?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Lymphedema is a chronic, debilitating disease that often requires life-long management. Predicting clinical manifestations and prognosis is crucial in clinical practice because the treatment of lymphedema should be individualized for best clinical outcome. The aim of this study is to explore the location and severity of lymphedema secondary to inguinal and/or iliac lymph node dissection (LND) in patients with melanoma.
Methods
Patients with melanoma who received LND at a single tertiary medical center between 1 January 2010 and 31 September 2022 were retrospectively reviewed. Patient who received inguinal LND only were designate as the inguinal group while those who received both ilioinguinal LND were included in the ilioinguinal group. Volumetric measurement was used to objectify the severity and location of lymphedema. Clinical data was acquired for 12–15 months of follow-up.
Results
Among 81 patients, 43 (53%) had developed lymphedema in the lower extremities at an average of 33 days after the surgery. Initially, patients manifested with medial thigh lymphedema in the inguinal group while patients were presented with whole leg lymphedema in the ilioinguinal group. Lower leg volume of the ilioinguinal group was significantly higher than the inguinal group. After more than 12 months of lymphedema treatment, upper leg volume was higher in the ilioinguinal group than the inguinal group (12.7% vs 5.4%, p < 0.05).
Conclusion
Lymphedema developed in early post-op period. The ilioinguinal group presented with a larger volume of lymphedema in the distal area of the legs. Even after sufficient treatment, predominant lymphedema remained in the proximal leg for the ilioinguinal group. Patients with both inguinal and iliac LND were associated with more severe lymphedema. Based on the dissection sites, the clinical manifestations and prognosis of leg lymphedema can vary widely. Thus, clinicians should consider the dissection site when approaching melanoma patients with lymphedema.
Introduction
Current clinical guidelines for the management of malignant melanoma includes lymph node dissection (LND) as a part of the treatment and recurrence prevention [Citation1–3]. Patients with melanoma with clinically positive nodes but no distant metastasis receive LND. LND is a commonly known risk factor for secondary lymphedema, a cancer-treatment related complication, which can deteriorate the quality of life for both cancer survivors and those undergoing cancer treatment [Citation4]. The 5-year survival rate for cutaneous melanoma reaches 92% [Citation5,Citation6], which leaves patients with melanoma prone to side effects and complications related to cancer treatment. Studies reported that incidences for cancer-related lymphedema for breast cancer are 19%, genitourinary cancer 12.9%, gynecological caner 23.9%, and lower extremity melanoma 30.5% [Citation7]. Moreover, as overall survival of cancer patients improves, the disease duration of lymphedema is also being prolonged, causing significant social and financial burden [Citation8,Citation9]. Thus, physicians in cancer centers should give attention not only to patients at risk of secondary lymphedema but also to methods that would predict the projectile of disease course.
Axillary, inguinal, iliac, para-aortic, and pelvic lymph nodes are all commonly dissected lymph node sites for various cancer types. However, not all lymph nodes seem to carry similar risks of developing secondary lymphedema. In gynecological cancer (uterine and ovarian cancer) surgery, pelvic and para-aortic LND did not increase the risk of lower extremity lymphedema; however, post-operative radiotherapy was found to be the main culprit [Citation10]. Axillary lymph nodes and inguinal lymph nodes are more prone to lymphedema development after dissection. Biglia et al. had investigated that in patients with endometrial cancer who underwent LND, removing the circumflex iliac lymph nodes (inguinal lymph nodes) significantly increased the risk of lower extremity lymphedema [Citation11]. For patients with breast cancer, axillary LND and postmastectomy radiotherapy were each identified as an independent risk factor for lymphedema in long-term follow-up [Citation12]. Moreover, previous studies on patients with melanoma reported that inguinal LND has a higher risk of developing lymphedema than axillary LND [Citation13,Citation14]. Furthermore, there were case reports of secondary lymphedema after axillary or inguinal sentinel lymph node biopsy for cutaneous melanoma [Citation15].
The higher rate of lymphedema in the lower extremities of patients with melanoma after LND is probably due to different physiopathology regarding the lymphatic drainage system. Recent guidelines recommend pelvic and/or para-aortic LND for patients with advanced gynecological and prostate cancer [Citation16–19]; however, superficial lymph nodes, which are commonly removed during LND for melanoma, are often preserved. Pelvic and para-aortic LND does not directly obstruct lymphatic flow of the lower extremities, whereas dissection of inguinal nodes, as in patients with melanoma, may savage the drainage. This is also partially supported by the fact that there are no reports of lower extremity lymphedema after melanoma excision and wide local excision without LND.
Providing accurate prognosis about how lymphedema will involve is crucial in treating patients with lymphedema because prescription of compressive garments must be individualized for each patient. Those with favorable prognosis may be able to taper the usage of compressive garment while others may need high compression persistently. Moreover, some patients may feel that despite some increase in the volume of leg, decreased duration of garment application is more compatible with their activities of daily living.
Although it has already been suggested that removal of more than 10 inguinal lymph nodes and cellulitis of the limb increase the risk of lower extremity lymphedema for patients with melanoma [Citation20,Citation21], no study to date has examined whether different LND sites may result in different clinical manifestations of lower extremity lymphedema. Thus, the aim of this study is to explore the clinical variations of lymphedema after patients with melanoma receive inguinal and/or iliac LND using both clinical and volumetric data.
Materials and methods
Patients
This study is a retrospective cohort study in a tertiary cancer center in South Korea. Patients with melanoma who received LND between 1 January 2010 and 31 September 2022 were screened for inclusion. All patients received LND by a specialized surgeon and were routinely referred to a board-certified cancer rehabilitation specialist as a part of the clinical pathway for early screening, active surveillance, and assessment of lymphedema immediately after postoperative management is finished.
All patients who received LND between 1 January 2010 and 31 September 2022 were screened. The inclusion criteria were those who had previously received local excision in only one of the distal part of extremities, those who received LND on the same extremity, and those whose initial biopsy results matches the biopsy from LND.
The exclusion criteria include those who were diagnosed with primary or secondary lymphedema before LND, who received mass dissection only, who received LND in the upper extremities, who received bilateral LND, who had melanoma recurrence during follow-up period, and who did not have initial volumetric data. Patients with lymphedema who received only inguinal LND were classified as the inguinal group while those with both inguinal and iliac nodes dissected were named the ilioinguinal group. Patients who received mass dissection were excluded from study because these patients received cutaneous or subcutaneous excision during the operation. Patients with upper extremity LND were excluded from this study due to anatomical differences of lymphatic drainage system in upper and lower extremities and also the number of cases were too small for subgroup analysis.
Clinical data including the operation date, the date of first visit to cancer rehabilitation clinic, LND site, the number of dissected nodes, biopsy results, adjuvant anticancer therapy (immunotherapy and radiotherapy), lymphedema sites, lymphedema treatments, and volumetric data of lower extremities were acquired from initial visit to 15 months of follow-up. Number of dissected sentinel nodes was included in the total number of dissected lymph nodes. Informed consent was waived by the Institutional Review Board due to retrospective study design.
Diagnosis and characterization of lymphedema
The diagnosis of lower extremity lymphedema was based on physical examination and confirmed by a board-certified physical medicine and rehabilitation (PM&R) physician specializing in cancer rehabilitation, who also performed a differential diagnosis between generalized edema after adjuvant systemic therapy, venous edema, and lymphedema. Physical examinations of lymphedema included the presence of Stemmer’s sign (inability to pinch the dorsum of the second toe with the thumb and the index finger), classical nonpitting signs, skin changes (hyperkeratosis and papillomatosis), skin turgor, and skin pitting [Citation22,Citation23]. Regions of lymphedema in the lower extremities were recorded based on the results of the physical exams. Volumetric difference of more than 5% was also regarded as symptomatic lymphedema when physical exam results were inconclusive. Patients had lymphedema either in proximal leg (medial thigh region) or in the whole leg (medial thigh region and below knee area including pretibial area, foot dorsum, and/or toe).
The severity and localization of lymphedema was also evaluated with volume measurement. Objective volumetric measurement of each leg was performed on every visit using an opto-electronic volumeter, perometer (Pero-System, Wupptertal, Germany) [Citation24–26]. Although the perometer is not yet a standard for volumetric measurement, it provides consistent measurements independently of the observers [Citation27]. To localize the manifestations of lymphedema in the lower extremities, upper leg volume (ULV) and lower leg volume (LLV) were recorded. ULV represents the proximal lower extremity volume, which includes the regions above the patella, the thigh, and below the inguinal area. LLV includes the areas from the patella to the ankle joint and represents the distal lower extremity volume. Whole leg volume (WLV) is the sum of ULV and LLV.
ULV, LLV, and WLV were compared separately to observe differences in regions of leg lymphedema during the follow-up periods. For comparison, percent difference of volume was used as follows:
Less than 10% volume difference in WLV was considered as mild lymphedema and 10% or more difference was considered as severe. International Society of Lymphology (ISL) defines 20% or less as mild and 40% or more as severe [Citation28]. The cut off for volume difference was decided differently from that of ISL because both physicians and patients at this cancer center are very highly attentive to secondary complications of cancer treatment and patients with lymphedema are managed appropriately with sensitivity.
Treatment and follow-up of lymphedema
All patients after immediate post-op care were referred to cancer rehabilitation specialist for initial screening of lymphedema. Patients without lymphedema at initial screening were followed-up in specialized oncology unit and were directly referred to the cancer rehabilitation specialist when any sign of swelling in extremities was noted or when patients complained of swelling. When a patient was diagnosed with lymphedema, the patient was also routinely followed up by cancer rehabilitation specialist for lymphedema assessment and treatment. The visit in which a patient was diagnosed to have lymphedema was considered as the initial point of this study.
All patients diagnosed with lymphedema had received comprehensive first-line therapy including size-appropriate medical compressive stockings and/or garments, lymphatic remedial exercise education in person and by video, and compliance education. Complex decongestive physical therapy was administered based on the severity of initial lymphedema and patient compliance. Patients with lymphedema were followed up at 3-month intervals up to 12–15 months with volumetric measurement. Patients were asked not to put on the compressive garments on the day of visits for proper volumetric measurement and physical examination.
Statistical analysis
Comparison between those diagnosed with lower extremity lymphedema and those without edema was performed. Pearson’s chi-square test was used to compare categorical variables. Continuous variables were tested with the independent sample t-test. The incidence of lower extremity lymphedema in both groups was calculated. The clinical manifestations of lymphedema in both groups were charted. Volumetric data of the two groups were compared using an independent sample t-test at each follow-up point. For all analyses, p < 0.05 was considered statistically significant. All analysis was performed using SPSS (SPSS version 29, IBM).
Results
A total of 104 patients had visited the outpatient clinic during the study time. None of the patients had been diagnosed with primary or secondary lymphedema before LND. A total of 81 patients received unilateral LND at the inguinal and/or iliac area. delineates the patient selection process and case categorization.
Of the 81 patients after lower extremity LND, 43 patients were diagnosed with lymphedema in the lower extremities (53%). Of the 94 patients after either upper or lower extremity LND, 45 patients (48%) developed lymphedema in either upper or lower extremities (). Prevalence of lymphedema is based on the physician’s evaluation of the patient.
Table 1. Description of patients with melanoma who received LND.
Mean duration to the first visit after surgery was 48 days for patients diagnosed with lower extremity lymphedema. Minimum duration was 11 days and maximum duration was 330 days. Three paid a delayed visit to the clinic (242, 203, and 330 days after surgery). These three patients reported that they had been disregarding their lower extremity edema for several months. After excluding these outlying values, the mean duration to first visit after surgery was 33 days ().
Comparison of the number of dissected lymph nodes and association with adjuvant radiotherapy or adjuvant systemic therapy
summarizes the comparison between those with or without lower extremity lymphedema. The mean number of dissected inguinal lymph nodes for patients with lymphedema was 11.35, while numbers in patients without lymphedema were significantly lower, with a mean of 6.75 (p < 0.001). The number of dissected iliac lymph nodes did not differ much between the two groups. The total number of dissected lymph nodes for the lymphedema group was on average 13.12, which was significantly higher than those without lymphedema, 8.05 (p < 0.001). Lymphedema was not associated with radiotherapy or adjuvant systemic therapy by chi-square tests. On , the total number of LND was 10.79 for the inguinal group and 19.5 for the ilioinguinal group (p < 0.001). Both groups were not associated with either radiotherapy or systemic therapy.
Table 2. Comparison between patients with or without lower extremity lymphedema.
Table 3. Comparison between the Inguinal group and ilioinguinal group.
Clinical manifestations of lower extremity lymphedema in the inguinal group and the ilioinguinal group
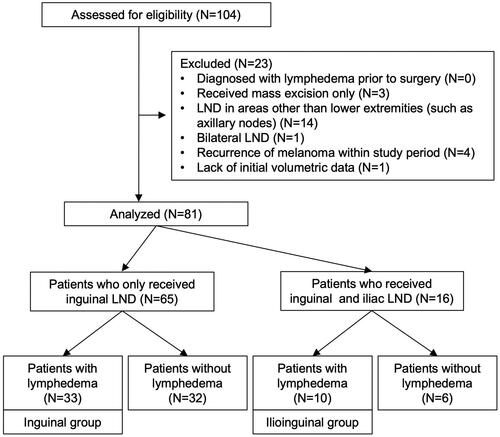
The incidence of lymphedema was 51% (33 patients) for the inguinal group and 62% (10 patients) for the ilioinguinal group (); 61% (20 patients) of the inguinal group manifested as medial thigh lymphedema and 39% (13 patients) presented as whole leg lymphedema. Only 40% (4 patients) of the ilioinguinal group manifested with medial thigh lymphedema, and 60% (6 patients) manifested as whole leg lymphedema ().
Figure 2. A Pie graph showing the incidences of lymphedema in patients who received inguinal LND only and those who received both inguinal and iliac LND.
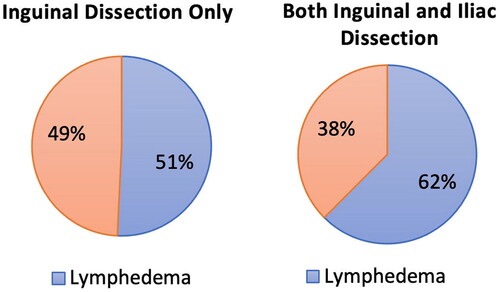
Figure 3. Clinical manifestations of lymphedema in the inguinal group and the ilioinguinal group. (A) Initial proportion of patients that presented with medial thigh lymphedema and whole leg lymphedema in each group. (B) Proportions of medial thigh lymphedema in the inguinal group throughout follow up. (C) Proportions of whole leg lymphedema in the ilioinguinal group throughout follow up.
Sixty-one percent of the inguinal group initially presented with lymphedema in the medial thigh region. At 3 months, 70% (14 patients) had medial thigh lymphedema. At 6 months, 67% (6 patients), and at the last follow-up, 64% of the inguinal group still had medial thigh lymphedema. The ilioinguinal group mostly manifested as whole leg edema. 60% and 63% of the ilioinguinal group had lymphedema in the whole leg initially and at 3 months follow-up. However, the proportion of whole leg edema generally decreased through the follow-up periods, and at 12–15 months follow-up, only 50% of patients had whole leg edema among the ilioinguinal group ().
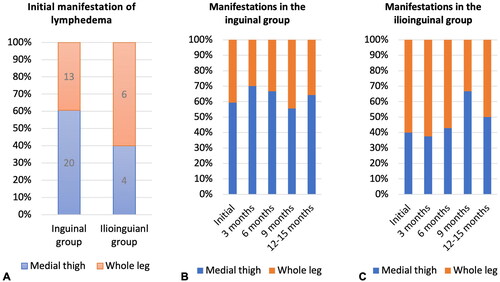
To assess severity of the lymphedema at the time of diagnosis, ULV was compared for those who presented with medial thigh lymphedema. In the inguinal group, only 16% (3 patients) had 10% or more difference in ULV, 42% (8 patients) with 5% to <10% difference, and 42% (8 patients) with <5% difference. For the ilioinguinal group, 50% (2 patients) had 10% or more difference, 50% (2 patients) had 5% to <10% difference, and none (0%) had <5% difference ().
Figure 4. Severity of lymphedema at time of diagnosis. (A) Severity of lymphedema in patients with medial thigh lymphedema. (B) Severity of lymphedema in patients with whole leg lymphedema.
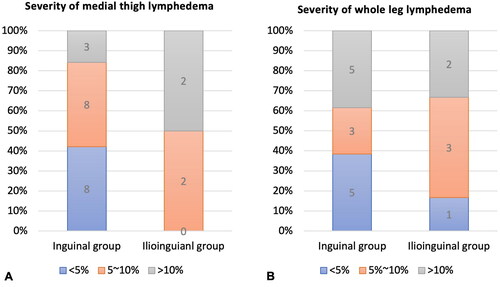
The severity of whole leg edema at the time of diagnosis was compared in two groups with WLV. In the inguinal group, 38% (5 patients) had 10% or more difference in WLV, 23% (3 patients) with 5% to <10% difference, and 38% (5 patients) with <5% difference. For the ilioinguinal group, 33% (2 patients) had 10% or more difference in WLV, 50% (3 patients) with 5% to <10% difference, and only 17% (1 patient) had <5% difference ().
Volumetric data analysis between the inguinal group and the ilioinguinal group
WLV, LLV, and ULV for the inguinal group and the ilioinguinal group were compared at each follow-up appointment. graphically displays the tends of volume changes throughout follow-ups. In the inguinal group, the slope of graphs for WLV, LLV, and ULV tends was slightly negative and fairly constant throughout. For the ilioinguinal group, WLV generally decreased until 9 months, but increased in 12–15 months. LLV initially was around 10% but the graph slopes downward until the last follow up. ULV also initially seemed to decrease until 9 months but increased in 12–15 months.
Figure 5. A linear graph presenting the Trends of percent differences of the volume between affected and unaffected lower extremity throughout follow-ups for both inguinal group and ilioinguinal group. Dashed line represents the inguinal group while solid line represents the ilioinguinal group. Asteroids signify statistically significant difference between two groups (p < 0.05). (A) Whole leg volume. (B) LLV. (C) ULV.
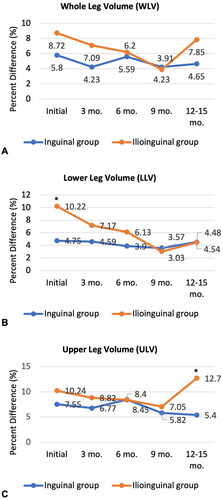
At the initial visit, LLV was statistically higher in the ilioinguinal group compared to the inguinal group (p < 0.05). However, ULV did not show significant differences. After 3, 6, and 9 months of lymphedema management, the difference between LLV and ULV was not significant. After 12–15 months of treatment, LLV was similar in both groups, but ULV was significantly higher in the ilioinguinal group (p < 0.05). Whole leg volume at 12–15 months was on average higher in the ilioinguinal group but failed to show statistical significance (p = 0.097).
Pearson’s chi-square test for association between the severity of edema and the two groups at 12-15 months was performed. Less than 10% volume difference in WLV was considered as mild and 10% or more difference was considered as severe. In the inguinal group, 11 patients were mild, and 2 patients were severe. For the ilioinguinal group, 2 patients were mild while 4 patients were severe. The chi-square test was statistically significant (p = 0.025). The ilioinguinal group was associated with more than 10% volume increase in the affected leg.
Discussion
Lower extremity lymphedema occurs very frequently and shortly after LND in patients with melanoma. In this study, more than half (53%) of the patients who received inguinal and/or iliac LND developed lower extremity lymphedema at an average of 33 days after LND. Considering that patients with breast cancer who underwent mastectomy and lymph node removal were diagnosed with lymphedema at a median of 12.6 months [Citation12], it is evident that lymphedema after lower extremity LND in patients with melanoma occurs rapidly after the iatrogenic assault.
Previous studies on risk factors for lymphedema in patients with melanoma after inguinal lymphadenectomy suggested that dissection of 10 or more lymph nodes can significantly increase the risk of lymphedema occurrence [Citation20]. The results of our study also demonstrate that patients with lymphedema had more lymph nodes dissected (11.35 nodes) compared to those without lymphedema (6.75 nodes).
Adjuvant radiotherapy and adjuvant systemic therapy, especially chemotherapy, are known risk factors for lymphedema in various types of cancer. Kim et al. demonstrated that postmastectomy radiotherapy increases the risk of arm lymphedema in patients with breast cancer [Citation12]. A study on patients with gynecological cancer (uterine and ovarian cancer) also identified postoperative radiotherapy as a risk factor for developing leg lymphedema [Citation10]. The results of this study seem to contradict previous results because lymphedema was not associated with radiotherapy and systemic therapy. A possible explanation is that the pathophysiology of leg lymphedema that develops after inguinal and/or iliac LND is more dependent on surgical disturbance of the anatomical structure of the lymphatic drainage system in the lower extremities. This hypothesis is further supported by the fact that the inguinal group and the ilioinguinal group displayed distinct volumetric changes during 12–15 months of follow-up. If adjuvant radiotherapy had more significant role than LND, the location of lymphedema would have not differed regardless of LND site because all eight patients with lymphedema (five from inguinal group and three from ilioinguinal group) received adjuvant radiotherapy on ilio-inguinal area.
In this study, the incidence of lymphedema was higher in the ilioinguinal group, which received both inguinal and iliac LND (62% vs. 51%). The mean number of dissected lymph nodes was 10 for the inguinal group and 20 for the ilioinguinal group. However, the higher incidence of lymphedema in the ilioinguinal group cannot be solely attributed to the larger number of removed lymph nodes. Previous studies have suggested that more than 10 removed nodes increase the risk of lymphedema [Citation20], but a linear relationship between the number of removed lymph nodes and the volume of the affected limb has not been established.
The inguinal group manifested mostly with lymphedema in the medial thigh rather than in the whole leg (). Initially, 61% patients presented with lymphedema in the medial thigh region. This pattern continued throughout the follow-ups (). This is an anatomically relevant clinical finding because the superficial lymphatics of the medial thigh region directly drain into the superficial inguinal lymph nodes. On the other hand, the ilioinguinal group mostly manifested as whole leg edema (). However, the proportion of whole leg edema generally decreased through the follow-up periods, and at 12–15 months follow-up, only 50% of patients had whole leg edema among the ilioinguinal group (). These results are in accordance with a previous study that indicates that lymphedema in the lower leg respond well to compression and decongestive therapies [Citation29].
On the first visit, LLV was significantly larger in the ilioinguinal group (p = 0.036) and ULV seemed to be higher in the ilioinguinal group but was not statistically significant (). This volumetric finding was expected because the ilioinguinal group presented with more whole leg edema clinically. This means that those who received inguinal and iliac LND had more severe lymphedema in the below knee-area compared to those who had only received inguinal LND. This could be a direct presentation of the anatomical structure of the inguinal and iliac lymph node system. Lymphatic fluid from the lower limb is mainly drained via the superficial inguinal lymph nodes, which in turn drains to the deep inguinal lymph nodes and external iliac lymph nodes. However, some of the lymphatic vessels from the anteromedial side of the lower leg are carried directly to the external iliac lymph nodes, which are the last gate keepers of lymphatic fluids from the leg. Thus, when the inguinal lymph nodes areas are dissected, the whole lower leg can swell, but when some of the vessels directly connected to iliac lymph nodes are still functional, below-knee edema may not be as severe. When the iliac lymph nodes are simultaneously removed, all possible drainage systems from the leg are disrupted, leading to whole leg edema.
The acute and more severe below-knee edema in the ilioinguinal group seems to respond well to conventional treatments. After 12-15 months of decongestive therapy and remedial exercise, the LLV was similar in both groups (). However, the ilioinguinal group still had a much larger ULV (ilioinguinal group ULV: 12.7%, inguinal group ULV: 5.4%, p < 0.01). Also, the ilioinguinal group was associated with more than 10% volume difference in the lower extremities (). This illustrated that receiving ilioinguinal LND in addition to inguinal LND increases the risk of treatment-resistant edema in the thigh region and more severe lymphedema.
As a post hoc analysis, the relationship between the number of dissected nodes (inguinal, iliac, and total) and leg volumes (LLV, ULV, and WLV) were explored with a linear regression model at each follow-up point. No statistically meaningful relationship was observed initially, at 3 months, 6 months, or 9 months follow-up. At 12–15 months follow-up, WLV displayed a positive and moderate correlation with the number of dissected inguinal nodes with an R-square value of 0.38. The observation of no initial correlation between the number of dissected nodes and volume of lymphedema may be due to individual differences in how the body responds to decreased lymphatic function. While some individuals may have less compensatory interstitial channels through the tissue third space which would take more time to compensate, others could have varying degrees of remaining collateral lymphatic vessels which could compensate for the loss rather quickly, so the volumetric measurement and the number of dissected nodes may not show a correlation initially. However, after decongestive therapy and a sufficient time interval for the body to compensate, the number of dissected inguinal nodes correlates moderately with the volume measurement of the whole limb.
In 2017, MSLT-II Clinical Trial concluded that LND after identification of positive sentinel node did not increase melanoma-specific survival among patients with melanoma and sentinel-node metastasis. Yet, LND may be helpful in providing prognostic information [Citation30]. However, more extensive LND is associated with development of more resistant and severe lymphedema. Thus, clinicians should contemplate about whether stringent staging provides more clinical value over patient’s quality of life.
This study utilized an objective measurement of lymphedema to study clinical manifestations following inguinal and/or iliac LND. Moreover, while many previous studies investigated lymphedema cross-sectionally, this study tried to describe lymphedema after LND in patients with melanoma at sequential time intervals and study how its clinical characteristics has changed throughout. However, not all patients were followed up strictly as in a cohort study. This was inevitable because most patients continued with anticancer melanoma treatment which requires periodic immunotherapy or radiotherapy. After receiving LND and establishment of an anticancer treatment plan, many patients preferred to be treated near home and were referred to other hospitals during routine anticancer treatment sessions. A Subgroup analysis with those who recurred within follow-up period could have yielded meaningful clinical data; however, after recurrence, too many patients missed the follow-ups on the cancer rehabilitation center. The data was too incomplete for analysis. The lymphedema staging according to ISL was not used in this study because the study population only included those who were not diagnosed with lymphedema prior to LND. Thus, lymphedema diagnosed at the initial study point were in the very early stage which correlates to Stage 1 of ISL classification. This was a single-center retrospective study, and the total number of cases were not very large despite reviewing 10 years of data. Because the overall incidence of melanoma in Korea is not as high as the western countries, a multicenter study could resolve this issue for further study of the clinical manifestations of lymphedema in patients with melanoma.
Conclusion
Iliac node dissection combined with inguinal LND for patients with melanoma results in a rather different patterns of lower extremity lymphedema as compared to those who only received inguinal node dissection. After both iliac and inguinal node dissection, distal lymphedema occurs more severely and acutely, presenting as whole leg lymphedema. With appropriate treatment, lymphedema in lower leg area may respond well to conventional comprehensive decongestive therapies; however, lymphedema around the thigh region is more resistant to treatment compared to lymphedema that occurs after inguinal dissection only. Clinical manifestations and prognosis of lower extremity lymphedema can vary depending on LND patterns. Physicians should consider the LND site when approaching secondary lymphedema that occurs following LND in patients with melanoma.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Raw data were generated at Samsung Medical Center. Derived data supporting the findings of this study are available from the corresponding author [JH] on request.
References
- Swetter SM, Thompson JA, Albertini MR, et al. NCCN guidelines(R) insights: melanoma: cutaneous, version 2.2021. J Natl Compr Canc Netw. 2021;19(4):364–376. doi: 10.6004/jnccn.2021.0018.
- Garbe C, Amaral T, Peris K, et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: diagnostics: update 2022. Eur J Cancer. 2022;170:236–255. doi: 10.1016/j.ejca.2022.03.008.
- Wright FC, Souter LH, Kellett S, et al. Primary excision margins, sentinel lymph node biopsy, and completion lymph node dissection in cutaneous melanoma: a clinical practice guideline. Curr Oncol. 2019;26(4):e541–e550. doi: 10.3747/co.26.4885.
- Pinto M, Marotta N, Caraco C, et al. Quality of life predictors in patients with melanoma: a machine learning approach. Front Oncol. 2022;12:843611. doi: 10.3389/fonc.2022.843611.
- Schadendorf D, van Akkooi ACJ, Berking C, et al. Melanoma. Lancet. 2018;392(10151):971–984. doi: 10.1016/S0140-6736(18)31559-9.
- Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66(4):271–289. doi: 10.3322/caac.21349.
- Bernas M, Thiadens SRJ, Stewart P, et al. Secondary lymphedema from cancer therapy. Clin Exp Metastasis. 2022;39(1):239–247. doi: 10.1007/s10585-021-10096-w.
- Ren Y, Kebede MA, Ogunleye AA, et al. Burden of lymphedema in long-term breast cancer survivors by race and age. Cancer. 2022;128(23):4119–4128. doi: 10.1002/cncr.34489.
- Roberson ML, Strassle PD, Fasehun LO, et al. Financial burden of lymphedema hospitalizations in the United States. JAMA Oncol. 2021;7(4):630–632. doi: 10.1001/jamaoncol.2020.7891.
- Tada H, Teramukai S, Fukushima M, et al. Risk factors for lower limb lymphedema after lymph node dissection in patients with ovarian and uterine carcinoma. BMC Cancer. 2009;9:47. doi: 10.1186/1471-2407-9-47.
- Biglia N, Zanfagnin V, Daniele A, et al. Lower body lymphedema in patients with gynecologic cancer. Anticancer Res. 2017;37(8):4005–4015.
- Kim N, Kim H, Hwang JH, et al. Longitudinal impact of postmastectomy radiotherapy on arm lymphedema in patients with breast cancer: an analysis of serial changes in arm volume measured by infrared optoelectronic volumetry. Radiother Oncol. 2021;158:167–174. doi: 10.1016/j.radonc.2021.02.033.
- Deban M, Vallance P, Jost E, et al. Higher rate of lymphedema with inguinal versus axillary complete lymph node dissection for melanoma: a potential target for immediate lymphatic reconstruction? Curr Oncol. 2022;29(8):5655–5663. doi: 10.3390/curroncol29080446.
- Friedman JF, Sunkara B, Jehnsen JS, et al. Risk factors associated with lymphedema after lymph node dissection in melanoma patients. Am J Surg. 2015;210(6):1178–1184; discussion 1184. doi: 10.1016/j.amjsurg.2015.08.014.
- Wrone DA, Tanabe KK, Cosimi AB, et al. Lymphedema after sentinel lymph node biopsy for cutaneous melanoma: a report of 5 cases. Arch Dermatol. 2000;136(4):511–514. doi: 10.1001/archderm.136.4.511.
- Parker C, Castro E, Fizazi K, et al. Prostate cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(9):1119–1134. doi: 10.1016/j.annonc.2020.06.011.
- Marth C, Landoni F, Mahner S, et al. Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv262. doi: 10.1093/annonc/mdy160.
- Colombo N, Sessa C, Du Bois A, et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Int J Gynecol Cancer. 2019;29(4):728–760. doi: 10.1136/ijgc-2019-000308.
- Chuang LT, Temin S, Camacho R, et al. Management and care of women with invasive cervical cancer: American society of clinical oncology resource-stratified clinical practice guideline. J Glob Oncol. 2016;2(5):311–340. doi: 10.1200/JGO.2016.003954.
- Chen T, Lin Y, Tan Q. Risk factors for lower extremity lymphedema after inguinal lymphadenectomy in melanoma patients: a retrospective cohort study. Surg Open Sci. 2022;8:33–39. doi: 10.1016/j.sopen.2022.02.001.
- Gjorup CA, Dahlstroem K, Hendel HW, et al. Factors associated with melanoma-related limb lymphoedema. Acta Oncol. 2021;60(6):779–784. doi: 10.1080/0284186X.2021.1905175.
- Kamijo E, Ishizuka K, Shikino K, et al. Physical findings and tests useful for differentiating lymphedema. J Gen Fam Med. 2021;22(4):227–228. doi: 10.1002/jgf2.425.
- Tiwari A, Cheng KS, Button M, et al. Differential diagnosis, investigation, and current treatment of lower limb lymphedema. Arch Surg. 2003;138(2):152–161. doi: 10.1001/archsurg.138.2.152.
- Armer JM, Hulett JM, Bernas M, et al. Best practice guidelines in assessment, risk reduction, management, and surveillance for post-breast cancer lymphedema. Curr Breast Cancer Rep. 2013;5(2):134–144. doi: 10.1007/s12609-013-0105-0.
- Campanholi LL, Baiocchi JMT, Batista BN, et al. Agreement between optoelectronic volumetry and circumferential girth measurements to diagnose lymphedema in patients submitted to axillary radical lymphadenectomy for treatment of cutaneous melanoma. Lymphat Res Biol. 2021;19(6):568–572. doi: 10.1089/lrb.2017.0081.
- Spillane AJ, Saw RP, Tucker M, et al. Defining lower limb lymphedema after inguinal or ilio-inguinal dissection in patients with melanoma using classification and regression tree analysis. Ann Surg. 2008;248(2):286–293. doi: 10.1097/SLA.0b013e31817ed7c3.
- Reza C, Norregaard S, Moffatt C, et al. Inter-observer and intra-observer variability in volume measurements of the lower extremity using perometer. Lymphat Res Biol. 2020;18(5):416–421. doi: 10.1089/lrb.2019.0063.
- International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2013 consensus document of the international society of lymphology. Lymphology. 2013;46(1):1–11.
- Kim YB, Hwang JH, Kim TW, et al. Would complex decongestive therapy reveal long term effect and lymphoscintigraphy predict the outcome of lower-limb lymphedema related to gynecologic cancer treatment? Gynecol Oncol. 2012;127(3):638–642. doi: 10.1016/j.ygyno.2012.09.015.
- Faries MB, Thompson JF, Cochran AJ, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376(23):2211–2222. doi: 10.1056/NEJMoa1613210.