Abstract
Background
The purpose was to investigate the treatment flow of patients with HER2-positive metastatic breast cancer (mBC), progression-free survival (PFS) and overall survival (OS) across treatment lines and adherence to guidelines (defined as trastuzumab, pertuzumab and chemotherapy first line, where 85% received vinorelbine as backbone and T-DM1 second line). Furthermore, we identified clinical markers to predict the risk of developing brain metastases.
Material and Methods
Patients with HER2-positive mBC, diagnosed between 01.01.2014–31.12.2019, registered in the database of the Danish Breast Cancer Group were included in this real-word study. Clinical follow-up was assessed until 01.10.2020 and complete follow-up for overall survival until 01.10.2021. Survival data were analyzed using the Kaplan-Meier method with guidelines adherence analyzed as a time-varying covariate, and the risk of CNS metastasis was estimated by the cumulative incidence function.
Results
631 patients were included. 329 (52%) patients followed the guidelines. The median OS for all patients was 42.3 months (95% Cl, 38.2–48.4), and significantly higher for the patients who followed guidelines; NA (95% CI, 78.2–NA). The median PFS for all patients was 13.4 months (95% Cl, 12.1–14.8), 6.6 (95% Cl, 5.8–7.6) and 5.8 (95% Cl, 4.9–6.9) for first, second and third line of treatment, respectively. Patients with ER-negative mBC had a higher risk of developing brain metastases and patients with high tumor burden had a higher risk of developing brain metastases with an adjusted HR of 0.69 (95% CI, 0.49–0.98), p = 0.047 and 2.69 (95% CI, 1.45–5.00), p = 0.002, respectively.
Conclusion
We found that only half of the patients with HER2-positive mBC, received first and second-line treatment according to national guidelines. Patients receiving treatment according to guidelines had a significantly higher median OS compared to patients who did not. We also found that patients with ER-negative disease or high tumor burden had a significantly higher risk of developing brain metastases.
Background
Breast cancer (BC) is the most frequent cancer affecting women on a worldwide basis, being the leading cause of cancer-related deaths in more than 100 countries [Citation1]. The prognosis of metastatic breast cancer (mBC) differs according to the specific subtype. The overexpression of the human epidermal growth factor receptor 2 (HER2) is present in approximately 20% of metastatic breast cancer patients [Citation2]. It was associated with a shorter overall survival (OS) and a higher risk of developing brain metastases compared to HER2-negative and ER-positive BC [Citation3], but the development of new targeted treatments has improved OS – also in real-world settings [Citation2].
The standard treatment of HER2-positive mBC according to international guidelines consists of regiments based on anti-HER2 targeted agents in addition to chemotherapy [Citation4,Citation5]. According to the present Danish national guidelines dual blockade of HER2 (trastuzumab and pertuzumab) in addition to chemotherapy is recommended as first-line treatment followed by second-line treatment with TDM-1. Until recently there was no standard choice of third-line treatment or beyond except continued HER2-targeted treatment. To the best of our knowledge, no studies have been published that investigate the flow of treatment of patients with HER2-positive mBC. Specifically, adherence to guidelines, treatment regimens, and the duration of the treatment lines.
Brain metastases (BM) play a major role in the mortality and morbidity of patients with HER2-positive mBC. Treatment of BM is primarily based on neurosurgery and/or radiation therapy often resulting in poor survival and a high level of adverse effects [Citation6]. Detecting BM early is in some studies associated with a better prognosis, but routine screening is not recommended according to international guidelines [Citation7,Citation8]. Identifying prognostic clinical markers associated with a high risk of developing BM could be helpful for the clinician to determine if an asymptomatic patient should be examined for BM and to be more vigilant toward patients with vague CNS-related symptoms – especially if there is a possibility to treat with therapies that work in the brain.
The Danish Breast Cancer Group (DBCG) was formed in 1976 to ensure optimal diagnostics and treatment of BC. From the beginning, a clinical database was established and since then, surgeons, pathologists and oncologists have systematically reported information on diagnosis, treatment and follow-up. Using real-world data from the DBCG database we aim to investigate the flow of treatment in HER2-positive mBC, examine progression-free survival (PFS) and OS and explore clinical characteristics that may be associated with a higher risk of developing BM.
Material and methods
Design
This study is an observational, retrospective, nationwide population-based study covering patients from all departments of oncology in Denmark utilizing the DBCG database.
Study population
Adult female patients with HER2-positive mBC, diagnosed with primary mBC or recurrent mBC between 01.01. 2014–31.12. 2019 was included. Clinical follow-up was assessed until 01.10. 2020 and complete follow-up for OS until 01.10. 2021. The index date was defined as the date of diagnosis of either metastatic recurrence or primary mBC. The disease had to be HER2-positive at first diagnosis of metastatic disease.
Data sources
The nationwide, population-based clinical DBCG database includes data on demographics, diagnosis, pathology, treatment and follow-up. From the DBCG database prospectively collected data concerning primary diagnosis and treatment were extracted. Retrospectively collected data from the DBCG database concerning metastatic disease included date of diagnosis and disease progression, location of metastases, treatment modalities with start and end date and reason for discontinuation. Date of death was retrieved from the Danish Civil Registration system to ensure complete information on the vital status.
Measures
The purpose was to investigate the treatment flow of patients with HER2-positive metastatic breast cancer (mBC), progression-free survival (PFS) and overall survival (OS) across treatment lines and adherence to guidelines. Furthermore, we analyzed the prognostic value of different clinical markers to assess the risk of developing brain metastases.
Baseline characteristics
Baseline characteristics were described by the number of observations (n), mean and proportions.
HER2-positivity was defined as either 3+ IHC or 2+ with ISH-positivity. ER-positivity was defined as ≥1% by IHC. The data were retrieved from the first biopsy in patients with de novo mBC or from the first biopsy diagnosing mBC in patients with recurrence. If a diagnostic biopsy at recurrence was not performed, the most recent biopsy was used.
De novo metastatic disease was defined as the presence of metastases at first diagnosis or within 90 days of first breast cancer diagnosis.
Aggressive disease was defined as having a metastatic recurrence within the lowest quartile of time to recurrence. Time to recurrence was defined as the time between the date of primary surgery and recurrence. Patients with primary metastatic disease were not included in this analysis.
High tumor burden was defined as having three or more metastatic sites and low tumor burden was defined as having less than three sites.
Adherence to guidelines
The patients’ treatment adhered to guidelines if they were treated with trastuzumab, pertuzumab and chemotherapy in the first line and T-DM1 in the second line. If a patient died before initiating second-line treatment but their treatment followed guidelines until death, they were analyzed as having received treatment as per guideline.
Progression-free survival and overall survival
OS was defined as the time from the index date until the death of any cause. PFS was defined as time from index date to progression or death in the first line of treatment. In the second and third lines of treatment, PFS was defined as the time from the progression date of the previous line to progression or death in the current line. PFS and OS were analyzed using the Kaplan-Meier method and results are presented with Kaplan-Meier curves and an estimate of median survival (with corresponding 95% confidence intervals). For the stratification of adherence to guidelines versus not, this was treated as a time-varying covariate, i.e., a patient was adherent to the guideline if their first-line treatment was double-blockade and chemotherapy, otherwise not, and would switch to not adherent at the start of second line if treatment was not T-DM1. Furthermore, we compared BMs at index and de novo versus recurrent disease. Comparisons were done with a log-rank test.
Clinical markers prognostic of brain metastases
Time to CNS metastases was defined as time between the index and development of CNS metastases treating death as a competing event. This was estimated by the cumulative incidence function. Patients with brain metastases at the index were not included in this analysis.
The clinical markers of disease presentation (de novo or recurrent mBC), ER status (positive or negative), aggressive disease, (yes or no) and tumor burden (1–2 or 3+ metastatic sites at the start of first line), were examined for prognostic value. They were evaluated using the Fine and Gray sub-distributional proportional hazard regression model and Gray’s test. Validation of Fine and Gray sub-distributional hazard proportionality was based on the cumulative sums of residuals. A time-dependent component was included to fulfill the assumption on proportional hazards.
Each marker was evaluated in a univariate analysis and included in multivariate analysis if p < 0.01. In the multivariate analysis, the significance level was set to 0.05.
Follow-up
Follow-up time for OS was calculated as the median time to either censoring (1/10/2021) or death. Follow-up time for PFS was calculated as the median time to either progression, death or censoring (1/10/2020) and for CNS metastases as the median time to either CNS metastases, death or censoring (1/10/2020).
Results
Baseline characteristics
In the study period, a total of 631 patients with HER2-postive mBC were included (). The patients had a mean age of 62 years (range 26–94). 403 (63%) of the patients were ER-positive, and 65 (10%) patients had brain metastases at diagnosis. 404 (65%) patients had recurrent disease and of these 67 (17%) and 238 (59%) patients had received neoadjuvant and adjuvant treatment, respectively. The lower quartile of time to recurrence was within 24 months, which was used as the cutoff point for aggressive disease. 101 (25%) patients had aggressive disease.
Table 1. Baseline characteristics.
Treatment flow in the metastatic setting
483 (77%) of the 631 patients received chemotherapy combined with trastuzumab and pertuzumab as first line treatment (). Vinorelbine was used as the chemotherapy backbone in 410 (85%) of these patients.
Table 2. Therapy regimen in each line.
196 (45%) patients received TDM-1 as second line treatment.
As third-line treatment 171 (64%) patients were given a combination of chemotherapy and a HER2 inhibitor, but there was no single regimen commonly used as third-line treatment.
185 (38%) of the patients who received second-line treatment with TDM-1, had received dual-HER2 blockade and chemotherapy as first-line treatment (Supplementary Table 1). A total of 329 (52%) patients with a mean age of 59 years received dual-HER2 blockade and chemotherapy as first-line treatment and TDM-1 as second-line treatment if applicable, in adherence to our aforementioned guidelines.
Overall survival
The median overall survival (mOS) for all patients was 42.3 months (95%Cl, 38.2–48.4), with a median follow-up of 55.0 months. For patients with recurrent mBC, the mOS was 37.1 months (95% CI, 33.1–43.6) compared to 56.8 months (95% CI, 43.1–NA) for patients with de novo mBC (p < 0.001). In the patients treated according to guidelines mOS was not reached (NA (95% CI, 78.2–NA), whereas mOS was 27.2 months (95% CI, 22.2–32.9) for patients who did not follow guidelines (p < 0.001). Patients with brain metastases at index had an mOS of 26.3 months (95% CI, 15.1–35.2) vs. 44.8 months (95% CI, 39.7–51.5) in patients without brain metastases (p < 0.0001) at index ().
Figure 1. Overall survival for all patients and according to subgroups. Adherence to guidelines analyzed as a time dependent variable. CNS metastases according to presence at index. Median overall survival (mOS) and log-rank test are shown together with patients at risk according to different time-point.
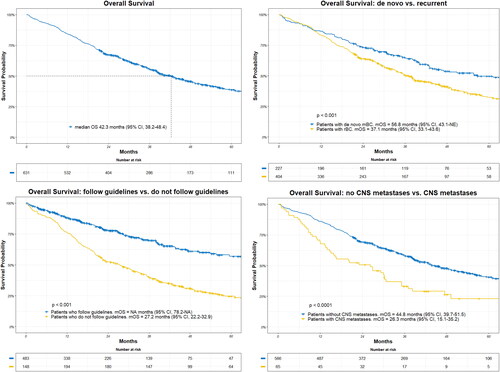
The mOS for patients receiving trastuzumab, pertuzumab and chemotherapy as first-line treatment was 46.1 months (95% CI, 40.1–55.7).
Progression-free survival
The median PFS for all patients was 13.4 months (95% Cl, 12.1–14.8), 6.6 months (95% Cl, 5.8–7.6) and 5.8 months (95% Cl, 4.9–6.9) for first, second and third line, respectively with a median follow-up of 32.7 months. The median PFS for patients receiving trastuzumab, pertuzumab and chemotherapy as first-line treatment was 14.2 months (95% Cl, 12.9–15.5).
Clinical markers prognostic of brain metastases
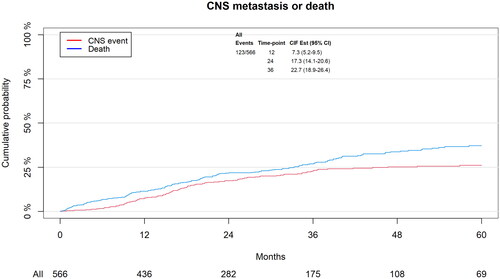
In total 123 (22%) of 566 patients developed brain metastases after index and 168 patients died as a competing event. Median follow-up was 23.8 months. The cumulative incidence of brain metastases at 12, 24 and 36 months was 7.3%, 17.3% and 22.7%, respectively ().
Figure 2. Cumulative incidence of brain metastases and death as competing event according to time since index. Patients at risk according to different time-point are listed below the x-axis.
After 36 months there was a 28.3% cumulative probability of developing brain metastases vs. 19.5% in patients with ER-negative mBC and ER-positive respectively (Supplementary Figure 1). ER-positive patients had a significantly lower risk of developing brain metastases with an HR of 0.69 (95% CI, 0.49–0.98), p = 0.047 in the adjusted analysis compared to ER-negative mBC (). After 24 months the cumulative probability of developing brain metastases in patients with high tumor burden was 26.0% vs. 13.1% in patients with low tumor burden (Supplementary Figure 2) and they had a significantly higher risk of developing brain metastases, with an adjusted HR of 2.69 (95% CI, 1.45–5.00), p = 0.002 after 12 months (). No statistically significant difference was shown for disease presentation (recurrent mBC vs. de novo mBC) or aggressive disease vs. non-aggressive disease.
Table 3. Fine and Gray sub-distributional proportional hazard models for risk of developing brain metastases.
Discussion
Using real-world data from the DCBG database we found that most patients with HER2-positive mBC (77%) received trastuzumab and pertuzumab combined with chemotherapy as first-line treatment. Approximately half (45%) of the patients received TDM-1 as second-line treatment. In correspondence with clinical experience, there was not a systematic choice of third-line treatment, but most patients (64%) received a HER2-targeted drug combined with chemotherapy. Only 329 (52%) patients followed guidelines by receiving dual HER2 blockade with chemotherapy followed by TDM1 if applicable. The relatively low degree of adherence to guidelines is somewhat difficult to explain. Both dual HER2 blockade with chemotherapy – where vinorelbine is mainly used in Denmark – and TDM1 are both regimens that are usually well tolerated. Some clinicians will choose endocrine treatment together with HER2-directed treatment due to poor performance, but if the patient is nevertheless offered a new line of treatment at a later point, the most effective treatments have been postponed.
In Denmark, the choice of first-line treatment is based on the results from the CLEOPATRA study [Citation9] and the HERNATA study [Citation10]. The CLEOPATRA study evaluated the addition of pertuzumab to trastuzumab combined with docetaxel as first-line treatment. The HERNATA study evaluated docetaxel vs. vinorelbine, both in combination with trastuzumab. The study failed to demonstrate superiority but found that vinorelbine was less toxic, hence it became the preferred choice of first-line chemotherapy together with HER2-targeted therapy in Denmark. In the recent end-of-study report of the CLEOPATRA trial, the median OS was 57.1 months (95% CI, 50–72) in the pertuzumab arm with a median follow-up of 99.9 months [Citation11].
In our study, we found a mOS for patients receiving trastuzumab, pertuzumab and chemotherapy (85% received vinorelbine) as first-line treatment of 46.1 months (95% CI, 40.1–55.7) and a PFS of 14.2 months (95% Cl, 12.9–15.5) which is inferior compared with the outcome in the CLEOPATRA study, although with wide confidence intervals. This difference is as expected when comparing survival data between real-world studies and clinical trials where patients are younger with fewer comorbidities and better performance status, as discussed in an earlier published paper [Citation12]. In our study, 85% of the patients received vinorelbine as the chemotherapy backbone in first-line treatment based on the HERNATA trial. In the ESMO and ASCO [Citation4,Citation5] guidelines, a taxane is recommended as backbone in first-line treatment, and it cannot be ruled out that a taxane is slightly better and therefore influence our results.
In patients who received dual-HER2 blockade and chemotherapy as first-line treatment and T-DM1 as second-line treatment, the mOS were almost doubled (NA (95% CI, 78.2–NA). However, this analysis was done with guideline adherence as a time-varying covariate. The result reflects that frail patients with poor performance status and consequently increased mortality, are not generally recommended for treatment according to guidelines. Conversely, these results should lead to thorough consideration with detailed patient involvement when a treatment strategy is to be planned. The expected better survival must be weighed against a less toxic regime with a significantly inferior effect.
In a large-scale French retrospective study investigating the survival of mBC in 22.000 patients, the authors found a mOS of 50.1 months (95% CI, 47.6–53.1) in patients with HER2-positive mBC [Citation2]. The OS is better than the findings in this study, but again with unprecise and non-conflicting estimates. The patients’ characteristics differed slightly between the studies. The patients in our study were older (mean age of 62 vs. a median age of 53), more patients had over 3 metastatic sites (34% vs 21%), and in contrast fewer patients with de novo mBC (35% vs. 40%). The French study included patients from Comprehensive Cancer Centers only, and this could affect outcomes as well.
The mOS for patients with brain metastases at index was 26.3 months (95% CI, 15.1–35.2) indicating less effectiveness of the treatments. Almost a third of all patients in this study had brain metastases at the index or developed brain metastases in the study period. We found that having an ER-negative disease or having a high tumor burden at the index is a prognostic marker of having a higher risk of developing brain metastases. Only a few studies have examined clinical markers associated with a higher risk of developing brain metastases in HER2-positive mBC. In a retrospective study, Paesmans M, et al. [Citation13] found that having lung metastases as the first site of relapse was an independent risk factor for developing brain metastases in HER2-positive mBC. However, they did not find ER status to be a significant risk factor.
In Denmark patients with mBC are not routinely screened for brain metastases at the time of diagnosis or during treatment unless they have symptoms. This study did not investigate whether there is a better outcome for patients if they are routinely screened for brain metastases. Our results could be used in clinical decision-making for lowering the threshold for examining brain metastases in patients who are ER-negative or in patients with a high tumor burden [Citation7,Citation8]. Identifying prognostic clinical markers associated with a high risk of developing BM could be helpful for the clinician to determine if an asymptomatic patient should be examined for BM and to be more vigilant toward patients with vague CNS-related symptoms - especially if there is a possibility to treat with therapies that work in the brain.
Since this study was initiated two new anti-HER2 drugs, have been introduced in the ESMO and ASCO guidelines for HER2-positive mBC as second- and third-line treatment (trastuzumab-deruxtecan and tucatinib respectively). They both have a highly relevant clinical effect on patients with HER2-positive mBC, also in patients with brain metastases. In the HER2CLIMB [Citation14] study, adding tucatinib to trastuzumab and capecitabine resulted in better OS (median of 21.6 months vs. 12.5 months) in patients with brain metastases. Trastuzumab-deruxtecan’s effect on brain metastases was investigated in the Tuxedo-1 trial [Citation15] where an intracranial response rate of 77.3% was found. These results could change the future strategies of screening for brain metastases, but further studies are needed to investigate the potential positive effects.
This study has different strengths and limitations. Using a large group of non-selected patients from a database with a high level of completeness makes this study robust. A limitation of this study is that the data are retrospectively gathered nationwide by different persons, which possibly could bring some irregularities in the database. Another limitation is that the database does not contain the patients’ comorbidities and performance status, which possibly could help understand the relatively low adherence to guidelines. A third limitation is that the group of patients with brain metastases is relatively small.
Conclusion
In this study using real-world data from the DBCG database we identified 631 patients with HER2-positive mBC between 2014–2019 that had an mOS of 42.3 months (95%Cl, 38.2–48.4) and a median PFS of 13.4 (95% Cl, 12.1–14.8), 6.6 (95% Cl, 5.8–7.6) and 5.8 months (95% Cl, 4.9–6.9) for first, second and third line of treatment, respectively. If patients received treatment according to guidelines, they had a significantly higher OS when compared to patients who did not follow guidelines. In this study, 65 patients had brain metastases at the time of diagnosis and a total of 123 patients developed brain metastases after index, resulting in almost a third of the patients being affected by brain metastases. We found that patients with ER-negative disease or high tumor burden have a higher risk of developing brain metastases.
Supplemental Material
Download MS Word (11.6 KB)Supplemental Material
Download TIFF Image (15.4 MB)Supplemental Material
Download TIFF Image (15.4 MB)Disclosure statement
Tobias Berg reports he received: Institutional grants from Pfizer, AstraZeneca, Novartis, Samsung Bieopis, Seagen, Merck, Eli Lilly and Danish Cancer Society. Personal grants (advisory board/presentation) from Merck, Astra Zeneca, Pfizer and Novartis. Personal grants (travel): Daiichi Sankyo. Ann Knoop reports she received: Institutional grants from Pfizer, AstraZeneca, Merck, Eli Lilly, Seagen, Roche, Novartis. Personal grants from Astra Zeneca and Daiichi Sankyo (travel + advisory board), MSD, Novartis, Seagen (Advisory Board). No conflicts of interest were reported by the remaining authors.
Data availability statement
The data supporting the findings of this study are not publicly available due to institutional restrictions. The data can be made available to qualified researchers through application to the Danish Breast Cancer Group.
Correction Statement
This article has been corrected with minor changes. These changes do not impact the academic content of the article.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492.
- Deluche E, Antoine A, Bachelot T, et al. Contemporary outcomes of metastatic breast cancer among 22,000 women from the multicentre ESME cohort 2008–2016. Eur J Cancer. 2020;129:60–70. doi: 10.1016/j.ejca.2020.01.016.
- Seshadri R, Firgaira FA, Horsfall DJ, et al. Clinical significance of HER-2/neu oncogene amplification in primary breast cancer. The South Australian breast cancer study group. J Clin Oncol. 1993;11(10):1936–1942. doi: 10.1200/JCO.1993.11.10.1936.
- Gennari A, André F, Barrios CH, et al. ESMO clinical practice guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann Oncol. 2021;32(12):1475–1495. doi: 10.1016/j.annonc.2021.09.019.
- Giordano SH, Franzoi MAB, Temin S, et al. Systemic therapy for advanced human epidermal growth factor receptor 2-positive breast cancer: ASCO guideline update. J Clin Oncol. 2022;40(23):2612–2635. doi: 10.1200/JCO.22.00519.
- Lee SS, Ahn J-H, Kim MK, et al. Brain metastases in breast cancer: prognostic factors and management. Breast Cancer Res Treat. 2008;111(3):523–530. doi: 10.1007/s10549-007-9806-2.
- Matsuo S, Watanabe J, Mitsuya K, et al. Brain metastasis in patients with metastatic breast cancer in the real world: a single-institution, retrospective review of 12-year follow-up. Breast Cancer Res Treat. 2017;162(1):169–179. doi: 10.1007/s10549-017-4107-x.
- Ramakrishna N, Anders CK, Lin NU, et al. Management of advanced human epidermal growth factor receptor 2-positive breast cancer and brain metastases: ASCO guideline update. J Clin Oncol. 2022;40(23):2636–2655. doi: 10.1200/JCO.22.00520.
- Swain SM, Kim S-B, Cortés J, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA study): overall survival results from a randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2013;14(6):461–471. doi: 10.1016/S1470-2045(13)70130-X.
- Andersson M, Lidbrink E, Bjerre K, et al. Phase III randomized study comparing docetaxel plus trastuzumab with vinorelbine plus trastuzumab as first-line therapy of metastatic or locally advanced human epidermal growth factor receptor 2–positive breast cancer: the HERNATA study. J Clin Oncol. 2011;29(3):264–271. doi: 10.1200/JCO.2010.30.8213.
- Swain SM, Miles D, Kim S-B, et al. Pertuzumab, trastuzumab, and docetaxel for HER2-positive metastatic breast cancer (CLEOPATRA): end-of-study results from a double-blind, randomised, placebo-controlled, phase 3 study. Lancet Oncol. 2020;21(4):519–530. doi: 10.1016/S1470-2045(19)30863-0.
- Christensen T, Berg T, Nielsen LB, et al. Dual HER2 blockade in the first-line treatment of metastatic breast cancer - A retrospective population-based observational study in Danish patients. Breast. 2020;51:34–39. Jundoi: 10.1016/j.breast.2020.03.002.
- Maurer C, Tulpin L, Moreau M, et al. Risk factors for the development of brain metastases in patients with HER2-positive breast cancer. ESMO Open. 2018;3(6):e000440. doi: 10.1136/esmoopen-2018-000440.
- Lin NU, Murthy RK, Abramson V, et al. Tucatinib vs placebo, both in combination with trastuzumab and capecitabine, for previously treated ERBB2 (HER2)-positive metastatic breast cancer in patients with brain metastases. JAMA Oncol. 2023;9(2):197–205. doi: 10.1001/jamaoncol.2022.5610.
- Bartsch R, Berghoff AS, Furtner J, et al. Trastuzumab deruxtecan in HER2-positive breast cancer with brain metastases: a single-arm, phase 2 trial. Nat Med. 2022;28(9):1840–1847. doi: 10.1038/s41591-022-01935-8.
