Abstract
Introduction
Proton radiation therapy (PT) has become a treatment option alongside photon therapy (XRT) for lower-grade gliomas (LGG). In this single-institution retrospective study, we investigate the patient characteristics and treatment outcomes, including pseudo-progression (PsP), for LGG patients selected for PT.
Method
Adult patients with grade 2–3 glioma consecutively treated with radiotherapy (RT) from May 2012 to December 2019 were retrospectively included in this cohort study. Tumor characteristics and treatment data were collected. The groups treated with PT and XRT were compared regarding treatment characteristics, side effects, occurrence of PsP, and survival outcomes. PsP was defined as new or growing lesions followed by either decrease or stabilization during a 12 month-period with no treatment.
Results
Out of 143 patients meeting the inclusion criteria, 44 were treated with PT, 98 with XRT and one with mixed PT + XRT. The patients receiving PT were younger, had a lower tumor grade, more oligodendrogliomas and received a lower mean brain and brainstem dose. PsP was observed in 21 out of 126 patients, with no difference between XRT and PT (p = .38). The rate of fatigue in immediate connection to RT (zero to three months after) was higher for XRT than for PT (p = .016). The PT patients had a significantly better PFS and OS than the XRT patients (p = .025 and .035), but in multivariate analysis radiation modality was non-significant. Higher average dose to both brain and brainstem was associated with inferior PFS and OS (p < .001). Median follow-up time were 69 months and 26 months for XRT and PT patients, respectively.
Conclusion
Contrary to previous studies, there was no difference in risk of PsP for XRT and PT. PT was associated with lower rates of fatigue <3 months after RT. The superior survival outcomes for PT indicates that the patients with the best prognosis were referred to PT.
Introduction
Modern radiation therapy (RT) of brain tumors can be given either as proton therapy (PT) or as conventional x-ray therapy (XRT). The use of intensity modulated proton therapy has been used for pediatric patients with brain tumors since the early 2000s [Citation1]. In the setting of RT, a beneficial characteristic of a proton beam is that it deposits most of its energy just before the protons come to a halt, resulting in a, so called, Bragg peak [Citation2] and meaning that PT can be tailored to target a tumor at a very precise depth, keeping the exit dose to a minimum. In practice, this means that for PT the radiation can be limited to a smaller volume, which may lead to less damage to healthy brain tissue [Citation3].
The majority of lower-grade gliomas (LGG) are diffuse gliomas WHO grade 2–3, i.e., less aggressive, diffusely infiltrating primary brain tumors (astrocytomas and oligodendrogliomas). A minor part of the patients have circumscribed gliomas (e.g., pleomorphic xantoastrocytomas and gangliogliomas) with less infiltrative nature.
When treating LGG, it is particularly important to minimize long term side effects, due to the long expected overall survival (OS). To avoid late toxicity, the EANO [Citation4] and Swedish national guidelines[Citation5] recommend that PT might be considered either if the tumor is close to organs at risk (OAR), or if the prognosis of the patient is deemed favorable, i.e., gliomas grade 1–2 and grade 3 with favorable prognostic factors (IDH-mutation and 1p/19q codeletion. Additionally, two studies have shown acceptable acute and late toxicity for PT [Citation6,Citation7], and a single arm prospective trial of PT for LGG found no decrease in cognitive function over time [Citation8]. In agreement with these clinical studies, a considerable amount of treatment planning simulation studies suggest dosimetric advantages for brain cancer patients treated with PT, compared to standard XRT [Citation9–11]. Only one clinical study comparing side effects of PT and XRT of glioblastomas in adults, have been conducted [Citation12], showing reduced toxicity and fatigue after PT, compared to XRT [Citation13]. Two ongoing randomized studies for grade 2–3 gliomas are ongoing [Citation14,Citation15].
With increasing use of PT, concerns have been raised regarding the frequency of pseudoprogression (PsP) compared to XRT. A meta-analysis of the few studies performed found no difference in pediatric LGG, but a significant heterogeneity between PT and XRT for adult LGG [Citation16]. An additional problem is that there are no standardized diagnostic criteria for PsP in LGG [Citation16], contrary to high grade glioma (HGG), where PsP is well studied [Citation17] and there are RANO criteria for the definition of PsP [Citation18]. Differentiation of PsP and true progression with indication for salvage treatment is a common and important clinical problem.
The overall purpose of this study was to describe characteristics and report outcomes for all patients treated primarily for lower grade glioma at Skåne University Hospital during the period when PT was introduced at the Department of Oncology. More specifically, the study aimed at retrospectively investigating what characterized the patients that were selected for PT, whether PsP is more common in that group, manifestation of acute and late side effects, progression-free survival (PFS) and OS in the group selected for PT, compared to the entire cohort.
Methods
Selection and description of participants
In this retrospective cohort study all adult patients with grade 2–3 gliomas that were planned for radiotherapy at the Department of Oncology at Skåne University Hospital from May 2012 to December 2019 were included. The patients were scanned and delineated at Skåne University Hospital, and subsequently received either PT at the Skandion Clinic, Uppsala during 2016–2019 or XRT at Skåne University Hospital during 2012–2019. The patients were followed up until March 31, 2021. In this study, we included patients several years before the introduction of proton therapy. In this way, patients with favorable clinical prognostic factors who were treated using both XRT and PT were studied and included in the multivariate analysis. Thus, we hope to reveal any differences related to the therapy rather than intrinsic bias in the selection of PT patients. We followed the EQUATOR guidelines for reporting, using the STROBE checklist for observational studies (checklist included in the Supplementary File) [Citation16]. The study was approved by the Swedish Ethical Review Authority (2020-04164).
Clinical data collection
Patients were found using treatment planning and record and verify software (Eclipse/Aria, Varian Medical Systems, Palo Alto, US). Follow-up images were retrieved from the local picture archiving and communication system (PACS). Medical records were scanned for demographics, tumor histology, prior surgery and chemotherapy, clinical condition, side effects, PFS, and OS. The WHO 2016 classification was used [Citation17] and for patients diagnosed earlier than 2016, the tumors were reclassified based on the pathology reports, accordingly. We did not reclassify all tumors per the WHO 2021 criteria [Citation18], since the necessary biomarkers were missing for several of the early patients. We classified ‘fatigue’ as the highest degree of either physical or mental fatigue. Side effects were scanned for retrospectively according to CTCAE v5.0, from the end of RT to progression/lost follow up. Extent of resection was estimated from the postoperative magnetic resonance imaging MRI, acquired within 48 h from surgery, and the following multidisciplinary conference, where gross total resection was defined as no evidence of tumor on the MRI, confirmed by a neuroradiologist at a multidisciplinary conference. Tumor location could be in several lobes. Insula and other deep structures were categorized as central tumor locations.
Radiation therapy
Starting in 2016, patients were considered for PT if they either had a grade 2 tumor or a oligodendroglioma or pleomorphic xantoastrocytoma grade 3. IDH-mutated anaplastic astrocytomas were in exceptional cases considered for PT, mainly younger patients or tumors with only minor parts with grade 3 characteristics. For patients referred for PT, comparative treatment plans were created, see , and were used to select the treatment of choice. The comparison took the following aspects into consideration for selecting treatment modality: target coverage, dose to OAR, as well as the dose to the entire brain volume minus the PTV. The proton and photon plans were discussed at a national multidisciplinary conference where representatives from all university hospitals participated, and where the decision was made. There were no exact criteria for choosing PT. Throughout the study the following definitions concerning the tumor volume are used. Gross tumor volume (GTV) is the actual tumor volume, as observed on MRI. In a postoperative setting, this equals the sum of the surgical cavity and residual tumor volumes. In grade 2 tumors, GTV is defined on FLAIR and in grade 3 tumors on T1 with contrast. Furthermore, the clinical target volume (CTV) is defined as the GTV plus a margin of 1.0–2.0 cm depending on tumor grade, not including natural barriers and the planning target volume (PTV) is the CTV plus a 3 mm margin. PT robust optimization was utilized, with range uncertainties of 3 mm/3.5%. For 51 of the XRT treatments, one or two Volumetric Modulated Arc Therapy (VMAT) co- or non-coplanar arcs with 6 MV was used. For 47 XRT patients, Helical Tomotherapy (Accuray Inc, Sunnyvale, CA, USA) with 1 cm or 2.5 cm field with 6 MV was used. For PT, two to three beams were used. Beam configuration was selected to avoid OAR, metal material such as surgical clips, but also to avoid spots from all beams accumulating dose in the same volume of a sensitive OAR, hence considering the possible Relative Biological Effectiveness (RBE) variation at the end of the Bragg peak, not to overdose in sensitive OARs. RBE for PT was set to 1.1. A range shifter was used in cases where the target volume was close to the patient outline. Energies that can be used ranges from 60 MeV to 230 MeV. Single Field Uniform Dose was preferred but Multifield Optimization was used when needed. Mainly 0.5 cm spotspacing was used.
Figure 1. Comparison between PT (left) and VMAT (right) for a patient with glioma. The patient was selected for PT based on lower doses to OARs, and a lower dose to the parts of the brain not including the PTV (brain-PTV); PT 6.7 Gy RBE vs VMAT 15.2 Gy.
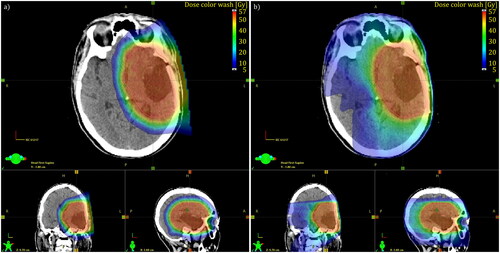
The constraints and objectives used are specified in the Swedish national guidelines [Citation5], which are based on the ICPU reports [Citation19]. Chiasm/optic nerve has the highest priority, followed by brainstem, GTV, PTV, retina, lenses, cochlea, pituitary, lacrimal gland and hippocampus in written order. Treatment planning and dosimetry data were extracted from the treatment planning software using the scripting API (Eclipse).
Follow up
Standard follow up practice for both therapy modalities included MRI scans every three months during the first year after therapy, and subsequently every three months for grade 3 tumors and every six months for grade 2 tumors, if stable. This study includes examination of all subsequent post-RT MRÍs until progression. An experienced neuroradiologist (LS) was involved in the examination to assess whether progression or PsP had occurred. Progression was defined by local standard, i.e., deemed present if one or more of the following criteria were met: findings resulting in new oncologic therapy, tissue diagnosis following surgery, worsened clinical symptoms and/or death. PsP was defined as new or enlarged lesions with abnormal contrast enhancement in the radiation field on post-RT MRIs mimicking tumor progression, which either decreased or stabilized for a period of 12 months without new oncologic therapy or was confirmed with biopsy. PsP was not only considered within a certain time frame following radiotherapy. Date of PsP and progression were backdated to the first date at which a lesion met the criteria for respective condition. The time to disease-related outcomes was calculated from the end date of RT. Patients without evidence of progression were censored from PFS analysis at date of last follow-up. The date of death was collected from the Swedish Cause of Death Register, using the record and verify software (Aria).
Statistics
The statistical analyses were performed with SPSS software (version 28, IBM, US) and Matlab (R2020b). All clinical data were descriptively summarized and compared by a non-parametric test and the pretreatment variables were analyzed with a spearman correlation test. PFS and OS were estimated using the Kaplan–Meier method. Cox regression analysis was used to investigate the association of different variables with outcome metrics. Fatigue over time was analyzed with the Wilcoxon rank sum test at each time-point, comparing the distribution over dichotomized data for PT and XRT, respectively. Missing data were censored. All the confidence intervals were set to 95%, pvalue cutoff for significance was set to <.05 and all tests were two-sided in this explorative, retrospective study.
Results
A total of 143 patients, treated during 2012–2019, were retrospectively identified, out of which 44 were treated with PT (2016–2019), and 98 were treated with XRT (2012–2019). One patient received PT and XRT consecutively, and data about that patient was missing. The most common diagnosis was astrocytoma followed by oligodendroglioma, pleomorphic xantoastrocytoma, oligoastrocytoma, and ganglioglioma. Patient characteristics are shown in . Three patients (1 PT, 2 XRT) were discontinued after 5.4 Gy, 18 Gy, and 46.8 Gy of radiation due to lowering of consciousness, imminent herniation followed by acute surgery, and pleuropneumonia in need of draining, respectively. All 142 patients were, however, included in pretreatment analyses (dosimetry and treatment planning data). The patients receiving 5.4, respectively, 18 Gy of radiation were excluded in analyses of outcomes (PsP, side effects, PFS and OS) but the patient receiving 46.8 Gy of radiation was included, since most of the treatment was given. Seven patients were lost to follow-up, and side-effect data were not available for another five patients receiving XRT.
Table 1. Patient characteristics.
At baseline the patients receiving PT were younger (p < .001), more often women (p = .023), had a lower tumor grade (p = .006), more IDH mutations (p = .019) and more commonly oligodendrogliomas (p = .008). They received less concomitant (p < .001) and more maintenance (p = .033) chemotherapy and received a lower mean brain (p < .001) and brainstem dose (p < .001). Patients with cognitive symptoms at diagnosis received XRT to a greater extent. The median prescription dose for XRT and PT was 59.4 Gy and 54.0 Gy, respectively. The XRT patients had an average follow-up time of 69 months (95% CI 62–77 months) and the PT patients 26 months (95% CI 20–31 months), estimated using the Kaplan–Meier method. See supplementary figure 1.
Patients with astrocytomas were more frequently men (Spearman’s ρ=−0.18, p = .041) and had centrally located tumors (ρ=−0.18, p = .038). 1p/19q codeletion correlated with a better ECOG performance status (ρ=−0.26, p = .023). IDH mutation was more common in younger patients (ρ=−0.24, p = .032) and was negatively correlated with both focal (ρ=−0.23, p = .043) and cognitive symptoms (ρ=−0.24, p = .032) at diagnosis. Worse EGOC performance status was more frequently observed in patients with centrally located tumors (ρ = 0.20, p = .017).
PsP was observed in 21 of 126 patients, out of which 17 patients were treated with XRT and 4 with PT. PsP occurred at a median of 4.5 months from the last day of RT (1.6–71 months). No significant difference in rate of PsP between XRT and PT was found using the Kaplan–Meier estimate (log rank, p = .38). Additional Cox regression analyses revealed no association between mean dose to the brain or GTV with PsP, see .
Table 2. Univariate Cox-models for pseudoprogression.
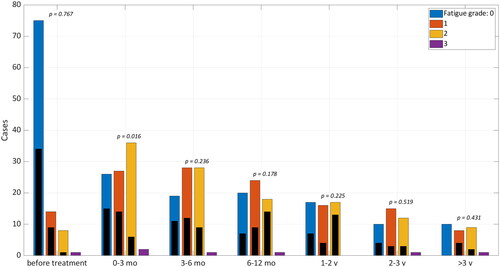
The association between fatigue and other variables is reported in Supplementary Table 1 and the fatigue status is shown in , where we found that the rate of fatigue as an acute side effect (zero to three months after RT) was greater after XRT than after PT (Wilcoxon, p = .016), but thereafter no difference was found. No difference was seen between PT and XRT for memory impairment after RT. A total of 12 patients experienced alopecia grade 2 at three months after RT (7 PT and 5 XRT). One XRT patient developed cognitive disturbance grade 2 after RT, one PT patient got tinnitus grade 1 and one PT patient got decreased range of motion in left temporomandibular joint grade 1.
Figure 2. Actuarial rate of fatigue, graded according to CTCAE for patients treated with photons (color) versus protons (black), before and after treatment. The groups are compared with Wilcoxon rank sum test.
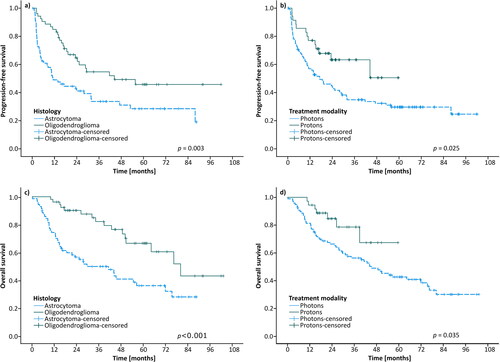
Recurrence occurred in 79 patients and 64 patients died during follow up. The median PFS was 24 months (95% CI 15–33) in the whole cohort and 17 months (95% CI 8–27) for XRT. Median PFS for PT had not been reached, though PFS was significantly longer for PT than for XRT (log-rank p = .025), see . Patients with astrocytoma had a shorter PFS than those with oligodendroglioma (11 months (95% CI 4–19) and 44 months (95% CI could not be counted) respectively (p = .003)). OS in the whole cohort was 51 months (95% CI 33–69 months), 45 months (95% CI 31–59 months) for XRT patients, while median OS was not yet reached for PT (p = .035). OS was 41 months (95% CI 22–61 months) and 80 months (95% CI 58–102 months, log rank p < .001) for astrocytoma and oligodendroglioma, respectively. The Cox regression analyses are summarized in Supplementary Table 2. In multivariate analysis, radiation modality was non-significant.
Figure 3. Kaplan–Meier curves of progression-free survival (PFS) and overall survival (OS). (a) PFS for patients with astrocytoma vs oligodendroglioma; (b) PFS for patients treated with photons vs protons; (c) OS for patients with astrocytoma vs oligodendroglioma; (d) OS for patients treated with photons vs protons.
Discussion
In this paper we report on our experience of transitioning from conventional XRT to PT for primary gliomas. We found that the patients receiving PT compared to XRT were younger, had a lower tumor grade, more oligodendrogliomas and more IDH mutations, all of which are good prognostic factors [Citation20,Citation21]. This is consistent with the Swedish national guidelines [Citation5] and hence as could be expected. Furthermore, they received less concomitant and more maintenance chemotherapy in accordance with former local guidelines with different treatment strategies due to histology. The mean brain and brainstem doses were lower for PT than XRT, due to lower prescription dose and the inherently improved sparing offered by the low exit dose in PT. Curiously, more women than men received PT (Χ2, p = .023), even though gliomas are more common in men [Citation22,Citation23]. In our cohort, more women had oligodendrogliomas, which could be a part of the explanation.
We found no association between PT and PsP. Previous trials have shown conflicting results for relationship between PsP and PT [Citation24–26], higher radiation dose[Citation26] and concomitant temozolomide [Citation25,Citation27]. In our study, no correlation was seen between chemotherapy and PsP. We found no significant prognostic factors for PsP, consistent with some studies [Citation24,Citation26] and there appears to be no consensus on this question. As a recent meta-analysis shows, different definitions of PsP in the various studies give difficulties in clinical interpretation of data and outcomes [Citation28], only two trials studied PsP after PT and one of those showed higher incidence. We chose no time limit in our analysis since PsP seems to appear later in LGG than HGG [Citation28]. Increasing T2/FLAIR changes were not considered an indication of PsP in HGG nor in our study [Citation29]. It would be desirable to find a common definition of PsP in order to be able to more reliably compare different studies, and even better prospective studies to further understand this phenomenon.
Fatigue is a considerable problem for patients with glioma, affecting quality of life, with a prevalence of 20–77%[Citation30], occurring before, during and after treatment [Citation31,Citation32]. In our study, acute fatigue (zero to three months after RT) occurred more frequently after XRT than after PT, but no difference was observed for long term fatigue. Since radiotherapy-induced fatigue declines over time [Citation33,Citation34], the impact of treatment modality may mostly affect the rate of fatigue early after treatment. The difference in onset of fatigue is probably multifactorial, but the radiation dose to the brain is known to affect the rate of fatigue [Citation35] and the PT patients did receive a lower radiation dose to the brain and brainstem. The radiation modality could be part of the explanation, due to dosimetric advantage of PT [Citation13]. The XRT group had worse PFS and OS, which is indeed associated with a higher degree of fatigue [Citation36,Citation37]. In addition, the patients receiving XRT had more concomitant and less maintenance chemotherapy, which could also affect the onset of fatigue [Citation34,Citation38]. In our study, worse ECOG performance status and fatigue before RT were the only factors that predicted fatigue after RT, which is consistent with other studies [Citation36,Citation39].
In our cohort, only age, ECOG performance status and histology were prognostic factors for both PFS and OS in multivariate analyses, consistent with earlier studies [Citation21,Citation40–45]. We found tumor located in the brainstem and multifocal tumor to be negative prognostic factors. Tumor location close to eloquent structures makes a gross total resection challenging which might partly explain the observed inferior outcome [Citation42].
In multivariate analyses, mean brain dose was found to be a significant predictor for PFS, while brainstem location and mean radiation brainstem dose had a significant correlation with OS, a correlation also found previously for glioblastoma [Citation46]. We did find that the PT patients had a better PFS and OS, as expected since patients with better prognostic factors should be referred to PT. However, in multivariate analyses, radiation modality was no significant prognostic factor. IDH mutation was strongly associated with a better PFS and OS but was not included in multivariate analysis due to missing data in several cases, and today, the IDH wild type-tumors would have been diagnosed as glioblastomas, according to the WHO 2021 classification. Our study cannot answer the question whether radiation modality affects survival. There is an ongoing prospective Nordic study [Citation15] where LGG patients are randomized to either photon or proton radiation therapy, that hopefully will help to answer the question whether treatment modality do affect survival.
The retrospective nature of this study is a major limitation, e.g., since tumors were reclassified according to the WHO 2016 guidelines if the diagnosis was made before its release. We have chosen not to reclassify the tumors according to the WHO 2021 classification [Citation18] mainly because molecular data is missing in many cases, and thus IDH wild type tumors are included in the study. Side effects were graded retrospectively from the medical records, probably leading to under-reporting. Also, PsP was graded retrospectively, which means that if a patient was assessed as having a true progression and thus starts salvage treatment, it is inherently impossible to find out whether the lesion would have stabilized into a PsP. Follow up was missing in a noticeable number of the PT patients which might introduce bias. Note that the prevalence of risk factors in the XRT cohort may change during the study period due to access of PT during the latter part of the period. In addition, the PT and XRT groups differed widely and comparisons between the groups should be made with caution.
In summary, we found significant lower rates of fatigue up to three months following therapy for patients treated with PT than for patients treated with XRT. The difference in fatigue appears to be associated with the performance status before therapy and/or potentially due to better dosimetry for proton patients. The difference in fatigue was not persistent over time.,
A noteworthy result in this study is that no significant difference in PsP was found between the radiation modalities. This result contrasts with previous studies and indicates that further investigation is required.
The superior PFS and OS outcomes for PT indicates that the patients with best prognosis were selected for PT. In multivariate analysis, we found that older patients, astrocytomas (vs oligodendrogliomas) and poor performance status were associated with inferior outcomes. Increased average dose to the brain was associated with inferior progression-free survival but increased mean brainstem dose with inferior overall survival.
Supplemental Material
Download MS Word (25.4 KB)Supplemental Material
Download MS Word (33.4 KB)Supplemental Material
Download MS Word (62.7 KB)Acknowledgments
We thank Hannes Mogensen for assisting radiotherapy data collection.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are available from the corresponding author, PM, upon reasonable request.
References
- Lomax AJ, Böhringer T, Bolsi A, et al. Treatment planning and verification of proton therapy using spot scanning: initial experiences. Med Phys. 2004;31(11):3150–3157.
- Newhauser WD, Zhang R. The physics of proton therapy. Phys Med Biol. 2015;60(8):R155–R209.
- Schulz-Ertner D, Tsujii H. Particle radiation therapy using proton and heavier ion beams. J Clin Oncol. 2007;25(8):953–964.
- Weller M, van den Bent M, Preusser M, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 2021;18(3):170–186.
- Swedish national guidelines tumors in brain and spinal cord; 2020 [accessed 2023 Jan 26]. Available from: https://kunskapsbanken.cancercentrum.se/diagnoser/hjarna/vardprogram/
- Muroi A, Mizumoto M, Ishikawa E, et al. Proton therapy for newly diagnosed pediatric diffuse intrinsic pontine glioma. Childs Nerv Syst. 2020;36(3):507–512.
- Tabrizi S, Yeap BY, Sherman JC, et al. Long-term outcomes and late adverse effects of a prospective study on proton radiotherapy for patients with low-grade glioma. Radiother Oncol. 2019;137:95–101.
- Shih HA, Sherman JC, Nachtigall LB, et al. Proton therapy for low‐grade gliomas: results from a prospective trial. Cancer. 2015;121(10):1712–1719.
- Boehling NS, Grosshans DR, Bluett JB, et al. Dosimetric comparison of three-dimensional conformal proton radiotherapy, intensity-modulated proton therapy, and intensity-modulated radiotherapy for treatment of pediatric craniopharyngiomas. Int J Radiat Oncol Biol Phys. 2012;82(2):643–652.
- Harrabi SB, Bougatf N, Mohr A, et al. Dosimetric advantages of proton therapy over conventional radiotherapy with photons in young patients and adults with low-grade glioma. Strahlenther Onkol. 2016;192(11):759–769.
- Dennis ER, Bussière MR, Niemierko A, et al. A comparison of critical structure dose and toxicity risks in patients with low grade gliomas treated with IMRT versus proton radiation therapy. Technol Cancer Res Treat. 2013;12(1):1–9.
- Chambrelant I, Eber J, Antoni D, et al. Proton therapy and gliomas: a systematic review. Radiation. 2021;1(3):218–233.
- Brown PD, Chung C, Liu DD, et al. A prospective phase II randomized trial of proton radiotherapy vs. intensity modulated radiotherapy for patients with newly diagnosed glioblastoma. Neuro-oncology. 2021;23(8):1337–1347.
- Oncology N. NRG-BN005: a phase ii randomized trial of proton vs. photon therapy (IMRT) for cognitive preservation in patients with IDH mutant, low to intermediate grade gliomas; 2022 [accessed 2022 Apr 04]. Available from: clinicalTrials.gov Identifier: NCT03180502
- PRO-GLIO: PROton versus photon therapy in IDH-mutated diffuse grade II and III GLIOmas; 2022. Available from: clinicalTrials.gov Identifier: NCT05190172
- Simera I, Moher D, Hoey J, et al. A catalogue of reporting guidelines for health research. Eur J Clin Invest. 2010;40(1):35–53.
- Louis DN, Perry A, Reifenberger G, et al. The 2016 world health organization classification of tumors of the Central nervous system: a summary. Acta Neuropathol. 2016;131(6):803–820.
- Louis DN, Perry A, Wesseling P, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23(8):1231–1251.
- International Commission on Radiation Units and Measurements; [cited 2023 Jan 26]. Available from: https://www.icru.org/reports/
- Sun H, Yin L, Li S, et al. Prognostic significance of IDH mutation in adult low-grade gliomas: a meta-analysis. J Neurooncol. 2013;113(2):277–284.
- Pignatti F, Van Den Bent M, Curran D, et al. Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol. 2002;20(8):2076–2084.
- Regionalt cancercentrum Norr. Nationellt kvalitetsregister för Hjärntumörer - Nationell rapport 1999-2017 (National Register of Brain tumors - National report 1999-2017). Regionala cancercentrum i samverkan; 2018.
- Ostrom QT, Cote DJ, Ascha M, et al. Adult glioma incidence and survival by race or ethnicity in the United States from 2000 to 2014. JAMA Oncol. 2018;4(9):1254–1262.
- Bronk JK, Guha-Thakurta N, Allen PK, et al. Analysis of pseudoprogression after proton or photon therapy of 99 patients with low grade and anaplastic glioma. Clin Transl Radiat Oncol. 2018;9:30–34.
- Dworkin M, Mehan W, Niemierko A, et al. Increase of pseudoprogression and other treatment related effects in low-grade glioma patients treated with proton radiation and temozolomide. J Neurooncol. 2019;142(1):69–77.
- van West SE, de Bruin HG, van de Langerijt B, et al. Incidence of pseudoprogression in low-grade gliomas treated with radiotherapy. Neuro-oncology. 2017;19(5):719–725.
- Gerstner ER, McNamara MB, Norden AD, et al. Effect of adding temozolomide to radiation therapy on the incidence of pseudo-progression. J Neurooncol. 2009;94(1):97–101.
- Lu VM, Welby JP, Laack NN, et al. Pseudoprogression after radiation therapies for low grade glioma in children and adults: a systematic review and meta-analysis. Radiother Oncol. 2020;142:36–42.
- Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–1972.
- van Coevorden-van Loon EM, Coomans MB, Heijenbrok-Kal MH, et al. Fatigue in patients with low grade glioma: systematic evaluation of assessment and prevalence. J Neurooncol. 2017;133(2):237–246.
- Schei S, Solheim O, Jakola AS, et al. Perioperative fatigue in patients with diffuse glioma. J Neurooncol. 2020;147(1):97–107.
- Struik K, Klein M, Heimans JJ, et al. Fatigue in low-grade glioma. J Neurooncol. 2009;92(1):73–78.
- Smets E, Visser M, Willems-Groot A, et al. Fatigue and radiotherapy:(A) experience in patients undergoing treatment. Br J Cancer. 1998;78(7):899–906.
- Schwartz A, Nail L, Chen R, et al. Fatique patterns observed in patients receiving chemotherapy and radiotherapy. Cancer Investig. 2000;18(1):11–19.
- Kiebert G, Curran D, Aaronson N, et al. Quality of life after radiation therapy of cerebral low-grade gliomas of the adult: results of a randomised phase III trial on dose response (EORTC trial 22844). Eur J Cancer. 1998;34(12):1902–1909.
- Brown PD, Ballman KV, Rummans TA, et al. Prospective study of quality of life in adults with newly diagnosed high-grade gliomas. J Neurooncol. 2006;76(3):283–291.
- Peters KB, West MJ, Hornsby WE, et al. Impact of health-related quality of life and fatigue on survival of recurrent high-grade glioma patients. J Neurooncol. 2014;120(3):499–506.
- Stasi R, Abriani L, Beccaglia P, et al. Cancer‐related fatigue: evolving concepts in evaluation and treatment. Cancer. 2003;98(9):1786–1801.
- Armstrong TS, Cron SG, Bolanos EV, et al. Risk factors for fatigue severity in primary brain tumor patients. Cancer. 2010;116(11):2707–2715.
- Daniels TB, Brown PD, Felten SJ, et al. Validation of EORTC prognostic factors for adults with low-grade glioma: a report using intergroup 86-72-51. Int J Radiat Oncol Biol Phys. 2011;81(1):218–224.
- Capelle L, Fontaine D, Mandonnet E, et al. Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases. J Neurosurg. 2013;118(6):1157–1168.
- Soffietti R, Baumert BG, Bello L, et al. Guidelines on management of low-grade gliomas: report of an EFNS-EANO Task Force. Eur J Neurol. 2010;17(9):1124–1133.
- Gittleman H, Sloan AE, Barnholtz-Sloan JS. An independently validated survival nomogram for lower-grade glioma. Neuro-oncology. 2020;22(5):665–674.
- Kaloshi G, Psimaras D, Mokhtari K, et al. Supratentorial low-grade gliomas in older patients. Neurology. 2009;73(24):2093–2098.
- Gorlia T, Wu W, Wang M, et al. New validated prognostic models and prognostic calculators in patients with low-grade gliomas diagnosed by Central pathology review: a pooled analysis of EORTC/RTOG/NCCTG phase III clinical trials. Neuro Oncol. 2013;15(11):1568–1579.
- af Rosenschold PM, Law I, Engelholm S, et al. Influence of volumetric modulated arc therapy and FET-PET scanning on treatment outcomes for glioblastoma patients. Radiother Oncol. 2019;130:149–155.
