Abstract
Purpose
To assess the long-term risks of infectious and thromboembolic events following inguinal (ILND) and pelvic (PLND) lymph node dissection in men with penile cancer.
Material and methods
A total of 364 men subjected to ILND with or without PLND for penile cancer between 2000 and 2012 were identified in the Swedish National Penile Cancer Register. Each patient was matched based on age and county of residence with six penile cancer-free men. The Swedish Cancer Register and other population-based registers were used to retrieve information on treatment and hospitalisation for selected infectious and thromboembolic events. Hazard ratios (HRs) with 95% confidence intervals (CIs) were estimated using Cox proportional hazard models with multiple imputation.
Results
The risk of infectious events remained increased for more than five years postoperatively in men with penile cancer compared with matched controls. The palpable nodal disease was the only predictor of these events, with risk increasing with the cN stage. The HR at one, three and five years and six months postoperatively was 8.60 (95% CI 5.16–14.34), 4.02 (95% CI 2.65–6.09) and 1.93 (95% CI 1.11–3.38), respectively. An increased risk of thromboembolic events persisted for three years postoperatively. The HR at one and three years postoperatively was 13.51 (95% CI 6.53–27.93) and 2.12 (95% CI 1.07–4.20). The results correspond well with the over-prescription of anticoagulants observed during this period. An association with bulky disease (cN3) was observed.
Conclusions
Lymph node dissection for penile cancer is associated with an increased risk of infectious and thromboembolic events. The findings of this population-based study show that the risks of these events remain increased more than five years for infectious and three years for thromboembolic events. Improved awareness of long-term complications following ILND is of importance both among patients and care givers to ensure early detection and treatment.
Introduction
Penile cancer (PeCa) is an uncommon neoplasm of men’s urinary tract, accounting for less than 1% of malignancies in men. The annual incidence in Sweden is approximately 2.1 per 100.000 with a mean age at diagnosis of 70 years [Citation1]. Most men are diagnosed with localised disease; however, approximately 14% of men will present with clinically suspicious inguinal lymph nodes (cN+) [Citation2]. Furthermore, about 20% of patients with clinically negative inguinal lymph nodes (cN0) harbour regional metastases [Citation2,Citation3]. Traditionally, most men have been referred to diagnostic inguinal lymph node dissection (ILND) [Citation4], a procedure associated with morbidity rates of 35–70% [Citation5–7]. Hence, a major challenge is to correctly select men for lymph node dissection. If all patients undergo diagnostic ILND, approximately 80% will be overtreated, with a risk of severe adverse effects on quality of life and without any survival benefits [Citation8,Citation9]. However, in men with lymph node metastases, delayed surgery is associated with worse outcome [Citation10,Citation11]. The introduction of dynamic sentinel node biopsy (DSNB) for lymph node staging represented a major step forward in correctly selecting men who benefit from lymph node dissection and avoid unnecessary untoward consequences [Citation3].
Therapeutic ILND is performed when palpable inguinal lymph nodes (cN+) are present or metastatic disease is confirmed by fine needle aspiration cytology (FNAC) or by DSNB. In the case of two or more positive inguinal lymph nodes or extranodal extension, ipsilateral pelvic lymph node dissection (PLND) is recommended [Citation12].
Several previous studies have assessed morbidity after ILND, with the majority focussing on early postoperative complications and most commonly report wound infection and dehiscence, seroma formation, haematoma, lymphocele, lymphedema of the leg and genitalia, skin-flap necrosis and thromboembolic complications [Citation6,Citation7,Citation13–17]. Less is known about delayed-onset complications following lymph node dissection in men with PeCa, apart from lymphedema being the most prevalent because of diminished lymphatic drainage from the lower extremities and genitalia [Citation13,Citation18]. Although all surgical procedures carry a risk for postoperative wound infections, abscess formations, or even sepsis [Citation5–7], patients with lymphedema of the leg and genitalia are particularly susceptible to late and recurrent skin and soft tissue infections (e.g., erysipelas, cellulitis, lymphangitis, etc.) [Citation18]. In general, patients undergoing surgical procedures, and especially those with underlying malignancies are also at increased risk of thromboembolic events several months after surgery [Citation15,Citation19,Citation20].
The aims of the present study were two-fold. First to assess long-term morbidity by investigating rates of infectious and thromboembolic events following ILND and PLND during a follow-up period up to 12 years. Second to identify clinical and pathological predictors available in the data at hand associated with the risk of these post-surgical complications.
Material and methods
Information in Penile Cancer Data Base Sweden (PenCBaSe), a register-based research database, was used for the purpose of this retrospective population-based study. PenCBaSe was generated by record-linkages between the Swedish National Penile Cancer Register (NPECR), the Swedish Cancer Register (SCR), the National Patient Register (NPR), the Cause of Death Register, the Swedish Prescribed Drug Register, the Longitudinal Integrated Database for Health Insurance and Labour market studies (LISA) and the Register of the Total Population.
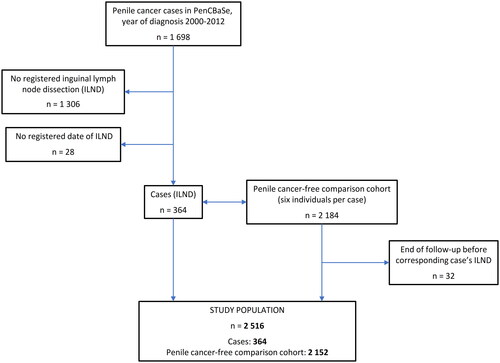
PenCBaSe includes data for the period between 2000 and 2012 and comprises 1698 men with penile cancer. Each man with PeCa was matched based on age and county of residence with six penile cancer-free men randomly selected in the Population Register, hereafter referred to as controls. Controls then constitute a limited representative comparison cohort with the possibility to retrieve the same individual-level information from the other registers as for the men with PeCa. The formation of PenCBaSe by record linkages between Swedish registers was possible by the use of the individually unique personal identity number assigned to all permanent residents in Sweden [Citation21]. All men registered in the NPECR who underwent ILND with or without PLND for PeCa during this period were included in the study. Cancer-specific variables, such as tumour grade, stage of the tumour and lymph nodes as well as demographic variables including a year of surgery, age at diagnosis, and county of residence were obtained. A more detailed description of PenCBaSe has been published previously [Citation2,Citation22].
The National Penile Cancer Register includes 99% of all men in Sweden diagnosed with PeCa compared to the SCR to which reporting is mandatory. The NPECR contains data on age at diagnosis, tumour characteristics according to the TNM classification and treatment [Citation2]. The NPR records information on all in-patient care in Sweden since 1987 including data on hospitalisations and discharge diagnostic codes according to the current International Classification of Diseases, ICD-10. Since 2001 the register also contains hospital outpatient care provided by both private and public caregivers. However, primary care is not covered by the register [Citation23,Citation24]. The Prescribed Drug Register includes data on all drugs prescribed and dispensed in Sweden since July 1st, 2005, except drugs administered during hospitalisations [Citation25].
For both men with PeCa and matched controls, information was obtained from the other population-based registers by means of record linkage. The National Patient Register was used to retrieve information on treatment and hospitalisation for infections of the lower limbs, groins, genitalia, trunk, and various septic conditions as well as thromboembolic events based on ICD-10 diagnostic codes for each event. Information on the prescription of anticoagulants 30 days after surgery was retrieved from the Prescribed Drug Register, assessed by ATC codes (the Anatomical Therapeutic Chemical (ATC) classification system). The prescription was used as a surrogate marker for thromboembolic events during the follow-up period. A complete list of ICD-10 and ATC codes used is presented in Supplement 1.
History of use of selected drugs (antidiabetics, corticosteroids, and immunosuppressants) was assessed by retrieval of ATC codes from the Swedish Prescribed Drug Register () that were used as a surrogate markers for underlying factors potentially contributing to the risk of infections and thromboembolism [Citation26–31]. The overall comorbidity burden was estimated by use of the Charlson Comorbidity Index (CCI) [Citation32] () based on information obtained from the National Patient Register.
Table 1. Baseline demographic characteristics of men diagnosed with penile cancer between 2000 and 2012 and matched penile cancer-free men in comparison cohort.
The STROBE cohort reporting guidelines and checklist were used when writing the manuscript [Citation33]. The study was approved by the Regional Ethics Committee in Uppsala (No.2012/021).
Statistical analyses
The null hypothesis of no difference in the distribution of characteristics between patients with PeCa and penile cancer-free comparison cohort was tested using a chi-squared test for categorical variables and the Mann–Whitney U-test for continuous variables.
Follow-up time was calculated as the time from ILND to the event of interest, death, emigration or December 31st, 2012, whichever came first. The net probability of an outcome was estimated using the Kaplan-Meier method by treating all other events than the outcome of interest as censoring events. The net hazard rate was estimated using a flexible parametric model [Citation34].
Univariate and multivariate Cox proportional hazard models were used to assess the effects of age, period, comorbidity, marital status, education, cT and cN stage, grade, and type of lymph node dissection (ILND ± PLND) on the net probability of the outcomes of interest among the PeCa patients. Results are presented as hazard ratios (HR) with 95% confidence intervals (CI).
Multiple imputation was used to handle missing data on the variables included in the models by use of the Multivariate Imputation by Chained Equations (MICE) algorithm [Citation35]. Data was imputed forty times using all variables as predictors in the MICE algorithm. Separate models were fitted to the dataset in each iteration and the results were then pooled using Rubin’s rules [Citation36].
In all analyses, a p-value <.05 was considered statistically significant. All analyses were performed using R [Citation37].
Results
In total, 392 men underwent lymph node dissection for PeCa between 2000 and 2012. Following the exclusion of 28 men with no registered date of the operation, 364 men were included in the final analysis (). Unilateral ILND was performed in 68 (18.7%) men and bilateral in 293 (80.5%) men. Information on the type of procedure was missing for 3 (0.8%) men. A total number of 657 ILNDs were performed in 364 men. PLND was performed in 108 (29.7%) men with unilateral and bilateral dissection accounting for 25% (n = 27) and 68.5% (n = 74), respectively. The type of PLND was not registered for seven men (). The median follow-up in men with PeCa and controls was 6.2 (IQR 3.2–9.1) and 6.2 (IQR 3.3–9.1) years, respectively. The longest registered follow-up exceeded 12 years.
Table 2. Tumour characteristics and the type of surgery performed in men with penile cancer between 2000 and 2012.
The chi-squared test was used to assess differences between men with and without PeCa. There was no difference in the distribution of CCI (p = .120) or use of antidiabetics (p = .645), corticosteroids (p = 1.000) and immunosuppressants (p = .531) prior to surgery. Educational level and marital status, indicators of socioeconomic status, were evenly distributed between men with and without PeCa and showed no association with increased risk of either infectious or thromboembolic events (Supplement 3). Detailed demographic characteristics of the study population are presented in .
Compared with their matched controls, the risk of infectious events remained increased up to 66 months postoperatively in men with PeCa who underwent ILND. The HR at 12, 36, and 66 months postoperatively was 8.60 (95% CI 5.16–14.34), 4.02 (95% CI 2.65–6.09), and 1.93 (95% CI 1.11–3.38), respectively (, Supplement 2). The palpable nodal disease was the only predictor of an increased risk of subsequent infectious events. The risk tended to increase with higher cN stage, with an HR of 1.64 (95% CI 0.99–2.74) for cN1, 1.92 (95% CI 1.13–3.27) for cN2 and 2.58 (95% CI 1.38–4.84) for cN3 disease following adjustment for age, period, comorbidity, marital status, education, cT and cN stage, grade and type of lymph node dissection (ILND ± PLND) in multivariable analyses (Supplement 3).
Figure 2. Net probability of infectious and thromboembolic events, and prescription of anticoagulants in men with and without penile cancer 2000–2012.
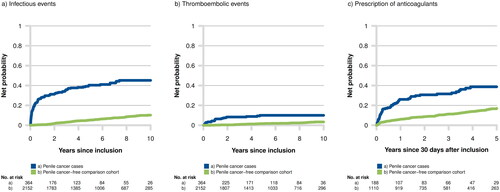
An elevated risk of thromboembolic events persisted for 36 months postoperatively. The HR at 12 and 36 months postoperatively was 13.51 (95% CI 6.53–27.93) and 2.12 (95% CI 1.07–4.20), respectively (, Supplement 2). An association with the bulky disease was observed, with an HR of 3.78 (95% CI 1.09–13.09) for the cN3 stage in the multivariate analysis (Supplement 3).
In patients undergoing ILND, PLND did not add further risk for infectious or thromboembolic events (Supplement 3).
The number of registered infectious and thromboembolic events in men with PeCa and the comparison cohort during the follow-up intervals 0–1, 1–2, 2–5, and more than 5 years, respectively, is presented in Supplement 4. The table contains all events, not only the first per individual, and is related to the person-years in each interval with the corresponding number of events per 1000 person-years.
Discussion
It is well known that men with penile cancer undergoing ILND with or without PLND are at increased risk of a variety of postoperative complications with estimated morbidity rates of up to 70% [Citation6]. The majority of studies to date addressing complications after lymph node dissection have primarily assessed early postoperative complications, classified as any complication experienced within the first 30 days of surgery. If assessed, the late complications are usually defined as all events emerging later than 30 days after surgery; however, the knowledge of long-term complications remains limited.
In the present study, we used a population-based approach to assess long-term morbidity with a focus on long-term risks of infectious and thromboembolic events. By use of information in Swedish nationwide registers of high quality and completeness with essentially complete follow-up, we were able to define a study population including 364 men with PeCa operated with ILND, representing the largest study to date based on the number of patients and ILND procedures included. The data at hand also allowed for consideration of factors that potentially could be associated with increased risk of infections and thromboembolism, e.g., use of antidiabetics, corticosteroids, and immunosuppressants [Citation26–31], and comorbidity by use of the Charlson Comorbidity Index (CCI) [Citation32].
Previous reports on complications following ILND for PeCa show variable results with estimated rates of postoperative complications between 35 and 70% [Citation6,Citation7,Citation13–16]. In line with our results, the highest rate has been observed during the early postoperative period. However, our study revealed a sustained long-term elevated risk for complications. The overall risk of infectious and thromboembolic events one year after ILND was 8 respectively 13 times higher compared to the penile cancer-free control group. This trend was observed up to more than five years postoperatively for infectious events and up to three years postoperatively for thromboembolic events. The results correspond well with the over-prescription of anticoagulants observed during the first postoperative years (, Supplement 2).
A large and detailed study by Gopman et al. reported that 181 (55.4%) out of 327 men with PeCa experienced at least one postoperative complication. Wound infection was the most common, comprising 35.4% of minor and 20.8% of major complications, respectively [Citation6]. Another large series on postoperative complications after ILND by Stuiver et al. observed complications in 58% of the procedures with wound infections occurring in 43% of the men [Citation7].
Patients with underlying malignancies are generally at greater risk of thromboembolism, with the highest risk during the first year after the cancer diagnosis [Citation38]. In concordance with our findings, Blom et al. found that the relative risk seems to decrease considerably two years after diagnosis, but remains elevated compared with cancer-free individuals [Citation39]. The surgery itself represents an independent risk factor for this severe complication with an increased risk remaining at least three months postoperatively [Citation20]. A series of previous studies have reported rates of thromboembolic events between 4 and 14% [Citation14,Citation15,Citation19,Citation40] after ILND for PeCa, albeit only Bouchot et al. and Perdona et al. reported time-to-event data with a median time of 3.6 months and 4.1 months, respectively.
Lymph node dissection is an essential part of the treatment of PeCa with regional metastases that can dramatically improve patient survival [Citation10]. A substantial proportion of the complications is wound related, mainly due to the nature of the open dissection. The nodal stage of the disease is an important factor since a higher nodal stage can increase the complexity, extent, and duration of surgery. Our results indicate that palpable nodal disease is a predictor of an increased risk of infectious and thromboembolic events.
While our study provides information about late complications following open dissection, results from recent studies on video endoscopic (VEIL) and robot-assisted video endoscopic inguinal lymphadenectomy (RAVEIL) show similar oncological outcomes, but lower rates of wound infections and major complications in men without the bulky disease [Citation41–43]. However, larger series with long-term follow-up are needed to make relevant comparisons.
Although our study is population-based, some limitations need mentioning. First, even though modified ILND is associated with less postoperative morbidity compared with radical ILND [Citation17], both procedures are classified under the same intervention code in the Swedish registers. However, modified ILND is only performed as a diagnostic procedure in men with cN0 PeCa and should be complemented with radical ILND if metastases are found. Moreover, with the introduction of DSNB [Citation44], the number of ILND procedures in the diagnostic setting have declined dramatically. Second, while we were able to adjust for multiple factors potentially associated with increased risk of infections and thromboembolism, no information was available on smoking history, BMI, and oncological treatment in the data set at hand.
Conclusions
Lymph node dissection is the gold standard in the treatment of men with penile cancer with regional metastases despite associated risks of post-operative morbidity. By use of population-based data we were able to examine the long-term impact of ILND with or without PLND on the risk of infectious and thromboembolic events which remained elevated up to more than five and three years, respectively. An improved awareness of possible long-term adverse events following lymph node dissection is of importance both among patients and care givers to ensure early detection and treatment to minimise the risk of untoward outcomes. While new minimally invasive techniques appear promising in reducing postoperative complications without compromising oncological outcomes, long-term data are needed before these methods can be widely implemented.
Supplemental Material
Download MS Word (19.2 KB)Supplemental Material
Download MS Word (33.9 KB)Supplemental Material
Download MS Word (11.4 KB)Supplemental Material
Download PNG Image (315.7 KB)Supplemental Material
Download MS Word (20.5 KB)Acknowledgements
The study was made possible by the continuous work of the NPECR steering group.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The data that support the findings of this study are from the NPECR which welcomes register-based research.
References
- Regionala Cancercentrum. Peniscancer–Nationell kvalitetsrapport för 2020. 2020.
- Kirrander P, Sherif A, Friedrich B, et al. Swedish national penile cancer register: incidence, tumour characteristics, management and survival. BJU Int. 2016;117(2):287–292.
- Pizzocaro G, Algaba F, Horenblas S, et al. EAU penile cancer guidelines 2009. Eur Urol. 2010;57(6):1002–1012.
- Algaba F, Horenblas S, Pizzocaro-Luigi Piva G, et al. EAU guidelines on penile cancer. Eur Urol. 2002;42(3):199–203.
- Bevan-Thomas R, Slaton JW, Pettaway CA. Contemporary morbidity from lymphadenectomy for penile squamous cell carcinoma: the M.D. Anderson cancer center experience. J Urol. 2002;167(4):1638–1642.
- Gopman JM, Djajadiningrat RS, Baumgarten AS, et al. Predicting postoperative complications of inguinal lymph node dissection for penile cancer in an international multicentre cohort. BJU Int. 2015;116(2):196–201.
- Stuiver MM, Djajadiningrat RS, Graafland NM, et al. Early wound complications after inguinal lymphadenectomy in penile cancer: a historical cohort study and risk-factor analysis. Eur Urol. 2013;64(3):486–492.
- Hegarty PK, Kayes O, Freeman A, et al. A prospective study of 100 cases of penile cancer managed according to european association of urology guidelines. BJU Int. 2006;98(3):526–531.
- Ercole CE, Pow-Sang JM, Spiess PE. Update in the surgical principles and therapeutic outcomes of inguinal lymph node dissection for penile cancer. Urol Oncol. 2013;31(5):505–516.
- Kroon BK, Horenblas S, Lont AP, et al. Patients with penile carcinoma benefit from immediate resection of clinically occult lymph node metastases. J Urol. 2005;173(3):816–819.
- Nazzani S, Catanzaro M, Biasoni D, et al. Clinical outcomes in clinical N0 squamous cell carcinoma of the penis according to nodal management: early, delayed or selective (following dynamic sentinel node biopsy) inguinal lymph-node dissection. J Urol. 2021;206(2):354–363.
- Hakenberg OW, Comperat EM, Minhas S, et al. EAU guidelines on penile cancer: 2014 update. Eur Urol. 2015;67(1):142–150.
- Koifman L, Hampl D, Koifman N, et al. Radical open inguinal lymphadenectomy for penile carcinoma: surgical technique, early complications and late outcomes. J Urol. 2013;190(6):2086–2092.
- Ravi R. Morbidity following groin dissection for penile carcinoma. Br J Urol. 1993;72(6):941–945.
- Bouchot O, Rigaud J, Maillet F, et al. Morbidity of inguinal lymphadenectomy for invasive penile carcinoma. Eur Urol. 2004;45(6):761–765, discussion 765–766.
- Nelson BA, Cookson MS, Smith JA, Jr., et al. Complications of inguinal and pelvic lymphadenectomy for squamous cell carcinoma of the penis: a contemporary series. J Urol. 2004;172(2):494–497.
- Jeanne-Julien A, Bouchot O, De Vergie S, et al. Morbidity and risk factors for complications of inguinal lymph node dissection in penile cancer. World J Urol. 2022;41(1):109–118.
- Okeke AA, Bates DO, Gillatt DA. Lymphoedema in urological cancer. Eur Urol. 2004;45(1):18–25.
- Perdona S, Autorino R, De Sio M, et al. Dynamic sentinel node biopsy in clinically node-negative penile cancer versus radical inguinal lymphadenectomy: a comparative study. Urology. 2005;66(6):1282–1286.
- Segon YS, Summey RD, Slawski B, et al. Surgical venous thromboembolism prophylaxis: clinical practice update. Hosp Pract (1995). 2020;48(5):248–257.
- Ludvigsson JF, Otterblad-Olausson P, Pettersson BU, et al. The swedish personal identity number: possibilities and pitfalls in healthcare and medical research. Eur J Epidemiol. 2009;24(11):659–667.
- Torbrand C, Wigertz A, Drevin L, et al. Socioeconomic factors and penile cancer risk and mortality; a population-based study. BJU Int. 2017;119(2):254–260.
- Ludvigsson JF, Andersson E, Ekbom A, et al. External review and validation of the swedish national inpatient register. BMC Public Health. 2011;11:450.
- Socialstyrelsen. National Patient Register. 2019 [cited 2023 Feb]. Available from https://www.socialstyrelsen.se/en/statistics-and-data/registers/national-patient-register/
- Wettermark B, Hammar N, Fored CM, et al. The new Swedish prescribed drug register–opportunities for pharmacoepidemiological research and experience from the first six months. Pharmacoepidemiol Drug Saf. 2007;16(7):726–735.
- Aberra FN, Lewis JD, Hass D, et al. Corticosteroids and immunomodulators: postoperative infectious complication risk in inflammatory bowel disease patients. Gastroenterology. 2003;125(2):320–327.
- Brassard P, Bitton A, Suissa A, et al. Oral corticosteroids and the risk of serious infections in patients with elderly-onset inflammatory bowel diseases. Am J Gastroenterol. 2014;109(11):1795–1802; quiz 1803.
- Abu-Ashour W, Twells LK, Valcour JE, et al. Diabetes and the occurrence of infection in primary care: a matched cohort study. BMC Infect Dis. 2018;18(1):67.
- Carey IM, Critchley JA, DeWilde S, et al. Risk of infection in type 1 and type 2 diabetes compared with the general population: a matched cohort study. Diabetes Care. 2018;41(3):513–521.
- Martin ET, Kaye KS, Knott C, et al. Diabetes and risk of surgical site infection: a systematic review and meta-analysis. Infect Control Hosp Epidemiol. 2016;37(1):88–99.
- Piazza G, Goldhaber SZ, Kroll A, et al. Venous thromboembolism in patients with diabetes mellitus. Am J Med. 2012;125(7):709–716.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies [Internet]. 2020. Available from: https://www.goodreports.org/strobe-cohort/
- Lambert P, Royston P. Further development of flexible parametric models for survival analysis. Stata J. 2009;9(2):265–290.
- Buuren S. Flexible imputation of missing data. Boca Raton (FL): CRC Press; 2012.
- Rubin DB. Multiple imputation for nonresponse in surveys. Vol. 81. Hoboken (NJ): John Wiley & Sons; 2004.
- R_Core_Team. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available from: https://www.R-project.org/. 2020
- Qureshi W, Ali Z, Amjad W, et al. Venous thromboembolism in cancer: an update of treatment and prevention in the era of newer anticoagulants. Front Cardiovasc Med. 2016;3:24.
- Blom JW, Doggen CJ, Osanto S, et al. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293(6):715–722.
- Sharma P, Zargar H, Spiess PE. Surgical advances in inguinal lymph node dissection: optimizing treatment outcomes. Urol Clin North Am. 2016;43(4):457–468.
- Singh A, Jaipuria J, Goel A, et al. Comparing outcomes of robotic and open inguinal lymph node dissection in patients with carcinoma of the penis. J Urol. 2018;199(6):1518–1525.
- Patel KN, Salunke A, Bakshi G, et al. Robotic-assisted video-endoscopic inguinal lymphadenectomy (RAVEIL) and video-endoscopic inguinal lymphadenectomy (VEIL) versus open inguinal lymph-node dissection (OILND) in carcinoma of penis: comparison of perioperative outcomes, complications and oncological outcomes. A systematic review and meta-analysis. Urol Oncol. 2022;40(3):112.e11–112.e22.
- Thyavihally YB, Dev P, Waigankar SS, et al. Comparative study of perioperative and survival outcomes after video endoscopic inguinal lymphadenectomy (VEIL) and open inguinal lymph node dissection (O-ILND) in the management of inguinal lymph nodes in carcinoma of the penis. J Robot Surg. 2021;15(6):905–914.
- Kirrander P, Andrén O, Windahl T. Dynamic sentinel node biopsy in penile cancer: initial experiences at a Swedish referral Centre. BJU Int. 2013;111(3 Pt B):E48–53.