?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Previous studies have shown that a large proportion of relapses in head-and neck squamous cell carcinoma (HNSCC) following radiotherapy (RT) occur in the pretreatment FDG-PET avid volume (GTV-PET). The aim of the current work was to see if this was valid also in an oropharynx squamous cell carcinoma (OPSCC) only population, and to compare the loco-regional relapse pattern between HPV positive and HPV negative patients.
Material and methods
Among 633 OPSCC patients treated between 2009 and 2017, 46 patients with known HPV (p16) status and isolated loco-regional relapse were included. Oncologists contoured relapse volumes (RV) on relapse scans (PET/CT, CT or MR), which were thereafter deformed to match the anatomy of the planning CTs. The point of origin (center of volume) of the deformed RVs were determined and analyzed in relation to the RT target volumes (GTV-PET, GTV and CTVs). The relapse pattern was compared between HPV positive and HPV negative patients using Fischer’s exact test.
Results
Sixty RVs were contoured in the 46 patients. 55% (95% CI 44–67%) of relapses originated in GTV-PET, while the other RT volumes harbored 12% (5–20%) (GTV), 18% (9–28%) (high risk CTV) and 5% (0–11%) (low risk CTV) of relapses. Six relapses were found outside the RT target volumes. No significant difference in relapse pattern between HPV positive and HPV negative patients was found (p = .95).
Conclusion
There were no signs of difference in loco-regional relapse pattern between HPV positive and HPV negative patients. In agreement with previous findings, GTV-PET was the most frequent RT target volume of relapse.
Background
Squamous cell carcinoma of the oropharynx (OPSCC) represents around one third of head-and-neck cancers [Citation1,Citation2]. The patient group is highly diverse regarding general condition and prognosis, with an increasing number of human papilloma virus (HPV) driven cancers [Citation3,Citation4]. Compared to other head and neck squamous cell carcinomas (HNSCC), HPV related OPSCC has a better prognosis and affects younger and often otherwise healthy patients [Citation5]. HPV status has been included as a factor in staging of OPSCC since 2016 [Citation6].
Most studies of the relapse pattern in HNSCC patients following primary radiotherapy state that the majority of relapses occur centrally, in the high-dose region [Citation7,Citation8]. Specifically, the volume with a high pretreatment uptake of 18 F-fluorodeoxyglucose (FDG) seems to harbor a large proportion of relapses [Citation9–11]. In these studies, however, few patients with OPSCC have been included.
Knowledge on the relapse pattern can help evaluate current treatment strategies, as well as guide studies of RT dose re-distribution, e.g., dose painting [Citation11,Citation12] or other strategies such as studies of de-escalation [Citation13]. Therefore, it is of interest to know if the previous findings – that most relapses occur in the FDG-PET avid volume [Citation9] – are valid also for OPSCC patients in general, and for HPV positive OPSCC patients in particular. It is known that HPV positive OPSCC is more radiosensitive than other HNSCC [Citation14], and that they differ in the distribution of M-site vs T/N-site relapses [Citation15]. The hypothesis of the current work was that the loco-regional relapse pattern in relation to pretreatment FDG-PET and radiotherapy target volumes differs between HPV positive and HPV negative patients.
Material and methods
Patients and treatment
Eligible patients were found among 633 patients treated for OPSCC with primary radiotherapy (RT) between 2009 and 2017 at our institution. The following criteria were required for inclusion:
Known HPV (p16) status
Isolated loco-regional relapse (LRR) between 3 months and 5 years after end of RT
Scan of relapse available (CT, PET/CT or MR)
Relapse verified by biopsy
Patients with isolated loco-regional relapse, i.e., with no simultaneous distant metastatic relapse, were identified. If a relapse occurred within 3 months after end of RT, it was classified as residual disease and excluded. If a relapse occurred later than 5 years, or if it was located in a new anatomical site, it was classified as a new primary tumor and was excluded.
Patients were treated with VMAT/IMRT to a total dose of 66 or 68 Gy according to DAHANCA guidelines, which are continuously updated [Citation16] (ref. to current version). Accelerated treatment with 2 Gy/fraction, 6 fractions/week was standard. All patients were offered the hypoxic sensitizer Nimorazole (1200 mg/m2 daily) and fit patients with T3–T4 or N + disease were also offered concurrent chemotherapy with weekly cisplatin 40 mg/m2. Patients were positioned with 5-point masks, and treated on linear accelerators Clinac, Novalis or TrueBeam (Varian Medial Systems, Palo Alto, CA). Daily cone-beam computed tomography was used for patient positioning as standard since 2010. After treatment, patients were evaluated clinically at 8 weeks and every 4 months during years 1–2 and biannually during years 3–5 until 2014 and every 6 months during years 1–2 and annually during years 3–5 after 2014. Clinical follow-up evaluations included a physical examination and endoscopic pharyngo-laryngoscopy.
Imaging and target definition
Delineation and treatment planning were performed based on pretreatment 18-fluoro-deoxy-glucose (FDG) PET/CT images with the patient immobilized in treatment position. The RT target volumes used in the current analysis were delineated as part of the primary treatment:
FDG-PET avid gross tumor volume (GTV-PET): Delineated by a nuclear medicine physician, based on visual assessment of malignant FDG uptake. Auto-contouring standard uptake value (SUV) thresholds could be used as a first estimation in this delineation process but would always be followed by editing by the specialist.
Gross tumor volume (GTV): Delineated by a radiologist and oncologist in collaboration. The GTV consisted of tumor tissue verified by imaging data and clinical examination, including the macroscopically involved lymph nodes.
Clinical target volume 1 (CTV1): Delineated by an oncologist. CTV1 formed a 10 mm margin to the GTV to account for uncertainties in tumor delineation. If the macroscopic tumor was >4 cm, the CTV1 received 68 Gy, otherwise it received 66 Gy.
CTV2: Delineated by an oncologist. The CTV2 encompassed CTV1 with a concentric margin of 2 mm and included involved lymph node levels and additional areas at high risk of subclinical spread. The prescription dose to CTV2 was 60 Gy. In some patients, the CTV2 was omitted.
CTV3: Delineated by an oncologist. CTV3 encompassed the CTV2 with an additional 2-mm margin and the low-risk regional elective lymph node regions (levels II-III bilaterally and level IV bilaterally in cases of level III involvement). The prescription dose to CTV3 was 50 Gy.
Imaging post-treatment was in the study period not prescribed in the follow-up guidelines but could be requested by the treating physician. Depending on site and purpose, the image modality could be either CT, PET/CT or MR. Generally, the use of post-treatment PET/CT increased during the study period [Citation17].
Relapse position analysis
Five oncologists (MB, AG, CAK, LS, JF) participated in the delineation of relapse volumes. Each patient’s relapse volume(s) was delineated in Eclipse Treatment Planning System (Varian Medical Systems, Palo Alto, CA) by one of the oncologists. All patient information, including medical records and images, were available to the oncologist. However – to avoid unintentional bias in the determination of position/extension of relapse volume – they were not allowed to look at the original target volume delineations.
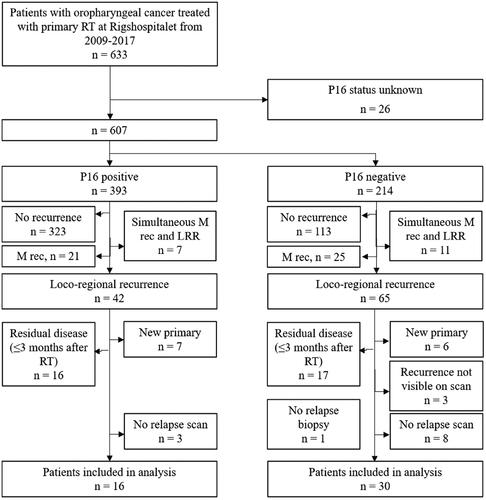
The relapse scans and relapse volume contours were exported to the Velocity software for deformation. The steps of a built-in function (Navigator) for structure deformation were followed. In short, an initial manual registration and a region of interest (ROI)-guided automatic rigid registration was followed by a deformable registration. The deformation algorithm was set to Extended Deformable Multipass, while MR corrected Deformable was used for MR images. The relapse volume contour was deformed based on the resulting deformation vector field and was transferred back to Eclipse ().
Figure 1. Illustration of the process for identifying the relapse point of origin (PO), defined as the center of volume: (a) RT target volume contours displayed on the planning CT: GTV-PET (cyan), GTV (red), CTV1 and CTV3 (pink), (b) T-site relapse volume (RV, purple contour) delineated on relapse scan, (c) relapse scan and RV deformed to the anatomy of the planning CT, and (d) the deformed relapse volume and corresponding PO (turquoise cross) copied onto the planning scan. As shown in (d), the point of origin for this relapse was inside GTV-PET.
The relapse volume point of origin (PO) was defined as the Center of volume of the relapse volume contour, following the nidus strategy of Due et al. [Citation18] and Zukauskaite et al. [Citation19]. The location of the relapse PO was determined in relation to the RT target structures (see section Imaging and target definition), assuming thereby that the relapse originated from the target structure in which the PO was found.
Statistics
The distribution of relapse PO locations (GTV-PET, GTV, CTV1, CTV2, CTV3 or outside), were compared between HPV positive and negative patients using Fisher’s exact test, with the null-hypothesis of equal distribution in the two groups. The number of relapses in a target volume was counted excluding the relapses in any inner target volumes incorporated. The relapse type (T or N site relapse) was similarly compared using Chi-squared test. In case of a significant overall difference in relapse location, it would be further investigated which RT target volume(s) was over- or under-represented in the groups.
For a test of trend, the RT target volumes were ordered from innermost to outermost with numbers 1–5. A spearman rank correlation coefficient was calculated based on the observed frequency of relapse POs found in the different RT target volumes. Confidence intervals were determined as the 2.5th and 97.5th percentile of the correlation coefficients calculated in 1000 bootstrap samples. Furthermore, the relapse density in each RT target volume (excluding any inner target volumes encompassed) was calculated:
Descriptive survival statistics were used to characterize the patient cohort. Median time to loco-regional relapse and 3-year disease-free survival (DFS) were calculated using the Kaplan-Meier method. Median potential follow-up time was calculated using the reverse Kaplan-Meier method [Citation20]. The cumulative incidence (absolute risks) of loco-regional relapse, distant metastasis and death with no evidence of disease (NED) were calculated using the competing risk method of Fine and Gray [Citation21].
Results
Of the 633 patients treated curatively for OPSCC in the study period, 62% were HPV positive and 34% HPV negative. HPV status was unknown for 26 patients (4%). The 3-year DFS was 77% for HPV positive and 36% for HPV negative patients (overall 3-year DFS 62%). The cumulative incidence of loco-regional relapse, distant metastasis and death NED is shown in supplemental Appendix Figure A1. With a median potential follow-up of 4.2 years (IQR 2.3–5.1), 42 (11%) of HPV positive and 65 (30%) of HPV negative patients had experienced an isolated loco-regional relapse. After excluding amongst other residual disease and new primary (details, see patient inclusion flowchart in ), 16 HPV positive and 30 HPV negative patients remained to be included in the relapse pattern analysis. In the analysis, the median time to isolated loco-regional relapse was 1.3 years (IQR 0.6–2.0) for HPV positive and 0.8 years (IQR 0.6–1.7) for HPV negative patients. Characteristics of the patients included, as well as those of the whole OPSCC cohort, are shown in . The relapse scans were 46% PET/CT, 37% CT and 17% MR, with no significant difference between HPV positive and negative patients (chi-squared test, p = .84).
Table 1. Patient characteristics of the HPV positive and negative OPSCC patients (n = 16 and 30, respectively) included in the analysis, as well as of the whole OPSCC patient cohort (n = 633).
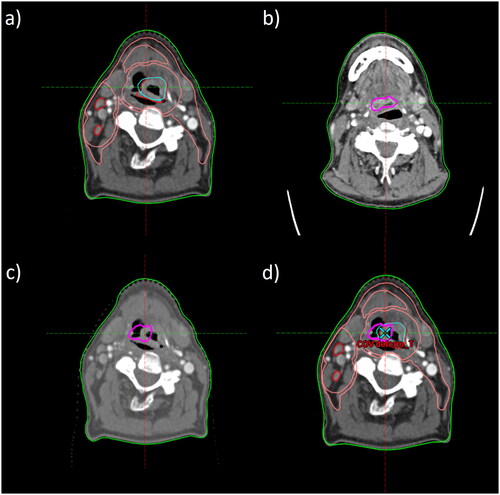
Sixty relapse volumes (21 HPV positive and 39 HPV negative) were found and analyzed in the 46 patients. The distribution of T-site and N-site relapses was approximately 48% vs 52% for HPV positive relapses and 62% vs 39% in HPV negative relapses, with no significant difference (). The distribution of relapse (PO) positions among HPV positive and HPV negative patients, as well as for all patients combined, are visualized in . The relapse pattern in relation to RT target volumes did not differ between HPV positive and HPV negative patients (p = .95) (). Most relapses − 55% – were found in GTV-PET, while the other RT target volumes harbored 12% (GTV), 18% (CTV1) and 5% (CTV3) of the relapses. Six relapses were found outside the target volumes and no relapses were found in CTV2. Of note, only 63% of patients had a CTV2 defined. The confidence intervals (CI) for relapse frequency in GTV-PET (44–67%) did not overlap with the CI:s of the other RT target volumes. The relapse frequency in GTV, CTV1, CTV3 and outside all had overlapping CI:s (). The trend of increased relapse density (relapses per cm3) for target volumes ordered from outer to inner was strong, with a correlation coefficient of −1.0 for more than 95% of bootstrap samples. The trend for relapse frequency was weaker, with corresponding correlation coefficients >0.3 (). The size of RT targets and relapse volumes are shown in supplemental Appendix Table A1.
Figure 3. Relapses visualized for the OPSCC patients included in the analysis; grouped by HPV status, as well as all patients combined. Each dot represents the point of origin of a T-site (red) or N-site (black) relapse. The relative areas of the circles correspond to the mean volume of the target volumes among the patients analyzed. CTV2 was omitted from the figure (only available in 63% of patients and harboring no relapses). Note that the positions of the ‘recurrence dots’ were randomly positioned within each target area.
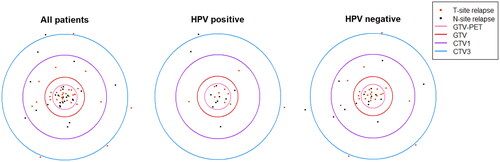
Figure 4. Distribution of relapse frequency [no. relapses] (a) and relapse density [no. relapses/cm3] (b) in the different RT target volumes.
![Figure 4. Distribution of relapse frequency [no. relapses] (a) and relapse density [no. relapses/cm3] (b) in the different RT target volumes.](/cms/asset/96ebc66e-9104-4e44-a8fe-6d39fbb77d58/ionc_a_2238889_f0004_b.jpg)
Table 2. Recurrence type and location for HPV +/− patients (frequency, percentage and 95% confidence interval).
Discussion
Our hypothesis of a different loco-regional relapse pattern in HPV positive patients was not supported by the data. On the contrary, the relapse pattern was very alike. Considering the low number of events in HPV positive OPSCC, we were not able to reach a sample size and statistical power to rule out small differences. However, with a p-value of 0.95 for the comparison, it seems unlikely that there would be an underlying clinically relevant difference between the groups. Radiobiological studies have shown that HPV related OPSCC is more radiosensitive than other HNSCC [Citation22]. That is in the current study reflected in the lower overall absolute risk of loco-regional relapse in the HPV positive group. The similarity in loco-regional relapse pattern between HPV positive and negative patients, however, suggests that within the tumor itself, similar mechanisms may determine the relative risk of relapse. Put in other words; if a relapse occurs, the likelihood of that relapse occurring in a certain RT target volume does not differ between HPV positive and HPV negative patients.
It was verified that the FDG-PET avid volumes harbor a large proportion of HNSCC relapses, also in an OPSCC-only population. We found 55% (CI: 44–67) of relapses in the FDG-PET avid volumes, which well matches the 54% (CI: 37–68) previously found in the mixed population of Due et al. [Citation9] When ordering the RT targets from outermost to innermost (outside, CTV3, CTV1, GTV and GTV-PET), it was clear that the relapse density, measured as relapses per cm3, significantly increased when moving in toward the center (). These results support the theoretical foundation for dose-painting strategies escalating the dose to FDG-PET avid sub volumes [Citation23,Citation24].
It has been shown that cisplatin cannot be omitted for HPV related OPSCC patients without a decrease in tumor control probability [Citation25]. Therefore, investigators have focused on other de-escalation strategies, including RT de-escalation [Citation13]. One such strategy was evaluated in Burr et al. [Citation26], where the irradiated volume was reduced for patients with HPV related OPSCC. Their observed failure pattern showed, similarly to our results, a majority of central relapses in the high-dose region. The present findings on the loco-regional relapse pattern in HPV related OPSCC may further contribute with empirical/theoretical foundation for the choice of RT dose de-escalation strategy in future studies. One should note, however, that persistent disease was not included in the current study, as the nidus strategy for relapse origin cannot be applied in that context.
The largest uncertainty in the current study is in the determination of the position of the PO. It includes uncertainty in the contouring of the relapse volume, as well as uncertainty in the deformation of the relapse scan/relapse volume to the anatomy of the planning CT. Based on previous studies, we estimate the uncertainty to be in the order of 5–10 mm for the PO [Citation9,Citation19], but with large variation between individual patients, as the influence of artifacts, tumor/relapse size and location, patient positioning differences etc. will influence both the contouring and deformation uncertainty. Unless there is a systematic difference in the uncertainty between HPV positive and HPV negative patients, this should not influence the results of the comparison of relapse location between the groups. In the analysis of relapse location, the uncertainty in PO position is unlikely to change the overall conclusion of a higher relapse density in the FDG-PET avid volume, as the probability of POs being placed in the smallest RT volume (GTV-PET) by chance is low.
In conclusion, the data do not suggest any difference in loco-regional relapse pattern in relation to pretreatment FDG-PET and RT target volumes between HPV positive and HPV negative OPSCC patients. In agreement with previous findings, the majority of relapses were found in the GTV-PET volume.
Supplemental Material
Download MS Word (186.1 KB)Supplemental Material
Download MS Word (16.1 KB)Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
Data not available due to ethical/legal restrictions.
Additional information
Funding
References
- DiGiulio S. Oropharyngeal cancer now most common head & neck cancer. Oncol Times. 2014;36(22):96–97. doi:10.1097/01.COT.0000457364.58827.33.
- Danish Head and Neck Cancer Group. Årsrapport 2020 for den kliniske kvalitetsdatabase DAHANCA. Danish Head and Neck Cancer Group (DK); 2020.
- Chaturvedi AK, Engels EA, Pfeiffer RM, et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J Clin Oncol. 2011;29(32):4294–4301. doi:10.1200/JCO.2011.36.4596.
- Zamani M, Grønhøj C, Jensen DH, et al. The current epidemic of HPV-associated oropharyngeal cancer an 18-year Danish population-based study with 2,169 patients the current epidemic of HPV-associated oropharyngeal cancer: an 18-year Danish population-based study with 2,169 patients. Eur J Cancer. 2020;134:52–59. doi:10.1016/j.ejca.2020.04.027.
- O’Sullivan B, Huang SH, Su J, et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 2016;17(4):440–451. doi:10.1016/S1470-2045(15)00560-4.
- Lydiatt WM, Patel SG, O’Sullivan B, et al. Head and neck cancers-major changes in the American Joint Committe on Cancer Eighth Edition Cancer Staging Manual. CA Cancer J Clin. 2017;67(2):122–137. doi:10.3322/caac.21389.
- Monika Studer G, Glanzmann C. Patterns of failure and toxicity after intensity-modulated radiotherapy for head and neck cancer: in regard to Schoenfeld et al. (Int J Radiat Oncol Biol Phys 2008;71:377–385). Int J Radiat Oncol Biol Phys. 2008;72(4):1271–1272. doi:10.1016/j.ijrobp.2008.07.022.
- Johansen S, Norman MH, Dale E, et al. Patterns of local-regional recurrence after conformal and intensity-modulated radiotherapy for head and neck cancer. Radiat Oncol. 2017;12(1):87. doi:10.1186/s13014-017-0829-5.
- Due AK, Vogelius IR, Aznar MC, et al. Recurrences after intensity modulated radiotherapy for head and neck squamous cell carcinoma more likely to originate from regions with high baseline [18F]-FDG uptake. Radiother Oncol. 2014;111(3):360–365. doi:10.1016/j.radonc.2014.06.001.
- Soto DE, Kessler ML, Piert M, et al. Correlation between pretreatment FDG-PET biological target volume and anatomical location of failure after radiation therapy for head & neck cancers. Radiother Oncol. 2008;89(1):13–18. doi:10.1016/j.radonc.2008.05.021.
- Mohamed ASR, Cardenas CE, Garden AS, et al. Patterns-of-failure guided biological target volume definition for head and neck cancer patients: FDG-PET and dosimetric analysis of dose escalation candidate subregions. Radiother Oncol. 2017;124(2):248–255. doi:10.1016/j.radonc.2017.07.017.
- Vogelius IR, Håkansson K, Due AK, et al. Failure-probability driven dose painting. Med Phys. 2013;40(8):081717. doi:10.1118/1.4816308.
- Patel RR, Ludmir EB, Augustyn A, et al. De-intensification of therapy in human papillomavirus associated oropharyngeal cancer: a systematic review of prospective trials. Oral Oncol. 2020;103:104608. doi:10.1016/j.oraloncology.2020.104608.
- Liu C, Mann D, Sinha UK, et al. The molecular mechanisms of increased radiosensitivity of HPV-positive oropharyngeal squamous cell carcinoma (OPSCC): an extensive review. J Otolaryngol Head Neck Surg. 2018;47(1):59. doi:10.1186/s40463-018-0302-y.
- Kjems J, Zukauskaite R, Johansen J, et al. Distant metastases in squamous cell carcinoma of the pharynx and larynx: a population-based DAHANCA study. Acta Oncol. 2021;60(11):1472–1480. doi:10.1080/0284186X.2021.1959056.
- Danish Head and Neck Cancer Group. Radiotherapy guidelines 2020. 2020. Available from: https://www.dahanca.dk/uploads/TilFagfolk/Guideline/GUID_DAHANCA_Radiotherapy_guidelines_2020.pdf
- Nøhr A, Gram SB, Charabi B, et al. PET/CT prior to salvage surgery in recurrent head and neck squamous cell carcinoma. Eur Arch Otorhinolaryngol. 2019;276(10):2895–2902. doi:10.1007/s00405-019-05550-1.
- Due AK, Korreman S, Bentzen SM, et al. Methodologies for localizing loco-regional hypopharyngeal carcinoma recurrences in relation to FDG-PET positive and clinical radiation therapy target volumes. Acta Oncol. 2010;49(7):984–990. doi:10.3109/0284186X.2010.498833.
- Zukauskaite R, Hansen CR, Brink C, et al. Analysis of CT-verified loco-regional recurrences after definitive IMRT for HNSCC using site of origin estimation methods analysis of CT-verified loco-regional recurrences after definitive IMRT for HNSCC using site of origin estimation methods. Acta Oncol. 2017;56(11):1554–1561. doi:10.1080/0284186X.2017.1346384.
- Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–346. doi:10.1016/0197-2456(96)00075-x.
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. doi:10.1080/01621459.1999.10474144.
- Bol V, Grégoire V. Biological basis for increased sensitivity to radiation therapy in HPV-positive head and neck cancers. Biomed Res Int. 2014;2014:696028. doi:10.1155/2014/696028.
- Berwouts D, Olteanu LAM, Duprez F, et al. Three-phase adaptive dose-painting-by-numbers for head-and-neck cancer: initial results of the phase I clinical trial. Radiother Oncol. 2013;107(3):310–316. doi:10.1016/j.radonc.2013.04.002.
- Rasmussen JH, Håkansson K, Vogelius IR, et al. Phase I trial of 18F-Fludeoxyglucose based radiation dose painting with concomitant cisplatin in head and neck cancer. Radiother Oncol. 2016;120(1):76–80. doi:10.1016/j.radonc.2016.03.005.
- Mehanna H, Robinson M, Hartley A, et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet. 2019;393(10166):51–60. doi:10.1016/S0140-6736(18)32752-1.
- Burr AR, Harari PM, Ko HC, et al. Reducing radiotherapy target volume expansion for patients with HPV-associated oropharyngeal cancer. Oral Oncol. 2019;92:52–56. doi:10.1016/j.oraloncology.2019.03.013.