se/en/statistics-and-data/registers/
- The National Board of Health and Welfare. Viktlistor för NordDRG: the National board of Health and Welfare [cited 2021 Aug 20]. Available from: https://www.socialstyrelsen.se/utveckla-verksamhet/e-halsa/klassificering-och-koder/drg/viktlistor/
- FASS. Farmacevtiska specialiteter i Sverige. 2020. [cited 2020 Oct 20]. Available from: https://www.fass.se
- Swedish Social Insurance Agency. [cited 2022 June 1]. Available from: https://www.forsakringskassan.se/
- SCB statistikdatabasen. 2020. Available from: http://www.statistikdatabasen.scb.se/
- The Riksbank (Sweden’s Central Bank). [cited 2021 Oct 7]. Available from: www.riksbank.se
- EuroQol Group. EuorQol [cited 2022 Sept 2]. Available from: https://euroqol.org/
- van Hout B, Janssen MF, Feng YS, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708–715.
- Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095–1108.
- Burström K, Sun S, Gerdtham UG, et al. Swedish experience-based value sets for EQ-5D health states. Qual Life Res. 2014;23(2):431–442.
- Devlin NJ, Shah KK, Feng Y, et al. Valuing health-related quality of life: an EQ-5D-5L value set for England. Health Econ. 2018;27(1):7–22.
- Kaur MN, Yan J, Klassen AF, et al. A systematic literature review of health utility values in breast cancer. Med Decis Making. 2022;42(5):704–719.
- Mishra SI, Scherer RW, Snyder C, et al. Exercise interventions on health-related quality of life for people with cancer during active treatment. Cochrane Database Syst Rev. 2012;2012(8):Cd008465.
- Fairman CM, Focht BC, Lucas AR, et al. Effects of exercise interventions during different treatments in breast cancer. J Community Support Oncol. 2016;14(5):200–209.