Abstract
Purpose
Triplet chemotherapy might be more effective than doublet chemotherapy in metastatic colorectal cancer (mCRC), but it may also be marked by increased toxicity. To investigate whether δ-tocotrienol, a vitamin E analogue, with possible neuroprotective and anti-inflammatory effects, reduces the toxicity of triplet chemotherapy, we conducted a randomized, double-blind, placebo-controlled trial in mCRC patients receiving first-line 5-fluorouracil, oxaliplatin and irinotecan (FOLFOXIRI).
Material and Methods
Seventy patients with mCRC were randomly assigned (1:1) to receive FOLFOXIRI plus either δ-tocotrienol or placebo at the Department of Oncology, Vejle Hospital, Denmark. Eligibility criteria were adenocarcinoma in the colon or rectum, age 18–75 years and ECOG performance status 0–1. FOLFOXIRI was given in eight cycles followed by four cycles of 5-fluorouracil. δ-tocotrienol 300 mg or placebo × 3 daily was added during chemotherapy and for a maximum of two years. The primary endpoint was time to hospitalization or death during treatment with chemotherapy.
Results
Median time to first hospitalization or death was 3.7 months in the placebo group (95% CI 1.93-not reached (NR)), and was NR in the δ-tocotrienol group (95% CI 1.87-NR) with a hazard ratio of 0.70 (95% CI 0.36–1.36). Grade 3–4 toxicities were uncommon in both groups, except for neutropenia, which occurred in 19 patients (58%) in the placebo group and 17 patients (50%) in the δ-tocotrienol group. There were no grade 3 or 4 peripheral sensory neuropathy. In the placebo group, 24 patients (71%) had oxaliplatin dose reductions compared to 17 patients (47%) in the δ-tocotrienol group (p = 0.047).
Conclusion
The addition of δ-tocotrienol to FOLFOXIRI did not statistically significant prolong the time to first hospitalization or death compared to FOLFOXIRI plus placebo. Toxicity was manageable and not statistically different. There was a statistically significant difference in dose reductions of oxaliplatin pointing to a possible neuroprotective effect of δ-tocotrienol.
Introduction
Colorectal cancer is still the second leading course of cancer-related deaths in developed countries [Citation1]. The overall 5-year survival rate is 55–60% [Citation2]. The combination of two-drug chemotherapy, a fluoropyrimidine and either oxaliplatin or irinotecan, is the standard first-line treatment of unresectable, metastatic colorectal cancer (mCRC) [Citation3]. Treatment may be further refined using monoclonal antibodies and taking the purpose of treatment into consideration. Treatment goals may be cured after downsizing of metastases, prolonged survival and symptom control [Citation4].
Several studies have suggested exposure to all three types of chemotherapy. In a phase III study conducted by the Gruppo Oncologico Nord Ovest (GONO), treatment with 5-fluorouracil, oxaliplatin and irinotecan (FOLFOXIRI) showed improved response rate (RR), progression-free survival (PFS), and overall survival (OS) as compared to 5-fluorouracil and irinotecan (FOLFIRI) [Citation5]. Also, the toxicity was manageable. Another randomized controlled trial, from the Hellenic Oncology Research Group (HORG), comparing FOLFIRI and FOLFOXIRI, did not find FOLFOXIRI to be superior in either RR, PFS or OS [Citation6]. The OLIVIA study comparing bevacizumab in combination with modified FOLFOX-6 (5-fluorouracil and oxaliplatin) or FOLFOXIRI showed improved RR and PFS in the group receiving FOLFOXIRI plus bevacizumab [Citation7]. In the TRIBE study [Citation8], comparing the addition of bevacizumab to FOLFIRI and FOLFOXIRI, respectively, PFS was higher in the group treated with FOLFOXIRI, but there was no significant difference in the resection rate. Compared to trials on FOLFOXIRI without addition of biologically targeted treatment an increased number of grade 3 and 4 adverse events was observed when adding bevacizumab.
Triplet compared to doublet chemotherapy may prove more effective in mCRC, but it is marked by increased toxicity leading to hospitalization, dose reduction, postponement, and partly or full discontinuation of treatment [Citation6,Citation9]. Two studies comparing FOLFOXIRI and FOLFIRI both found an increased grade of peripheral neurotoxicity as well as the increased grade of neutropenia and diarrhea, respectively, when treated with FOLFOXIRI [Citation5,Citation6]. Additional measures to reduce the toxicity of triplet chemotherapy are required.
Tocotrienol is a derivative of vitamin E. The chemical structure of tocotrienol is formed by an aromatic chromanol ring and an isoprenoid chain containing three unsaturated methyl side chains [Citation10]. δ-tocotrienol, a vitamin E analogue, has been shown to affect angiogenesis and stimulate different pathways in in-vitro and in-vivo animal experiments [Citation11]. Other studies have shown that δ-tocotrienol has neuroprotective and anti-inflammatory effects [Citation12,Citation13], which might reduce the toxic effect of chemotherapy. A reduction in peripheral neuropathy, a well-known potentially chronic side effect of oxaliplatin occurring due to a wide range of molecular and cellular mechanisms [Citation14], could in theory be expected.
The clinical experience of δ-tocotrienol is sparse, but several studies are ongoing [Citation15]. To date, few trials have published data on δ-tocotrienol in humans with cancer, but the toxicity seems minimal or non-existing. In a non-randomized trial in breast cancer, the recurrence rate was reduced when combining tamoxifen and δ-tocotrienol, and there was no toxicity from δ-tocotrienol after five years of treatment [Citation16]. A phase I study used a very high dose of δ-tocotrienol (3.250 mg daily) without signs of toxicity [Citation17]. In a phase II study δ-tocotrienol was given in addition to bevacizumab in chemotherapy refractory recurrent ovarian cancer and suggested an additive effect with no increase in toxicity [Citation18].
To investigate whether δ-tocotrienol with its possible neuroprotective and anti-inflammatory effects reduces the toxicity of FOLFOXIRI, we conducted a phase II trial comparing FOLFOXIRI with and without δ-tocotrienol.
Material and methods
Trial design and study population
The trial was designed as a single center, randomized, double-blind, placebo-controlled phase II study at the Danish Colorectal Cancer Center South, Vejle Hospital, Denmark. Patients with potentially resectable or non-resectable mCRC eligible for first line treatment were enrolled at the Department of Oncology, Vejle Hospital, Denmark.
The inclusion criteria were age 18–75 years, ECOG performance status (PS) 0–1 (patients above 70 years were eligible if PS = 0), adenocarcinoma in the colon or rectum, evaluable but not necessarily measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 [Citation19] and adequate biochemistry. Previous adjuvant chemotherapy for radical treatment of stage II or III colorectal cancer was allowed in patients proven disease-free for more than 6 months. The detailed eligibility criteria are listed in the Supplementary Info File.
The study was approved by The Regional Committee on Health Research Ethics for Southern Denmark (S-20150185) and the Danish Medicines Agency (2015122439), and was prospectively registered with Clinicaltrials.gov (Identifier NCT02705300, 10/03/2016). The investigation was conducted in accordance with the Declaration of Helsinki and adhered to the guidelines for good clinical practice (GCP). All participating patients provided informed consent.
Study data were collected and managed using the Research Electronic Data Capture (REDCap) tool [Citation20,Citation21] hosted by Open Patient data Explorative Network (OPEN), Odense University Hospital, Region of Southern Denmark. Data were monitored by the regional GCP Unit.
Randomization
A randomization list (1:1) was generated using www.randomization.com and kept at the hospital pharmacy via the Clinical Research Unit of Department of Oncology, Vejle Hospital, each patient was assigned a unique code referring to the randomization list. The pharmacy would then allocate the patient to arm A or B. The treating staff and researchers were blinded to the allocation. In case of urgent need for unblinding, the unique codes were kept in sealed envelopes in a locked room in the Department of Oncology.
Treatment and procedures
FOLFOXIRI was given as the standard regimen developed by the GONO group [Citation22] and administered biweekly for eight cycles with irinotecan 165 mg/m2 i.v for 30 min, leucovorin 200 mg/m2 i.v. for 120 min, oxaliplatin 85 mg/m2 i.v. for 30 min followed by a continuous infusion of 5-fluorouracil 3200 mg/m2 i.v. for 46 h. After the eight cycles of FOLFOXIRI, treatment was continued with 5-fluoruracil and leucovorin biweekly for four cycles, equivalent to approximately six months of treatment.
Capsules of δ-tocotrienol 300 mg × 3 daily or placebo × 3 daily were administered for a maximum of two years. The tocotrienol capsule consisted of the isomers δ-tocotrienol (90%) and γ-tocotrienol (10%) while the placebo capsule contained olive oil. δ-tocotrienol and placebo were made by the same manufacturer (American River Nutrition, Inc., Hadley, MA, USA) and had the same appearance.
Dose delays and modifications of chemotherapy were permitted based on toxicity and done according to the summaries of product characteristics.
In patients needing an intervention such as surgery or hepatic radiofrequency ablation (RFA) of primary tumor or metastases during study treatment chemotherapy was suspended according to departmental guidelines and δ-tocotrienol/placebo paused 7 days before and after the intervention.
Treatment effect was evaluated by CT scan according to the RECIST 1.1 criteria every eight weeks during chemotherapy, then every three months for a year, every six months the following two years, and yearly the fourth and fifth year after end of chemotherapy. In case of progression, patients were treated according to departmental guidelines.
Blood sampling and biobanking
Before each treatment cycle, blood was sampled for complete blood count, liver function, electrolytes and renal function.
Plasma samples from all patients were collected at baseline, before every treatment cycle and at every follow-up visit for later translational research.
Outcome measures
The primary endpoint was ‘time to first non-planned hospitalization or death’ calculated from start of first cycle of FOLFOXIRI and seven months ahead in order to investigate if δ-tocotrienol reduced toxicity during the planned six months of chemotherapy. Secondary endpoints were number and duration of non-planned hospitalizations during chemotherapy; death during chemotherapy; RR, defined as the proportion of patients achieving partial or complete response according to RECIST 1.1; PFS; OS; radical resection rate; toxicity, and quality of life.
Toxicity assessment
Registration of adverse events was performed before treatment started, before each cycle, after the last cycle of chemotherapy and at every follow-up until progression. Adverse events were classified according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0 (CTCAE 4.0).
Quality of life assessment
Quality of life was evaluated by the European Organization for Research and Treatment of Cancer (EORTC) QLQ-C30 and EORTC QLQ-CR29 questionnaires before the start of treatment, at every response evaluation and follow-up visit.
Statistical analysis
In a previous study with FOLFOXIRI, around half of the patients died or had grade 3-4 toxicities usually requiring hospitalization [Citation5]. Using randomized phase II screening trial statistics [Citation23], we wanted to test if the time to first hospitalization or death could be reduced with a hazard ratio (HR) of 0.5 by adding δ-tocotrienol to FOLFOXIRI. With a power of 80% and a two-sided significance level of 5%, 70 patients should be included. An enrollment period of 36 months was planned.
Time to first hospitalization or death was calculated and reported according to the Kaplan–Meier estimator. PFS and OS were calculated from the day of enrollment as median survival and according to the Kaplan-Meier estimator. Kaplan-Meier curves illustrating survival and time to first hospitalization or death were compared by the log-rank test, and a simple Cox regression model was used to estimate HR with a corresponding 95% confidence interval (CI). The assumptions of proportional hazard were assessed by log-log plots, observed vs. estimated survival curves and the Schoenfeld residuals, and they were accepted. Non-parametric methods were used to calculate and compare the number of admissions, responses and toxicity. The EORTC QLQ-C30 was calculated as means with 95% CI and change in means from baseline to second response evaluation (week 16). The EORTC summary score was calculated as the mean of 13 of the QLQ-C30 scales (the Global Health Status and the Financial Impact scale is not included). Comparison of the two groups relied on the Student’s t-test.
Statistical calculations were performed using Stata/BE 17.0 (StataCorp LLC, TX, USA).
Results
Between 6 May 2016 and 19 December 2018, 70 patients with metastatic colorectal cancer were randomly assigned to receive FOLFOXIRI plus either δ-tocotrienol (n = 36) or placebo (n = 34). Follow-up is ongoing.
The data cutoff was 20 March 2023.
The median age was 64 years (range 40–75 years), 69% had ECOG PS 0, and 39% were female. Age, PS and gender were well balanced between the two arms. Of the total population, 67% had synchronous metastatic disease with 74% and 61% in the placebo and δ-tocotrienol group, respectively. Of note, BRAF mutations known to correlate with a negative prognosis tended to be overrepresented in the δ-tocotrienol group ().
Table 1. Baseline patient characteristics.
Patient compliance to δ-tocotrienol/placebo was monitored by the hospital pharmacy by counting the capsules returned. The actual intake of capsules in relation to the expected intake was counted by the study nurse at the Department of Oncology. Compliance data were available for 30 and 35 patients in the placebo and δ-tocotrienol groups, respectively. An intake of at least 70% of the prescribed capsules was documented for 87% and 80% of the patients in the placebo and δ-tocotrienol groups, respectively (p = 0.78).
Hospitalization and death
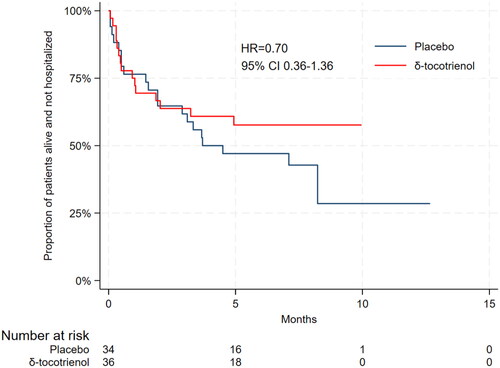
A total of 70 patients were eligible for analysis of the primary outcome. Median time to first hospitalization or death was 3.7 months in the placebo group (95% CI 1.93-not reached (NR)), and in the δ-tocotrienol it was NR (95% CI 1.87-NR) with a hazard ratio (HR) of 0.70 (95% CI 0.36–1.36) (). The proportion of patients who were hospitalized or died within seven months from the start of treatment was 57% for placebo vs 42% for δ-tocotrienol (p = 0.15).
Figure 1. Kaplan–Meier estimate of time to first hospitalization or death according to treatment group.
From the start of FOLFOXIRI to one month after the end of treatment, there were 32 hospitalizations in the placebo group and 25 in the δ-tocotrienol group. The mean number of hospitalizations was 0.94 and 0.69, respectively (p = 0.35). One patient in the placebo group represented eight of the 32 hospitalizations and in the δ-tocotrienol group, one patient represented six of the 25 hospitalizations. The median duration of hospitalizations was 5.8 days in the placebo group and 5.9 days in the δ-tocotrienol group (p = 0.92).
One of 34 patients (3%) in the placebo group and 2/36 (6%) in the δ-tocotrienol group died during chemotherapy. The patient in the placebo group died from cardiac arrest during the first 5-FU infusion. The autopsy did not clarify the cause of death further. The patients in the δ-tocotrienol group died from cardiac arrest and cerebral progressive disease, respectively. No autopsy was performed on these patients.
Adverse events
The median number of administered cycles was 12 in both groups. Dose modifications of FOLFOXIRI were done in 24 (71%) and 18 (51%) patients in the placebo and δ-tocotrienol groups, respectively (p = 0.103). Data regarding the postponement of treatment were available for 33 patients in the placebo group and 35 patients in the δ-tocotrienol group. In both groups, chemotherapy was postponed in 23 patients (p = 0.726). Data regarding adverse events were available for 69 patients. The most common grade 1 and 2 toxicities were nausea, diarrhea, peripheral sensory neuropathy, pain, fatigue, and anemia (). Grade 3-4 toxicities were uncommon in both groups, except for neutropenia, which occurred in 19 patients (58%) in the placebo group and in 17 patients (50%) in the δ-tocotrienol group. Four patients in each group had febrile neutropenia (11% vs. 12%, p = 0.86). There were no grade 3 or 4 peripheral sensory neuropathy in any of the groups. During treatment with FOLFOXIRI the dose of oxaliplatin was reduced in 24 (71%) and 17 (47%) patients in the placebo and δ-tocotrienol group, respectively (p = 0.047). The dose was reduced to 75% in 17 and nine patients. Seven patients in both groups had oxaliplatin discontinued. There was no statistically significant difference in the dose reduction of 5-FU or irinotecan between the groups. In general, the adverse events were manageable.
Table 2. Adverse events of special interest, according to treatment groups.
Efficacy
Sixty-five patients (93%) were evaluable for response. One patient died and one had major surgery before the first evaluation. Three patients did not have measurable disease. The response rate was 62% in the placebo group and 52% in the δ-tocotrienol group (p = 0.30).
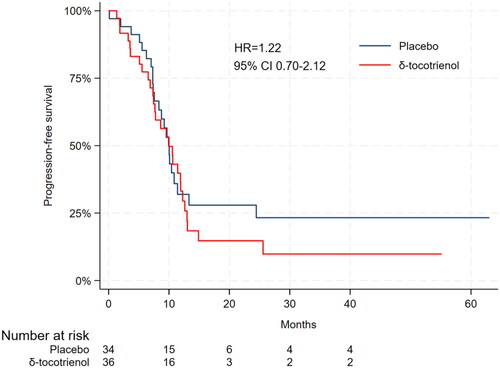
Disease progression occurred in 62 patients (89%) of which 30 (48%) were in the placebo group and 32 (52%) in the δ-tocotrienol group. The median PFS in the placebo group was 9.9 months (95% CI 7.6–11.4) compared to 10.0 months (95% CI 7.4–11.9) in the δ-tocotrienol group (HR 1.22, 95% CI 0.70–2.12) ().
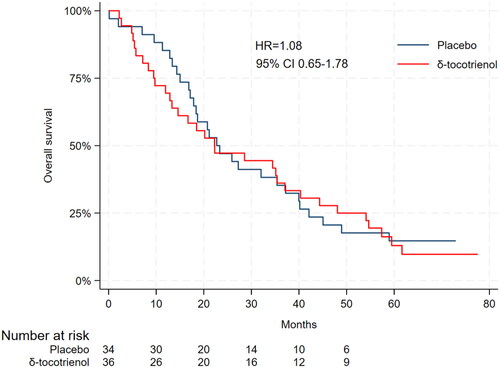
The median duration of follow-up was 64.7 months (95% CI 60.5–73.0). At the time of statistical analysis the median OS was 22.7 months (95% CI 17.1–37.2) in the placebo group and 22.3 months (95% CI 12.9–37.1) in the δ-tocotrienol group (HR = 1.08, 95% CI 0.65–1.78) (). The preliminary 4-year survival rate was 18% in the placebo group and 25% in the δ-tocotrienol group.
Radical resection rate
During FOLFOXIRI treatment 16 patients (23%) underwent radical surgery for metastases (R0-resection), i.e., 12 (35%) and four (11%) patients in the placebo and δ-tocotrienol group, respectively (p = 0.023). In 15/16 patients the primary tumor was also resected. Nine patients in the placebo group and three patients in the δ-tocotrienol group experienced relapse after surgery.
Quality of life
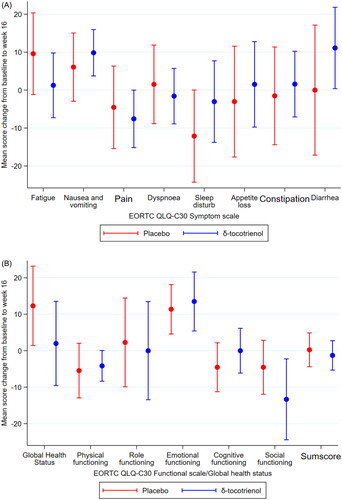
At baseline 66 of the 70 patients completed quality of life questionnaires, while 46 patients completed at week 16. Questionnaires from 41 patients were evaluable for analyses of changes from baseline to week 16. There was no significant differences in quality of life between the placebo and the δ-tocotrienol group in the subscores, nor was there any significant difference in the EORTC QLQ-C30 summary score between the two groups (). Within groups, a few significant differences was observed, i.e., improvement of sleep disturbance in the placebo group and worsening of nausea and vomiting in the δ-tocotrienol group.
Discussion
This randomized, double blind, placebo-controlled single center phase II study investigated if the addition of δ-tocotrienol to FOLFOXIRI would improve tolerability defined as the time to hospitalization or death. The combined endpoint of ‘hospitalization or death’ was chosen due to its robustness, valid registration, manageability, and clinical relevance.
We found a numerically reduced risk over time of hospitalization or death in patients treated with FOLFOXIRI plus δ-tocotrienol compared to placebo indicated by an HR of 0.70. This could be of clinical importance, although it was not statistically significant (95% CI 0.36-1.36) in this phase II trial powered to detect an HR of 0.50 or less. The possible anti-inflammatory effect of δ-tocotrienol did not lead to improved tolerability of chemotherapy in this study. One can argue if the daily dose of δ-tocotrienol was sufficient to reach the possible anti-inflammatory potential of the vitamin. The dose of δ-tocotrienol in this study was higher than the conventional and recommended use of vitamin E, but we found no specific toxicity related to δ-tocotrienol and it was well tolerated for up to two years of treatment. This is in agreement with the phase I study using a very high dose of δ-tocotrienol (3.250 mg daily) without signs of toxicity [Citation17].
Peripheral neuropathy rarely requires hospitalization and is most often handled in the outpatient clinic, which is supported by the CTCAE grading of 1-2 in our total population and patients were not hospitalized for this adverse event. Interestingly, δ-tocotrienol was associated with fewer dose reductions of oxaliplatin (p = 0.047). This supports the potential neuroprotective effect of δ-tocotrienol and motivates further studies. Neuroprotection in treatment with oxaliplatin has also been tested in other studies. A study tested n-3 polyunsaturated fatty acids compared to placebo and found, that 47% in the intervention group did not develop neuropathy compared to 11% in the placebo group (p = 0.002) [Citation24]. Another study investigated the neuroprotective potential in riluzole, a glutamate antagonist, compared to placebo and found that riluzole does not minimize neuropathy, but might even worsen it [Citation25]. A review from 2014 found, that neither of the drugs studied, i.e., chelating agents, antioxidants, trophic factors, or antiepileptic agents, had proven prevention of oxaliplatin-induced neuropathy [Citation26]. Although some studies suggest optimism regarding vitamin supplementation to chemotherapy, it is important to be cautious. A study of α-tocopherol and beta-carotene in male smokers, showed a higher incidence in lung cancer, in patients receiving beta-carotene [Citation27]. Vitamin supplementation can be harmful, why this approach should always be tested in trials.
The purpose of a phase II trial is to gain insight into a treatment and provide data for dimensioning a possible phase III trial. We suggest a future phase III trial with a minimal clinically important difference in hospitalizations and death of HR = 0.70 and a secondary endpoint of oxaliplatin induced neurotoxicity.
The observed fewer dose reductions of oxaliplatin in the δ-tocotrienol group did not translate into any difference in efficacy. Of note, the trial was not powered to detect difference in efficacy and all comparisons are prone to type II errors. There was no difference in median PFS; 9.9 months in the placebo group versus 10.0 months in the δ-tocotrienol group. The overall effect of triplet chemotherapy in our study is in agreement with the phase III trial from the Italian GONO group (9.8 months) [Citation5] and the phase III trial from the Greek HORG group (8.4 months) [Citation6]. There was no difference in median OS between the placebo and the δ-tocotrienol group (22.7 months (95% CI 17.1–37.2) and 22.3 months (95% CI 12.9-37.1), respectively). An antineoplastic or chemosensitizing effect of δ-tocotrienol was not found in this trial as opposed to γ-tocotrienol in in-vitro and in-vivo animal experiments [Citation28]. Median OS was also in agreement with the trials from the GONO and HORG groups.
Since 2010 several trials have been investigating the combination of FOLFOXIRI plus bevacizumab for metastatic, unresectable colorectal cancer [Citation29]. In the updated analysis of the TRIBE study [Citation30] comparing FOLFIRI plus bevacizumab and FOLFOXIRI plus bevacizumab, the FOLFIRI group had a PFS and OS of 9.7 and 25.8 months. FOLFOXIRI improved the survival with a PFS of 12.3 months and an OS of 29.8 months. Unfortunately, the effect of adding bevacizumab to FOLFOXIRI is not evaluable in their trial design, but some guidelines now suggest FOLFOXIRI plus bevacizumab as an option in first line for selective patients with good performance and without comorbidities [Citation31,Citation32].
A recent phase II study in chemotherapy refractory ovarian cancer combining δ-tocotrienol and bevacizumab [Citation18] showed higher PFS and OS compared to similar studies suggesting an additive angiogenic effect of δ-tocotrienol to bevacizumab. The angiogenic effect has been seen in in-vitro studies where it suppressed the vascular endothelial growth factor (VEGF) and inhibited proliferation of endothelial cells resulting in reduced tube formation [Citation33,Citation34]. Our group is currently exploring the potential synergism between δ-tocotrienol and bevacizumab in colorectal cancer (https://clinicaltrials.gov/ct2/show/NCT04245865).
Curatively intended resection of metastases and, if present, primary tumor was performed in 23% of patients, which is in agreement with phase II and III trials conducted by the GONO group [Citation35]. Remarkably, there was a statistically significant difference between the groups; 12 patients (35%) in the placebo group had potentially curative surgery compared to 4 patients (11%) in the δ-tocotrienol group (p = 0.023). However, the number of patients who underwent surgery is small and did not have an impact on the prognosis. The overrepresentation of BRAF mutations with negative prognostic effect in the intervention group may be of importance in interpreting these results.
The present study did not find any significant differences in quality of life between the two groups. However, it was only possible to compare changes in quality of life in approximately 60% of patients in both groups.
The strengths of this study include the fact that it is the first randomized, double-blind, placebo-controlled design to evaluate the potential effect of δ-tocotrienol as to increased tolerability of FOLFOXIRI. Also, the study explored the effects of δ-tocotrienol on treatment outcomes in colorectal cancer.
The small study population based on phase II statistics rendering findings preliminary is a limitation of the study, and the results are primarily hypothesis-generating. The reporting on peripheral neuropathy according to CTCAE 4.0 was made by physicians and there are no specific questions regarding neuropathy in the chosen EORTC QLQ questionnaires. In future trials, an objective specific endpoint testing peripheral neuropathy, i.e., the total neuropathy score-reduced (TNSr), may be considered [Citation36, Citation37]. Another limitation is the single-center setup reducing external validity. Also, adherence reporting regarding δ-tocotrienol/placebo intake was limited by patients failing to return unused capsules.
Conclusion
In this randomized, double-blind, placebo-controlled trial, we found no statistically significant difference between placebo and δ-tocotrienol in terms of time to first hospitalization or death when given as a supplement to FOLFOXIRI in patients with potentially resectable or non-resectable metastatic colorectal cancer in good performance. δ-tocotrienol 300 mg three times daily was well tolerated. There was a statistically significant difference in dose reductions of oxaliplatin pointing to a possible neuroprotective effect of δ-tocotrienol. However, given the limited sample size, these findings should be confirmed in larger trials.
Ethical approval
This study was performed in line with the principles of the 1964 Helsinki Declaration. Approval was granted by The Regional Committee of Health Research Ethics for Southern Denmark (S-20150185) and the Danish Medicines Agency (2015122439). Clinicaltrials.gov identifier NCT02705300, 10/03/2016.
Consent form
Informed consent was obtained from all participants included in the study.
Supplemental Material
Download MS Word (14.5 KB)Acknowledgements
We would like to acknowledge the support by OPEN, Open Patient data Explorative Network, Odense University Hospital, Region of Southern Denmark. Study data were collected and managed using REDCap electronic data capture tools hosted by OPEN.
We are thankful for the logistic work by study coordinator Monica Tronhjem. Finally, we express gratitude to Karin Larsen for linguistic editing of the manuscript.
Disclosure statement
No potential conflict of interest was reported by the authors.
Data availability statement
Data can be shared for transparency purposes if requested.
Additional information
Funding
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492.
- Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383(9927):1490–1502. doi: 10.1016/S0140-6736(13)61649-9.
- Venook AP, Niedzwiecki D, Lenz H, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer. JAMA. 2017;317(23):2392–2401. doi: 10.1001/jama.2017.7105.
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422. doi: 10.1093/annonc/mdw235.
- Falcone A, Ricci S, Brunetti I, et al. Phase III trial of infusional fluorouracil, leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) compared with infusional fluorouracil, leucovorin, and irinotecan (FOLFIRI) as first-line treatment for metastatic colorectal cancer: the gruppo oncologico nor. J Clin Oncol. 2007;25(13):1670–1676. doi: 10.1200/JCO.2006.09.0928.
- Souglakos J, Androulakis N, Syrigos K, et al. FOLFOXIRI (folinic acid, 5-fluorouracil, oxaliplatin and irinotecan) vs FOLFIRI (folinic acid, 5-fluorouracil and irinotecan) as first-line treatment in metastatic colorectal cancer (MCC): a: multicentre randomised phase III trial from the hellenic oncolog. Br J Cancer. 2006;94(6):798–805. doi: 10.1038/sj.bjc.6603011.
- Gruenberger T, Bridgewater J, Chau I, et al. Bevacizumab plus mFOLFOX-6 or FOLFOXIRI in patients with initially unresectable liver metastases from colorectal cancer: the OLIVIA multinational randomised phase II trial. Ann Oncol. 2015;26(4):702–708. doi: 10.1093/annonc/mdu580.
- Loupakis F, Cremolini C, Masi G, et al. Initial therapy with FOLFOXIRI and bevacizumab for metastatic colorectal cancer. N Engl J Med. 2014;371(17):1609–1618. doi: 10.1056/NEJMoa1403108.
- Maniadakis N, Pallis A, Fragoulakis V, et al. Economic analysis of a multicentre, randomised, phase III trial comparing FOLFOXIRI with FOLFIRI in patients with metastatic colorectal cancer in Greece. Curr Med Res Opin. 2007;23(9):2251–2257. doi: 10.1185/030079907X223765.
- Sen C, Khanna S, Rink C, et al. Tocotrienols: the emerging face of natural vitamin E. Vitam Horm. 2007;76:203–261.
- Husain K, Centeno B, Chen D-T, et al. Vitamin E δ-tocotrienol prolongs survival in the LSLKrasG12D/+; LSL-Trp53R172H/+;pdx-1-Cre (KPC) transgenic mouse model of pancreatic cancer. Cancer Prev Res (Phila). 2013;6(10):1074–1083. doi: 10.1158/1940-6207.CAPR-13-0157.
- Cardenas E, Ghosh R. Vitamin E: a dark horse at the crossroad of cancer management. Biochem Pharmacol. 2013;86(7):845–852. doi: 10.1016/j.bcp.2013.07.018.
- Aggarwal BB, Sundaram C, Prasad S, et al. Tocotrienols, the vitamin E of the 21st century: it’s potential against cancer and other chronic diseases. Biochem Pharmacol. 2010;80(11):1613–1631. doi: 10.1016/j.bcp.2010.07.043.
- Boyette-Davis JA, Hou S, Abdi S, et al. An updated understanding of the mechanisms involved in chemotherapy-induced neuropathy. Pain Manag. 2018;8(5):363–375. doi: 10.2217/pmt-2018-0020.
- Sailo BL, Banik K, Padmavathi G, et al. Tocotrienols: the promising analogues of vitamin E for cancer therapeutics. Pharmacol Res. 2018;130:259–272. doi: 10.1016/j.phrs.2018.02.017.
- Nesaretnam K, Selvaduray KR, Abdul Razak G, et al. Effectiveness of tocotrienol-rich fraction combined with tamoxifen in the management of women with early breast cancer: a pilot clinical trial. Breast Cancer Res. 2010;12(5):R81. http://breast-cancer-research.com/content/12/5/R81. doi: 10.1186/bcr2726.
- Springett GM, Husain K, Neuger A, et al. A phase I safety, pharmacokinetic, and pharmacodynamic presurgical trial of vitamin E δ-tocotrienol in patients with pancreatic ductal neoplasia. EBioMedicine [Internet]. 2015;2(12):1987–1995. doi: 10.1016/j.ebiom.2015.11.025.
- Thomsen CB, Andersen RF, Steffensen KD, et al. Delta tocotrienol in recurrent ovarian cancer. A phase II trial. Pharmacol Res. 2019;141:392–396. doi: 10.1016/j.phrs.2019.01.017.
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026.
- Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)-A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010.
- Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. doi: 10.1016/j.jbi.2019.103208.
- Masi G, Allegrini G, Cupini S, et al. First-line treatment of metastatic colorectal cancer with irinotecan, oxaliplatin and 5-fluorouracil/leucovorin (FOLFOXIRI): results of a phase II study with a simplified biweekly schedule. Ann Oncol. 2004;15(12):1766–1772. doi: 10.1093/annonc/mdh470.
- Rubinstein LV, Korn EL, Freidlin B, et al. Design issues of randomized phase II trials and a proposal for phase II screening trials. J Clin Oncol. 2005;23(28):7199–7206. doi: 10.1200/JCO.2005.01.149.
- Esfahani A, Somi M h, Ayromlou H, et al. The effect of n-3 polyunsaturated fatty acids on incidence and severity of oxaliplatin induced peripheral neuropathy: a randomized controlled trial. Biomark Res. 2016;4:13. doi: 10.1186/s40364-016-0066-3.
- Trinh T, Park SB, Murray J, et al. Neu-horizons: neuroprotection and therapeutic use of riluzole for the prevention of oxaliplatin-induced neuropathy—a randomised controlled trial. Support Care Cancer. 2021;29(2):1103–1110. doi: 10.1007/s00520-020-05591-x.
- Zedan AH, Hansen TF, Svenningsen ÅF, et al. Oxaliplatin-induced neuropathy in colorectal cancer: many questions with few answers. Clin Colorectal Cancer. 2014;13(2):73–80. doi: 10.1016/j.clcc.2013.11.004.
- O. Heinonen DA. The effect of vitamin E and beta carotene on the incidence of lung cancer and other cancers in male smokers. N Engl J Med. 1994;330(15):1029–1035. doi: 10.1056/NEJM199404143301501.
- Kunnumakkara AB, Sung B, Ravindran J, et al. γ-Tocotrienol inhibits pancreatic tumors and sensitizes them to gemcitabine treatment by modulating the inflammatory microenvironment. Cancer Res. 2010;70(21):8695–8705. doi: 10.1158/0008-5472.CAN-10-2318.
- Tomasello G, Petrelli F, Ghidini M, et al. FOLFOXIRI plus bevacizumab as conversion therapy for patients with initially unresectable metastatic colorectal cancer: a systematic review and pooled analysis. JAMA Oncol. 2017;3(7):e170278. doi: 10.1001/jamaoncol.2017.0278.
- Cremolini C, Loupakis F, Antoniotti C, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol. 2015;16(13):1306–1315. doi: 10.1016/S1470-2045(15)00122-9.
- Cervantes A, Adam R, Roselló S, et al. Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up ⋆. Ann Oncol. 2023;34(1):10–32. doi: 10.1016/j.annonc.2022.10.003.
- Colon Cancer Colon Cancer. NCCN Clin Pract Guidel Oncol (NCCN Guidel [Internet]. 2023;Version 1. Available from: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf.
- Inokuchi H, Hirokane H, Tsuzuki T, et al. Anti-angiogenic activity of tocotrienol. Biosci Biotechnol Biochem. 2003;67(7):1623–1627. doi: 10.1271/bbb.67.1623.
- Weng-Yew W, Selvaduray KR, Ming CH, et al. Suppression of tumor growth by palm tocotrienols via the attenuation of angiogenesis. Nutr Cancer. 2009;61(3):367–373. doi: 10.1080/01635580802582736.
- Masi G, Loupakis F, Pollina L, et al. Long-term outcome of initially unresectable metastatic colorectal cancer patients treated with 5-fluorouracil/leucovorin, oxaliplatin, and irinotecan (FOLFOXIRI) followed by radical surgery of metastases. Ann Surg. 2009;249(3):420–425. doi: 10.1097/SLA.0b013e31819a0486.
- Cornblath DR, Chaudhry V, Carter K, et al. Total neuropathy score: validation and reliability study. Neurology. 1999;53(8):1660–1664. doi: 10.1212/wnl.53.8.1660.
- Cavaletti G, Jann S, Pace A, et al. Multi-center assessment of the total neuropathy score for chemotherapy-induced peripheral neurotoxicity. J Peripher Nerv Syst. 2006;11(2):135–141. doi: 10.1111/j.1085-9489.2006.00078.x.