Abstract
Purpose
Patients with synchronous metastatic head and neck squamous cell carcinomas (mHNSCC) are at risk of locoregional progression associated with significant morbidity and mortality. The aim of this study is to assess whether the addition of aggressive locoregional treatment to systemic therapy could be associated with an improved overall survival (OS) compared to systemic therapy alone in upfront mHNSCC patients.
Material and methods
This retrospective study included patients presenting with previously untreated mHNSCC who underwent first-line systemic therapy at a single institution between 1998 and 2018. Locoregional treatment was defined as either exclusive locoregional radiotherapy (RT) or surgery with or without adjuvant RT.
Results
One hundred forty-eight patients were included. Eighty patients were treated with systemic therapy alone and 68 patients were treated with a combination of locoregional treatment and systemic therapy. Median overall survival (OS) was 13 months [10.7–15] and median progression free survival (PFS) was 7.7 month [6.5–8.9]. The addition of a locoregional treatment to systemic therapy compared to systemic therapy alone was associated with improved survival (1-year OS, 65.8% vs. 41.1%, p < .001, and 1-year PFS, 42.5% vs. 18.5%, p < .001). Moreover, RT dose equal to 70 Gy was associated with even longer OS compared to a RT dose below 70 Gy and to no locoregional treatment (23.4 vs. 12.7 vs 7.5 months respectively). In a subgroup analysis on 75 patients presenting with a responding or stable metastatic disease after first-line systemic therapy, oropharyngeal primary tumor site and the addition of a locoregional treatment, especially a high radiation dose of 70 Gy, were evidenced as independent prognostic factors for improved OS.
Conclusion
The addition of a high-dose RT locoregional treatment to systemic therapy is associated with prolonged OS in patients with synchronous mHNSCC and should be discussed for patients who respond to or have a stable disease after first-line systemic therapy.
Introduction
Head and neck squamous cell carcinomas (HNSCC) are metastatic at diagnosis in 10% of cases [Citation1]. Standard-of-care for synchronous metastatic HNSCC (mHNSCC) is systemic therapy, using a combination of platinum-based chemotherapy and cetuximab [Citation2]. Recently, immunotherapy has become a first-line alternative option following the KEYNOTE-048 results. Pembrolizumab used as monotherapy or associated with chemotherapy has shown improved overall survival (OS) compared to the traditional EXTREME regimen [Citation3]. Even though median OS rates reach 15 months with these recent advances, prognosis of mHNSCC remains dismal. Treatment of the primary tumor in the context of synchronous metastatic initial presentation has been investigated in many solid tumors (colon cancer, renal carcinoma, lung cancer…), with essentially retrospective series demonstrating survival advantage [Citation4], while few prospective trials have been published [Citation5–7].
As synchronous mHNSCC usually present with associated advanced locoregional disease, the main cause of morbidity and mortality is primary tumor progression [Citation8,Citation9]. Because of the lethal risk of locoregional failure (hemorrhage, airway obstruction), locoregional treatment may be proposed in addition to systemic therapy. Aside from symptomatic palliation, emerging evidence show that the use of locoregional treatment, mainly radiation therapy (RT), is associated with improved survival in synchronous mHNSCC patients [Citation10–13]. However, due to the retrospective nature of available data, no guidelines have been established to determine the place of locoregional treatment of the primary tumor, including surgery and/or high-dose RT, in the initial management of mHNSCC patients. The aim of this study was to assess the impact of aggressive locoregional treatment on the survival of patients with synchronous mHNSCC and to determine which subset of patients would benefit most from this strategy.
Material and methods
Patients
This retrospective study included all synchronous metastatic HNSCC patients diagnosed in Gustave Roussy from 1998 to 2018. Included patients had histologically proven invasive squamous cell carcinoma (SCC) of the oropharynx, oral cavity, hypopharynx, or larynx. Synchronous metastatic disease was defined by the presence of distant metastases or metastatic lymph nodes located below the clavicles on initial imaging studies (computed tomography (CT) scan and/or 18-fluorodeoxyglucose positron emission tomography combined with CT scan). Patients were excluded if histology was non-SCC, if the treatment was done in another institution, if death occurred within 30 days following diagnosis and if no systemic therapy was administered.
Treatment information and follow up
Locoregional treatment of the primary tumor and nodal involvement, if present, included either surgery, RT or both. Patients who received such treatment were part of ‘locoregional treatment’ group. Total dose, number of fractions, technique and concomitant chemotherapy regimen were recorded if RT was the proposed treatment. Patients who did not receive locoregional irradiation were part of ‘systemic only’ group. Chemotherapy regimen, duration, and toxicity were recorded.
Follow-up was carried out with a cervicothoracic CT scan throughout and after completion of the treatment, at the physician’s discretion and in function of the evolving follow-up recommendations. Disease progression was assessed using RECIST 1.1 criteria. Follow up was defined from time of diagnosis to time of last follow-up or death.
Objectives
The primary objective of this study was to assess whether the addition of high dose RT (± surgery) to systemic therapy could be associated with an improved OS compared to systemic therapy alone in patients with mHNSCC at diagnosis. OS was defined from the time of diagnosis to the time of death from any cause.
Secondary objectives were to assess the impact of locoregional treatment on progression free survival (PFS) and to identify prognostic factors associated with OS. PFS was defined from time of diagnosis to time of disease progression (locoregional, metastatic or both) or time of death from any cause if death occurred first.
Statistical analysis
The following variables were included for analysis: age, performance status (PS) at diagnosis, smoking status, malnutrition, comorbidities, histologic grade, primary tumor site (oral cavity, oropharynx, hypopharynx or larynx), T and N classification according to AJCC TNM staging, number of metastases seen on initial imaging studies, metastatic sites (lung or other sites), chemotherapy regimen (polychemotherapy or monochemotherapy) and RT dose (no RT, dose less than 70 Gy, or dose equal to 70 Gy). PS was retrospectively treated as a dichotomous variable, good (PS 0 or 1) or poor (PS ≥2). Malnutrition was defined by a body mass index (BMI) below 18.5 kg/m2.
Baseline characteristics for the entire cohort were compared using the chi-square test. OS and PFS were assessed using the Kaplan Meier method and compared using log-rank test. Cox proportional hazards model was used for univariate and multivariate analyses. Multivariate analyses were carried out using two different models according to the treatment classified in two or three categories: one is based on a 2-category variable (systemic therapy with or without locoregional treatment) and the other is based on a 3-category variable (systemic therapy alone, locoregional RT <70 Gy, or RT = 70 Gy). Such analyses were performed on OS in the entire cohort, OS in patients with metastatic disease response, and PFS. To account for the significant proportion of missing data on the number of metastases, a multiple imputations method has been used in our analyses. To account for selection bias favoring the administration of RT in patients with better prognosis, a planned subgroup analysis was performed in patients with stable disease or disease response after first-line systemic therapy. To assess prognostic factors of response to RT, a subgroup analysis on the ‘locoregional group’ was performed. All statistical analyses were performed using SAS software (SAS Institute, Cary, NC, USA) with 2-sided tests and a significance level of .05.
Results
Population
We identified 190 patients diagnosed with synchronous mHNSCC. Patients whose deaths occurred within 30 days following date of diagnosis were excluded (n = 12) as well as patients who did not receive any systemic therapy including patients treated with upfront locoregional treatment without any subsequent systemic therapy (n = 30). A total of 148 patients were included in the analysis, of which 80 (54%) received systemic therapy alone and 68 (46%) received locoregional and systemic treatment. Patient characteristics at diagnosis are presented in . Median age was 59 years old [26–93]. The number of patients with good PS was significantly higher in the ‘locoregional group’ (p = .02). Of note, p16 status was tested in 19 of the 61 patients (31%) with an oropharyngeal primary tumor, population representing 41% of the whole cohort. P16 was positive in 5 patients (26% of the patients tested).
Table 1. Initial patients characteristics.
Systemic therapy
First-line polychemotherapy was administered in 138 patients (93%) while only 8 patients received monochemotherapy (6%). The type of chemotherapy was unknown in 2 patients (1%). Most frequently used protocols were TPEx (docetaxel, cisplatin and cetuximab), EXTREME and cisplatin-5FU. Median treatment duration was 4 months. Systemic therapy was interrupted in 13 patients (9%) for toxicity, of which nine patients were in the ‘systemic only’ group and four in the ‘locoregional’ group. Frequently used chemotherapy regimens were EXTREME (6 patients, 46%) and cisplatin-5FU (4 patients, 31%). Two deaths were related to chemotherapy administration and both received TPF. After initial chemotherapy, 75 patients (51%) had disease response or stable disease in metastatic lesions according to RECIST criteria, including 56 patients (38%) with partial or complete response.
Locoregional treatment
Sixty-eight (46%) patients received locoregional treatment in addition to the systemic therapy, majority (91%) after first-line chemotherapy. Median duration of systemic therapy before locoregional treatment was 4.3 months. Among these 68 patients, 49 (72%) were responders or had stable metastatic disease after first-line chemotherapy. Six patients were treated with surgery, followed by adjuvant RT in four of them. Sixty-two patients were treated with exclusive RT, either combined to concomitant cetuximab (26%), cisplatin 100 mg/m2 every three weeks (11%), other chemotherapy (13%) or without any concomitant systemic therapy (50%).
Thirty-three patients received a RT dose equal to 70 Gy, representing 50% of the 66 patients treated with RT either exclusively or after surgery. 3D-conformal RT was used in 70% of patients and 20% had intensity-modulated radiotherapy (IMRT). Data was missing in 10% of the patients who underwent RT. Three patients discontinued RT, one due to grade 4 mucositis and two due to the inability to tolerate immobilization.
Survival outcomes
Median follow-up in the entire cohort was 11.8 months [1–121]. One hundred twenty-six patients (85%) were dead at the time of analysis.
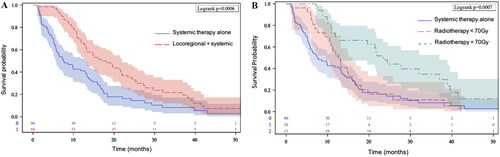
Median OS was 13 months [10.7–15]. The addition of locoregional treatment to systemic therapy was associated with longer median OS (16.8 vs. 7.5 months), 1-year OS (65.8% vs. 41.1%, p < .001) and 2-year OS (35.2% vs. 16.2%, p < .001) compared to systemic therapy alone (). Introducing the total radiotherapy dose in the second multivariate model, RT dose of 70 Gy, versus a dose below 70 Gy and no locoregional treatment, was significantly associated with a prolonged median OS (23.4 vs. 12.7 vs 7.5 months, respectively) and improved 1-year OS (69% vs. 53% vs. 41% respectively, p < .001) ().
Figure 1. Overall survival. (A) Overall survival for patients receiving locoregional plus systemic therapy versus systemic therapy. (B) Overall survival for patients receiving systemic therapy plus locoregional radiotherapy with a dose equal to 70 Gy versus a dose inferior to 70 Gy versus systemic therapy alone.
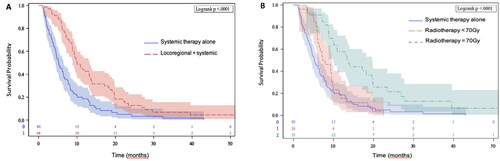
Median PFS in the entire cohort was 7.7 months [6.5–8.9]. The addition of locoregional treatment to systemic therapy was also significantly associated with longer PFS (10 vs. 5.2 month) compared to systemic therapy alone. One- and 2-year PFS were respectively 42.5% and 12.8% in the ‘locoregional’ group versus 18.5% and 5% in the systemic therapy only group (p < .001) (). High-dose RT of 70 Gy, compared to a dose below 70 Gy and no locoregional treatment, was significantly associated with a prolonged median PFS (13.7 vs. 7.5 vs. 5.2 months, respectively) and improved 1-year PFS (57.6% vs. 15.3% vs. 18.5%, respectively, p < .0001) ().
Figure 2. Progression free survival. (A) Progression free survival for patients receiving locoregional plus systemic therapy versus systemic therapy. (B) Progression free survival for patients receiving systemic therapy plus locoregional radiotherapy with a dose equal to 70 Gy versus a dose inferior to 70 Gy versus systemic therapy alone.
In patients with complete or partial response or stable disease in metastatic lesions after first-line systemic therapy (n = 75), the addition of locoregional treatment was associated with significantly longer median OS (21.9 vs. 14.7 months, p = .047).
Prognostic factors for OS
On univariate analysis, locoregional treatment, RT dose equal to 70 Gy, good PS, nonsmoker status, early N stage, oropharyngeal primary tumor site, and polychemotherapy were factors significantly associated with prolonged OS. On multivariate analysis, OS was significantly improved in patients with good PS, early N stage and locoregional treatment, especially those receiving a radiation dose of 70 Gy ().
Table 2. Univariate and multivariate analysis for overall survival in entire cohort (n = 148).
In the subgroup of responders or patients with stable metastatic disease, the oropharyngeal primary tumor site and again locoregional treatment with a radiation dose of 70 Gy were significant independent factors associated with an improved OS ().
Table 3. Univariate and multivariate analysis for overall survival in patients with partial, complete or stable metastatic response (n = 75).
In a subgroup analysis on the ‘locoregional’ group alone, improved OS was observed in patients with oropharyngeal primaries and RT dose of 70 Gy. Duration of chemotherapy before locoregional treatment was not a significant factor for OS (Table S1, Supplementary material).
Discussion
In this retrospective study, we investigated the impact of adding aggressive locoregional treatment to systemic therapy in synchronous mHNSCC. Nearly half of the patients in this cohort received combined locoregional and systemic therapy. Our results showed a 9-month improvement in median OS with the addition of locoregional treatment to systemic therapy compared to systemic therapy alone, 16.8 vs. 7.5 months, respectively. Furthermore, a curative dose of radiation, 70 Gy, was significantly associated with a prolonged median OS compared to a dose below 70 Gy and no locoregional treatment (23.4 vs. 12.7 vs 7.5 months, respectively).
Of note, the median OS in patients treated with systemic therapy alone (7.5 months) is inferior to the results published in the pivotal study by Vermorken et al. on metastatic and/or recurrent HNSCC who had a median OS of 10.1 months in the group that received chemotherapy plus cetuximab, but there is no available data on the specific population of synchronous metastatic patients [Citation14]. The difference may be explained by our cohort spanning over 20 years, with corresponding changes in the systemic treatments used, with current standard regimens like EXTREME or TPEx only being prescribed more recently. Furthermore, a significant proportion of patients were not able to receive such protocols due to poor PS, comorbidities, and treatment related toxicities. Lastly, no patients have been treated with immunotherapy. Nevertheless, even when compared with survival outcomes of standard chemotherapy regimens in the metastatic setting, superior median OS was achieved with the addition of locoregional treatment in our cohort. This observed OS benefit is consistent with the findings of previous published retrospectives studies [Citation11,Citation12] including those of Rambeau et al. showing prolonged OS in patients receiving a locoregional treatment compared to systemic therapy alone (median OS 16.1 vs. 7.5 months, respectively) [Citation13].
In our cohort, half of the patients treated with RT received 70 Gy, the dose commonly prescribed in the curative setting. On multivariate analysis, a radiation dose of 70 Gy was associated with longer OS. To address the selection bias whereby patients with good response to chemotherapy were more likely to receive high radiation dose, we performed a subgroup analysis in patients with complete or partial response or stable metastatic disease after first-line systemic therapy. The improvement in OS was maintained in patients who received locoregional treatment, especially those receiving a high dose of 70 Gy. Our results are concordant with those published by Zumsteg et al. who have shown that patients receiving a ‘curative’ dose defined as superior or equal to 60 Gy had longer OS compared to those receiving less than 60 Gy [Citation12]. Similarly, in another retrospective study, patients treated with RT biologically effective dose (BED) superior or equal to 72Gy10 had better OS than those treated with BED inferior to 72Gy10 [Citation11]. Cumulative retrospective data thus suggest that the use of local high-dose RT in non-metastatic HNSCC is beneficial in selected patients.
The most appropriate timing of locoregional RT in the initial management of mHNSCC remains an issue. The risk of metastatic progression is a significant concern in the absence of effective concurrent systemic therapy during the time of RT, which lasts up to seven weeks [Citation15]. Thus, in our cohort, for most patients, locoregional treatment has preferably been used as consolidation therapy following response to first-line systemic therapy. In Rambeau et al.’s study, there was a trend toward improved OS when locoregional RT was administered as consolidation therapy after chemotherapy rather than up-front (median OS of 22.1 and 15.5 months respectively) [Citation12]. Our data confirm this treatment strategy: in 75 patients for whom partial response or stable disease in metastatic lesions was obtained after first line systemic therapy, a significant improvement in OS was observed among patients who received locoregional treatment compared to those who did not (21.9 and 14.7 months, respectively). Moreover, performance status and N stage which were prognostic factors initially associated with OS in the whole cohort were no longer evidenced in this subset. Thus, starting with systemic treatment may help select patients and avoid indiscriminately administering aggressive RT to patients for whom such local treatment may not be beneficial and could even be deleterious due to its non-negligible adverse effects. Response to chemotherapy may be a tool for patient selection, with the hypothesis that chemosensitivity would be a predictive factor for better RT effectiveness.
This approach was successfully assessed in de novo metastatic nasopharyngeal carcinoma (NPC) in a recent randomized controlled trial on 126 patients. Inclusion criteria were patients with complete or partial response after three cycles of chemotherapy. The 24-month OS was significantly longer in the chemotherapy plus high-dose RT compared to the chemotherapy-alone group (76.4% vs. 54.5%). Local radiotherapy added to chemotherapy thus improved survival in patients with chemotherapy-sensitive metastatic NPC [Citation16].
Furthermore, Zumsteg et al. reported better OS in patients treated with high-intensity locoregional treatment no more than six months after the diagnosis versus greater than six months [Citation11] and explained this finding by the declining efficacy of systemic therapy after a 6-month period. In our cohort, median duration of chemotherapy before locoregional radiotherapy was only 4.3 months, which may explain why the timing of RT had no significant impact on survival in the subgroup analysis performed on the ‘locoregional’ group.
Some limitations must be acknowledged in our study. Its retrospective nature induces selection and immortal-time bias. Patients with good PS were more likely to receive locoregional treatment rather than systemic therapy alone (97% vs 86%, p = .02). In all analyses, we adjusted for performance status but certain baseline characteristics that could influence survival such as comorbidities were not documented and could not be accounted for. We thus performed a subgroup analysis in patients with complete or partial response or stable metastatic disease after first line chemotherapy to account for the potential confusion bias. Another issue was the missing data on the number of metastases in 60% of the cohort, whether the disease was oligometastatic (1–3 metastatic lesions) or polymetastatic was unclear in many patients. Consequently, we used a multiple imputations method to account for the proportion of missing data. Despite analyses including adjustment for this factor, no significant association between survival and the number of metastases has been shown, contrary to other recent studies [Citation15,Citation16]. In our cohort, the therapeutic strategy for both oligo- and polymetastatic disease was generally the same. Interestingly, Zumsteg et al. showed that aggressive local therapy still improved survival with a similar magnitude even when the number and location of organs with metastases were controlled in propensity score–matched and multivariate analyses [Citation12].
Another limitation is the absence of p16/HPV status in 69% of patients with a primary oropharyngeal tumor. Nevertheless, multivariate analyses were adjusted for primary tumor localization and this factor did not significantly impact the OS in the entire cohort. One potential explanation for this finding is that proportion of individuals with p16/HPV-positive status in the general population was less important before 2010 than it is today. In a cohort of 801 patients with oropharyngeal cancer treated at Gustave Roussy between 2007 and 2016, only 293 patients (37%) were p16-positive, of which 12% were finally HPV-negative. Overall, the prevalence of HPV-positive oropharyngeal cancers was around 32% during this period [Citation17]. However, in two subgroup analyses, in patients with partial response or stable metastatic disease and in the subset of patients treated by the combined systemic and locoregional treatment, oropharyngeal primary tumor site appeared to be an independent factor associated with an increased OS. Given the higher radiosensitivity of HPV-driven oropharyngeal cancers [Citation18,Citation19] and the favorable patient characteristics (younger age, better PS and less comorbidities), it would be of interest to prospectively evaluate whether an aggressive locoregional treatment can provide a survival advantage in this specific population. Of note, insufficient information on long-term side effects of radiation did not allow assessment of quality of life, which remains an essential consideration in these patients where the administration of high-dose locoregional RT aimed to prevent the onset of severe local symptoms from an uncontrolled primary tumor.
Of course, high intensity RT might not be appropriate in all mHNSCC. In order to optimize the risk-benefit ratio of aggressive locoregional irradiation in metastatic patients, several approaches may be considered. One is decreasing the number of fractions. Radiobiological modeling suggested that a hypofractionated RT protocol delivering 54 Gy in 18 fractions of 3 Gy was more effective for tumor control and for reduction of late effects probability, compared to the standard 2-Gy fractionation in locally advanced HNSCC [Citation20]. Another could be reducing irradiated volumes by treating only the gross tumor with no prophylactic nodal irradiation. As tested in patients with HNSCC who respond to induction chemotherapy, this can lead to reduced toxicity and improved quality of life, without altering survival outcomes [Citation21].
With the recent therapeutic advances in mHNSCC, this locoregional approach is yet to be tested in combination with immunotherapy. In the ongoing phase III PembroMetaRT trial (NCT04747054), a hypofractionated scheme with reduced irradiated volumes will be used in association with pembrolizumab compared to pembrolizumab alone in synchronous mHNSCC presenting a combined positive score ≥1. In this context, new predictive factors based on dynamic imaging modalities or liquid biopsies will probably be of significant help for selecting patients who would benefit from aggressive locoregional therapy.
In this retrospective study, high-dose RT of 70 Gy after first-line systemic treatment showed an improvement in in OS in patients with mHNSCC at diagnosis compared to patients receiving less than 70 Gy or no locoregional treatment. Response to first-line chemotherapy should be used to select patients likely to benefit from such locoregional treatment. Lastly, this combined strategy seemed to be more beneficial in oropharyngeal primaries.
Conclusion
High-dose radiation therapy used for locoregional treatment of the primary tumor in patients with synchronous mHNSCC may improve OS and should be considered in patients who respond or have stable disease after first-line systemic therapy.
Ethical standards statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Supplemental Material
Download MS Word (27.3 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
- Marur S, Forastiere AA. Head and neck squamous cell carcinoma: update on epidemiology, diagnosis, and treatment. Mayo Clin Proc. 2016;91(3):386–396.
- Guigay J, Aupérin A, Fayette J, et al. Cetuximab, docetaxel, and cisplatin versus platinum, fluorouracil, and cetuximab as first-line treatment in patients with recurrent or metastatic head and neck squamous-cell carcinoma (GORTEC 2014-01 TPExtreme): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2021;22(4):463–475.
- Burtness B, Harrington KJ, Greil R, et al. Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): a randomised, open-label, phase 3 study. Lancet. 2019;394(10212):1915–1928.
- Ferrand F, Malka D, Bourredjem A, et al. Impact of primary tumour resection on survival of patients with colorectal cancer and synchronous metastases treated by chemotherapy: results from the multicenter, randomised trial Fédération Francophone de Cancérologie Digestive 9601. Eur J Cancer. 2013;49(1):90–97.
- Méjean A, Ravaud A, Thezenas S, et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N Engl J Med. 2018;379(5):417–427.
- Shiarli A-M, McDonald F, Gomez DR. When should we irradiate the primary in metastatic lung cancer? Clin Oncol. 2019;31(12):815–823.
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy for the comprehensive treatment of oligometastatic cancers: long-term results of the SABR-COMET phase II randomized trial. J Clin Oncol. 2020;38(25):2830–2838.
- Coatesworth AP, Tsikoudas A, MacLennan K. The cause of death in patients with head and neck squamous cell carcinoma. J Laryngol Otol. 2002;116(4):269–271.
- Slootweg PJ, Bolle CW, Koole R, et al. Cause of death in squamous cell carcinoma of the head and neck an autopsy study on 31 patients. J Craniomaxillofac Surg. 1992;20(5):225–227.
- Patel TD, Marchiano E, Chin OY, et al. Utility of surgery/radiotherapy in distant metastatic head and neck squamous cell carcinoma: a population-based approach. Otolaryngol Head Neck Surg. 2016;154(5):868–874.
- Kabarriti R, Baliga S, Ohri N, et al. Radiation therapy for patients with newly diagnosed metastatic head and neck squamous cell carcinoma. Head & Neck. 2018;41(1):130–138. 10.1002/hed.25476.
- Zumsteg ZS, Luu M, Yoshida EJ, et al. Combined high-intensity local treatment and systemic therapy in metastatic head and neck squamous cell carcinoma: an analysis of the national cancer data base: local treatment in metastatic HNSCC. Cancer. 2017;123(23):4583–4593.
- Rambeau A, Bastit V, Thureau S, et al. Impact of locoregional irradiation in patients with upfront metastatic head and neck squamous cell carcinoma. Oral Oncol. 2019;93:46–51.
- Vermorken J, Mesia R, Rivera F, et al. Platinum-Based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. 2008;359(11):1116–1127.
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051–2058.
- Leeman JE, Patel S, Anderson ES, et al. Long-term survival in oligometastatic head and neck cancer. Int J Radiat Oncol Biol Phys. 2017;99(2):S138.
- Mirghani H, Leroy C, Chekourry Y, et al. Smoking impact on HPV driven head and neck cancer’s oncological outcomes? Oral Oncol. 2018;82:131–137.
- Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363(1):24–35.
- Pike LRG, Hwang WL, Royce TJ, et al. HPV status predicts for improved survival following chemotherapy in metastatic squamous cell carcinoma of the oropharynx. Oral Oncol. 2018;86:69–74.
- Shuryak I, Hall EJ, Brenner DJ. Optimized hypofractionation can markedly improve tumor control and decrease late effects for head and neck cancer. Int J Radiat Oncol Biol Phys. 2019;104(2):272–278.
- Villaflor VM, Melotek JM, Karrison TG, et al. Response-adapted volume de-escalation (RAVD) in locally advanced head and neck cancer. Ann Oncol. 2016;27(5):908–913.
