Abstract
Background
This study aimed to investigate the impact of adjuvant chemotherapy on long-term survival in unselected patients with high-risk stage II colon cancer including an analysis of each high-risk feature.
Materials and Methods
Data from the Danish Colorectal Cancer Group, the National Patient Registry and the Danish Pathology Registry from 2014 to 2018 were merged. Patients surviving > 90 days were included. High-risk features were defined as emergency presentation, including self-expanding metal stents (SEMS)/loop-ostomy as a bridge to resection, grade B or C anastomotic leakage, pT4 tumors, lymph node yield < 12 or signet cell carcinoma. Eligibility criteria for chemotherapy were age < 75 years, proficient MMR gene expression, and performance status ≤ 2. The primary outcome was 5-year overall survival. Secondary outcomes included the proportion of eligible patients allocated for adjuvant chemotherapy and the time to first administration.
Results
In total 939 of 3937 patients with stage II colon cancer had high-risk features, of whom 408 were eligible for chemotherapy. 201 (49.3%) patients received adjuvant chemotherapy, with a median time to first administration of 35 days after surgery. The crude 5-year overall survival was 84.9% in patients receiving adjuvant chemotherapy compared with 66.3% in patients not receiving chemotherapy, p < 0.001. This association corresponded to an absolute risk difference of 14%.
Conclusion
5-year overall survival was significantly higher in patients with high-risk stage II colon cancer treated with adjuvant chemotherapy compared with no chemotherapy. Adjuvant treatment was given to less than half of the patients who were eligible for it.
Keywords:
Introduction
Colorectal cancer is the third most frequent malignancy worldwide and the incidence rate has increased over the last decades. In 2020, almost 2 million new cases and around 900.000 deaths were confirmed [Citation1]. The quality of diagnostic techniques, screening procedures and surgical as well as oncological treatment have all drastically improved [Citation2], which is reflected in a steadily increasing long-term survival rate [Citation3]. Nevertheless, 30–40% of patients with colorectal cancer undergoing potentially curative surgery relapse and die from metastatic disease [Citation4–6].
Adjuvant chemotherapy has an established role in reducing the risk of recurrence in patients with stage III colon cancer, improving disease-free as well as overall survival [Citation7–9]. However, the same convincing results have not been demonstrated for patients with stage II colon cancer [Citation10,Citation11]. Current international and national guidelines only recommend adjuvant chemotherapy for certain patients with high-risk stage II colon cancer [Citation10,Citation12]. However, these recommendations are based on older studies from the 1990s, mainly demonstrating a non-significant benefit from adjuvant chemotherapy [Citation13,Citation14]. Since then, lymph node staging, preoperative imaging, and oncological treatment have improved. Surgical treatment has also been refined, with the widespread adoption of minimally invasive techniques following the principles of complete mesocolic excision and high tie ligation, which have also improved outcomes.
Newer studies underline the discordance in the role of adjuvant chemotherapy for patients with stage II colon cancer. In 2007, The QUASAR study concluded that patients diagnosed with stage II colon cancer had a borderline significant benefit from adjuvant chemotherapy in terms of overall survival [Citation4]. However, due to a lack of pathological data, they were not able to investigate the difference between high-and low-risk stage II patients. A more recent study from 2018 demonstrated a significant difference in the benefit of treating stage II colon cancer patients with adjuvant chemotherapy, dependent on the type and number of high-risk features [Citation15]. This suggests that high-risk features should be taken into consideration when recommending adjuvant chemotherapy to patients with stage II colon cancer. The estimated improvement in overall survival with adjuvant chemotherapy in these patients is between 3 and 5% [Citation4,Citation8]. Despite this, the role of adjuvant chemotherapy for patients with stage II colon cancer remains poorly defined [Citation2,Citation16,Citation17].
The aim of the present study was to investigate the impact of adjuvant chemotherapy on overall survival in patients with high-risk stage II colon cancer and further explore the adherence to national guidelines [Citation18].
Patients and methods
Study population and variables
This was a retrospective nationwide cohort study using data from the database of the Danish Colorectal Cancer Group (DCCG.dk), the National Patient Registry, and the Danish Pathology Registry. The DCCG.dk database includes > 97% of all Danish patients with colorectal cancer [Citation19]. Data sources were merged using the unique 10-digit personal identification code given to all Danish citizens, to investigate the effect of adjuvant chemotherapy on survival in patients with high-risk stage II colon cancer.
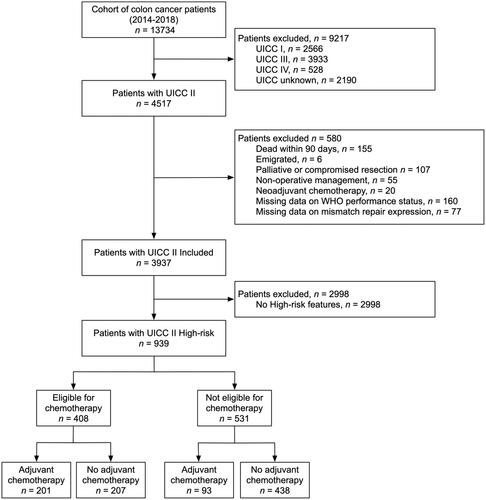
Patients, 18 years or older, with a first-time pathological diagnosis of stage II colon cancer between 2014 and 2018 were identified in the DCCG.dk database. Patients surviving > 90 days after curatively intended resection were included in analyses. Exclusion criteria included non-operative management, neoadjuvant chemotherapy, and missing data on mismatch repair (MMR) gene expression and WHO performance score (). Follow-up was commenced from the operation.
Primary outcome was overall survival. Secondary outcomes included overall survival for each high-risk feature, and adherence to the DCCG.dk guidelines with regard to eligibility for adjuvant chemotherapy, allocation of each high-risk feature for adjuvant chemotherapy, and time to first administration of adjuvant chemotherapy.
According to the DCCG.dk recommendations, patients < 75 years with proficient MMR gene expression and a performance status of ≤ 2 were eligible for adjuvant chemotherapy. Patients not fulfilling these criteria were designated as not eligible (Supplemental Figure 1). The recommended adjuvant chemotherapy regime was 6 months of fluorouracil as monotherapy.
The variable of interest, ‘high-risk’, was defined as the presence of any of the risk features outlined in the recommendations from the DCCG.dk on adjuvant chemotherapy in stage II colorectal cancer. These features include emergency presentation requiring treatment within 24 h, including self-expanding metal stents (SEMS)/loop-ostomy as a bridge to surgery, grade B or C anastomotic leakage [Citation20], pT4 tumors, lymph node yield <12, or signet cell carcinoma. Other explaining variables included age, gender, year of diagnosis, American Society of Anesthesiology (ASA) score, Charlson comorbidity index score (categorized in 0, 1, 2, and 3+) [Citation21], type of resection, and surgical approach.
Statistical analysis
Categorical variables are expressed as numbers and percentages, and continuous variables are expressed as medians and interquartile ranges. Differences between groups were investigated using the χ2 test or the Mann-Whitney test, accordingly.
Overall survival was examined using the Kaplan-Meier method and comparisons between patients receiving adjuvant chemotherapy or not were made using the Log rank test. Potential confounding was assessed using multivariable Cox regression analyses. All patients alive were censored at 5 years. Potential confounders used are listed in conjunction with the results. At least age, gender, and comorbidity were included in the final models. Age was included as a continuous variable. Schoenfeld residuals were used to verify the assumption of proportional hazards. An estimate of the adjusted absolute risk reduction was calculated by multiplying the crude mortality estimate in patients not receiving adjuvant chemotherapy (1-survival) by the adjusted hazard ratio, under the assumption of a constant hazard over time [Citation22]. All statistical analyses were 2-sided and p < 0.05 was considered statistically significant. Data were analyzed using SPSS version 28 (IBM Corp, Armonk, NY).
Results
A total of 4517 patients with stage II colon cancer were identified and after exclusion of 580 patients undergoing non-operative management, palliative surgery, or dying within 90 days, 3937 patients were included for initial analyses (). Of these, 939 (24%) patients were defined as ‘high-risk’. In general, these patients had a higher ASA class and poorer WHO performance status. They were also prone to undergo total colectomy or open surgery (Supplemental Table 1). A slight reduction in the proportion of patients with high-risk features was noted from 2014 to 2018. Crude overall long-term survival for patients surviving > 90 days after surgery was lower in patients with high-risk features (Supplemental Figure 2), which was confirmed after adjustment for age, gender, year of surgery, comorbidity, surgical procedure, surgical approach and MMR gene expression (hazard ratio (HR)= 1.82, 95% confidence interval (CI) 1.53–2.18, p < 0.001). We performed a similar plot for stage IIa vs. IIb-c however, the curves were very similar to (data not shown) and thus not reported.
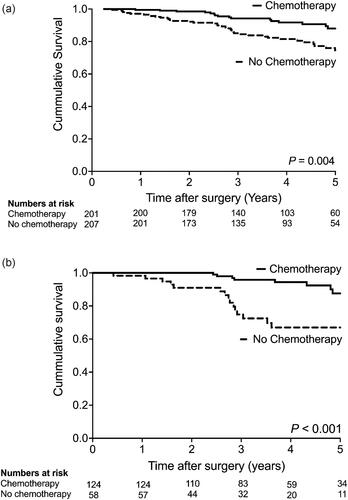
Figure 2. a. Kaplan-Meier survival curve of 408 eligible high-risk UICC stage II colon cancer patients. b. Kaplan-Meier survival curve of 182 eligible high-risk UICC stage II colon cancer patients with pT4 tumors.
The most abundant high-risk feature was pathological T4 stage (pT4) (59%) followed by an emergency presentation (29%), anastomotic leakage (17%), low lymph node yield (9%) and signet cell carcinoma (2%) (). There was no difference in crude overall survival between patients with the 3 most abundant high-risk features; emergency presentation, anastomotic leakage, or pT4 tumors, p = 0.163.
Table 1. The distribution of high-risk features in 939 patients with UICC stage II colon cancer alive 90 days after resection.
Of the 939 patients with high-risk stage II colon cancer, 408 (43.5%) were eligible for adjuvant chemotherapy. Of these, a total of 201 (49.3%) received chemotherapy. In contrast, chemotherapy was also given to 93 (17.5%) of the 531 patients who were not eligible according to national guidelines. As such, national guidelines were adhered to in 639 out of 939 patients (68.1%) and a total of 294 patients (31.3%) with high-risk stage II colon cancer received adjuvant chemotherapy. Details of the types of chemotherapy used are provided in . The median time to first administration was 35 days (IQR 28–43 days).
Table 3. Type of adjuvant chemotherapy in 939 patients with high-risk UICC stage II colon cancer.
The crude 5-year overall survival was 84.9% in all patients receiving adjuvant chemotherapy compared with 66.3% in patients not receiving chemotherapy (p < 0.001), which was confirmed after adjustment for age, gender, year of surgery, comorbidity, surgical procedure, surgical approach and MMR gene expression (HR = 0.57, 95% CI 0.39–0.83, p = 0.004).
The patients not eligible for chemotherapy had primarily pT4 tumors (78.5%) or multiple high-risk features (10.8%). Eligible patients receiving chemotherapy were slightly younger, predominantly female, and with less comorbidity (). There was a significant difference in adherence to the guideline for eligible patients depending on the specific high-risk feature. Only 10 of the 66 (15.2%) patients with anastomotic leakage as a single high-risk feature received adjuvant chemotherapy compared with 124 of 182 (68.1%) patients with pT4 tumors ().
Table 2. Differences in 408 eligible patients with high-risk UICC stage II colon cancer according to administration of adjuvant chemotherapy.
The crude overall 5-year survival in the 201 eligible patients receiving adjuvant chemotherapy was 88.0% compared with 76.0% in 207 patients not receiving chemotherapy, p = 0.004 (). This difference was confirmed after adjustment for age, gender, comorbidity, and high-risk features (HR = 0.45, 95% CI 0.22 to 0.82, p = 0.009), corresponding to an absolute risk difference in mortality of 14.2%. In a subgroup analysis of patients with pT4 tumors, patients receiving adjuvant chemotherapy had a greater reduction in long-term mortality than patients not receiving adjuvant chemotherapy. (), which was confirmed after adjusting for confounding (HR = 0.33, 95% CI 0.15–0.75, p = 0.008) and corresponding to an absolute risk reduction of 10.9% (Supplemental Table 3).
A total of 4 different chemotherapy regimens were used. Most patients received capecitabine as monotherapy (). In eligible patients, combination chemotherapy was primarily used for patients with pT4 tumors or with multiple high-risk features (Supplemental Table 4), while slightly more of the patients not eligible received combination chemotherapy.
Discussion
Patients with high-risk stage II colon cancer have inferior long-term survival compared to patients without risk factors. These results indicate that relative to observation alone, adjuvant chemotherapy was associated with increased overall survival in patients with high-risk stage II colon cancer undergoing surgery with curative intent and who were considered eligible for adjuvant chemotherapy. The survival benefit corresponded to an absolute risk difference in 5-year mortality of 14%, which is approximately 3 times greater than the reported results from the QUESAR study.
In patients with radical resected high-risk stage II colon cancer not treated with chemotherapy, Osterman et al. reported a 5 years recurrence rate of 23% compared to a recurrence rate of 16% for patients who did receive chemotherapy [Citation23]. In the current study, only data regarding overall survival was available. Whilst other previous studies have demonstrated improved survival with adjuvant chemotherapy in stage II disease, they have been limited by an inability to investigate the effect with respect to high-risk features [Citation4,Citation11,Citation24,Citation25]. As such, the most important question of which patients with which high-risk features stand to benefit most from adjuvant chemotherapy remained unanswered.
We aimed to stratify the effect of adjuvant chemotherapy for each high-risk feature. Unfortunately, this was only possible for pT4 tumors, as too few eligible patients with other high-risk features were treated with chemotherapy. There was a robust association between chemotherapy and increased survival in patients with pT4 disease. Kumar et al. likewise demonstrated increased 5-year overall survival in patients with high-risk stage II colon cancer treated with adjuvant chemotherapy compared with no chemotherapy (75.3% vs. 69.3%). This benefit was mainly seen in the subgroup of patients with pT4 tumors [Citation26]. Verhoeff et al. likewise reported a significantly increased 3-year survival in stage II patients with pT4 tumors treated with adjuvant chemotherapy (91% with adjuvant chemotherapy vs. 73% without chemotherapy). However, only 42% of the whole pT4 population received adjuvant chemotherapy in that study [Citation27]. In comparison, the results from the present study demonstrated that 62% of eligible patients with pT4 tumors received chemotherapy treatment (Table S1). As such, there seems to be an unfulfilled potential to further allocate patients with pT4 tumors for adjuvant chemotherapy. Based on the most recently published data from the Foxtrot study neo-adjuvant oxaliplatin-based chemotherapy may also be an option in patients with clinically staged high-risk colon cancer for example wise cT4 tumors [Citation28].
One explanation for the poor adherence to the Danish guideline could be the ongoing debate on the limited benefit of adjuvant chemotherapy in the stage II population [Citation29]. However, the specific reasons for the omission of chemotherapy in these patients, including patient choice after shared decision making, are not well understood and should be explored further. Even though we aimed to minimize bias, it can’t be excluded that the superior survival seen in patients treated with adjuvant chemotherapy was partly driven by selection favoring the choice of adjuvant chemotherapy in the fittest patients. Potential differences in short-term mortality were addressed by only including patients surviving at least 90 days after surgery, which minimized the impact of emergency procedures and anastomotic leakage on mortality. In addition, only patients younger than 75 years, with proficient MMR gene expression and a performance status ≤ 2 were considered eligible for adjuvant chemotherapy, minimizing but not excluding the risk of omitting chemotherapy in the elderly and comorbid patients in whom poorer survival would be expected.
The elderly population is very heterogenous ranging from the very frail patient to a fit patient. Consequently, a selection based on age alone could potentially deprive elderly fit patients of the potential benefit of adjuvant chemotherapy in high-risk stage II CRC. In daily clinical practice, fit elderly patients often receive adjuvant treatment and this could explain why 17.5% of non-chemo-eligible patients received chemotherapy (Table S2). The effect of adjuvant chemotherapy may differ by age group but there is no clear evidence for omitting adjuvant chemotherapy based on age alone [Citation4,Citation30,Citation31]. However, given that elderly patients tend to be omitted from randomized clinical studies, there is limited direct data to inform decision-making for this age group.
Presuming that the benefit from adjuvant chemotherapy is mainly dependent on reducing the risk of disease recurrence, the causality between each high-risk feature and the potential benefit from adjuvant chemotherapy is important. For pT4 tumors, this is biologically plausible as they have a greater risk of disease recurrence per se [Citation32]. For other high-risk features, the association may not be as convincing.
The investigation of the prognostic benefit of adjuvant chemotherapy for stage II colon cancer patients with emergency presentation is limited. Verhoeff et al. reported a non-significant benefit from adjuvant chemotherapy based on a low number of cases, the majority of whom presented with bowel obstruction [Citation27]. In the current study, the emergency presentation included both tumor perforation and obstruction, where only tumor perforation has a plausible biological explanation for an increased risk of recurrence [Citation33].
Regarding anastomotic leakage, the authors of the present study have previously demonstrated that adjuvant chemotherapy in stage III colon cancer in patients with anastomotic leakage increased overall survival because of a reduction in distant recurrence [Citation34]. However, the increased recurrence rate was mainly because of the omission of or delayed chemotherapy. As such, there does not seem to be substantial data favoring adjuvant chemotherapy in patients with stage II colon cancer and anastomotic leakage.
The prognostic value of low lymph node yield (<12 lymph nodes) is well examined. Babcock et al. reported that less than 12 lymph nodes examined were associated with a poor overall prognosis, which was not altered by the administration of adjuvant chemotherapy [Citation15]. However, subsequent studies have demonstrated that adjuvant chemotherapy increased overall survival in patients subjected to low lymph node yield [Citation25]. Since then, surgery has improved dramatically because of centralization and the introduction of the concept of complete mesocolic excision, leaving low lymph node yield a rarity that primarily should be handled by ensuring proper surgery.
Signet-ring cell carcinoma has been associated with a substantially poorer prognosis [Citation35]. The frequency of signet-ring cell carcinoma is rare and often combined with more advanced tumor stages. Given the low number of patients with this histological subtype in the current study, it is not possible to draw any significant conclusions as to the degree of benefit of adjuvant chemotherapy in this subgroup. Other biological features such as lymphovascular invasion (LVI), venous- and perineural invasion, tumor budding, and high histological grade are no longer considered high-risk features in the Danish national guidelines, unlike ASCO and ESMO, and were therefore not investigated here. There is however evidence that these features are associated with more unfavorable biology. For instance, LVI was recently associated with a 30–40% risk of lymph node metastasis in pT1 colorectal cancer [Citation36].
Iveson et al. investigated the impact of the number of high-risk features on disease-free survival and reported that patients with two or more high-risk features had significantly lower disease-free survival compared to patients with only one high-risk feature [Citation37]. Due to a lack of statistical power, we were not able to investigate the additional effect of several risk features.
An alternative and promising tool to identify high-risk patients is the use of circulating tumor DNA (ctDNA) in blood. Tie et al. investigated a ctDNA-guided approach to the treatment of stage II colon cancer. Not only was the absence of ctDNA after surgery associated with lower risks of recurrence, but the use of a ctDNA-guided approach for patient selection for adjuvant therapy was associated with a reduced number of patients being treated with chemotherapy without compromising recurrence-free survival [Citation38].
There are several limitations to this study. Most importantly, the retrospective design increased the risk of selection bias as discussed previously. In addition, eligible and non-eligible patients receiving adjuvant chemotherapy were younger and with less comorbidity. This difference was in part counteracted by adjustment for age, gender, and comorbidity. Data on disease recurrence was not available and as such we were not able to examine disease-free survival. The size of the cohort was relatively small, mainly because of the strict eligibility criteria, which resulted in limited statistical power. Finally, data regarding the number of cycles of chemotherapy received by each patient was not available.
In conclusion, adjuvant chemotherapy was significantly associated with an increased 5-year overall survival in patients with high-risk stage II colon cancer. The survival difference was higher than the difference reported in previous randomized trials. This may be explained by treatment with adjuvant chemotherapy; however, some degree of selection bias cannot be ruled out. Adherence to the Danish national guidelines was relatively low in the group of chemo-eligible patients, which might be driven by patient preferences. Novel prognostic tools such as ctDNA, may be able to identify which patients with stage II colon cancer benefit the most from adjuvant chemotherapy.
Supplemental Material
Download MS Word (13.5 KB)Supplemental Material
Download MS Word (14.1 KB)Supplemental Material
Download MS Word (16.2 KB)Supplemental Material
Download MS Word (16.3 KB)Supplemental Material
Download MS Word (60.6 KB)Supplemental Material
Download MS Word (109.9 KB)Disclosure statement
No potential conflict of interest was reported by the author(s). There are no disclaimers such as conflicts of interest, use of off-label or unapproved drugs or products, or use of previously copyrighted material. There is no grant of support or financial relationship for this project.
Data availability statement
Due to the nature of the research and due to ethical and legal restrictions, data is not available.
References
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/CAAC.21660.
- Pahlman LA, Hohenberger WM, Matzel K, et al. Should the benefit of adjuvant chemotherapy in colon cancer be re-evaluated? J Clin Oncol. 2016;34(12):1297–1299. doi: 10.1200/JCO.2015.65.3048.
- Wong MCS, Huang J, Lok V, et al. Differences in incidence and mortality trends of colorectal cancer worldwide based on sex, age, and anatomic location. Clin Gastroenterol Hepatol. 2021;19(5):955–966.e61. doi: 10.1016/J.CGH.2020.02.026.
- QUASAR Collaborative Group. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet. 2007;370(9604):2020–2029. doi: 10.1016/S0140-6736(07)61866-2.
- Jeffery M, Hickey BE, Hider PN, et al. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2016;11(11):CD002200. doi: 10.1002/14651858.CD002200.PUB2/MEDIA/CDSR/CD002200/REL0002/CD002200/IMAGE_N/NCD002200-CMP-009-01.PNG.
- Sargent DJ, Wieand HS, Haller DG, et al. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23(34):8664–8670. doi: 10.1200/JCO.2005.01.6071.
- Arkenau HT, Bermann A, Rettig K, et al. 5-Fluorouracil plus leucovorin is an effective adjuvant chemotherapy in curatively resected stage III colon cancer: long-term follow-up results of the adjCCA-01 trial. Ann Oncol. 2003;14(3):395–399. doi: 10.1093/ANNONC/MDG100.
- André T, De Gramont A, Vernerey D, et al. Adjuvant fluorouracil, leucovorin, and oxaliplatin in stage II to III colon cancer: updated 10-year survival and outcomes according to BRAF mutation and mismatch repair status of the MOSAIC study. J Clin Oncol. 2015;33(35):4176–4187. doi: 10.1200/JCO.2015.63.4238.
- Haller DG, Tabernero J, Maroun J, et al. Capecitabine plus oxaliplatin compared with fluorouracil and folinic acid as adjuvant therapy for stage III colon cancer. J Clin Oncol. 2011;29(11):1465–1471. doi: 10.1200/JCO.2010.33.6297.
- Baxter NN, Kennedy EB, Bergsland E, et al. Adjuvant therapy for stage II colon cancer: ASCO guideline update. J Clin Oncol. 2022;40(8):892–910. doi: 10.1200/JCO.21.02538.
- Figueredo A, Coombes ME, Mukherjee S. Adjuvant therapy for completely resected stage II colon cancer. Cochrane Database Syst Rev. 2008;2008(3):CD005390. doi: 10.1002/14651858.CD005390.PUB2.
- Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–1422. doi: 10.1093/ANNONC/MDW235.
- Moertel CG, Fleming TR, Macdonald JS, et al. Intergroup study of fluorouracil plus levamisole as adjuvant therapy for stage II/dukes’ B2 colon cancer. J Clin Oncol. 1995;13(12):2936–2943. doi: 10.1200/JCO.1995.13.12.2936.
- Erlichman C, O’Connell M, Kahn M, et al. Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International multicentre pooled analysis of B2 colon cancer trials (IMPACT B2) investigators. J Clin Oncol. 1999;17(5):1356–1363. doi: 10.1200/jco.1999.17.5.1356.
- Babcock BD, Aljehani MA, Jabo B, et al. High-risk stage II colon cancer: not all risks are created equal. Ann Surg Oncol. 2018;25(7):1980–1985. doi: 10.1245/S10434-018-6484-8.
- Kannarkatt J, Joseph J, Kurniali PC, et al. Adjuvant chemotherapy for stage II colon cancer: a clinical dilemma. J Oncol Pract. 2017;13(4):233–241. doi: 10.1200/JOP.2016.017210.
- Lee JJ, Chu E. Adjuvant chemotherapy for stage II colon cancer: the debate goes On. J Oncol Pract. 2017;13(4):245–246. doi: 10.1200/JOP.2017.022178.
- Klinisk Retningslinje │Kraeft DCCG. 2021. Available from: https://dccg.dk/wp-content/uploads/2021/01/DCCG_Adj-KRC_st_II_AdmGodk220121.pdf
- Ingeholm P, Gögenur I, Iversen LH. Danish colorectal cancer group database. Clin Epidemiol. 2016;8:465–468. doi: 10.2147/CLEP.S99481.
- Rahbari NN, Weitz J, Hohenberger W, et al. Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the international study group of rectal cancer. Surgery. 2010;147(3):339–351. doi: 10.1016/J.SURG.2009.10.012.
- Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8.
- Fisher S, Bennett C, Hennessy D, et al. Comparison of mortality hazard ratios associated with health behaviours in Canada and the United States: a population-based linked health survey study. BMC Public Health. 2022;22(1):478. doi: 10.1186/S12889-022-12849-Y/FIGURES/2.
- Osterman E, Hammarström K, Imam I, et al. Completeness and accuracy of the registration of recurrences in the Swedish colorectal cancer registry (SCRCR) and an update of recurrence risk in colon cancer. Acta Oncol. 2021;60(7):842–849. doi: 10.1080/0284186X.2021.1896033.
- Wilkinson NW, Yothers G, Lopa S, et al. Long-term survival results of surgery alone versus surgery plus 5-fluorouracil and leucovorin for stage II and stage III colon cancer: pooled analysis of NSABP C-01 through C-05. A baseline from which to compare modern adjuvant trials. Ann Surg Oncol. 2010;17(4):959–966. doi: 10.1245/S10434-009-0881-Y.
- Casadaban L, Rauscher G, Aklilu M, et al. Adjuvant chemotherapy is associated with improved survival in patients with stage II colon cancer. Cancer. 2016;122(21):3277–3287. doi: 10.1002/CNCR.30181.
- Kumar A, Kennecke HF, Renouf DJ, et al. Adjuvant chemotherapy use and outcomes of patients with high-risk versus low-risk stage II colon cancer. Cancer. 2015;121(4):527–534. doi: 10.1002/CNCR.29072.
- Verhoeff SR, Van Erning FN, Lemmens VEPP, et al. Adjuvant chemotherapy is not associated with improved survival for all high-risk factors in stage II colon cancer. Int J Cancer. 2016;139(1):187–193. doi: 10.1002/IJC.30053.
- Morton D, Seymour M, Magill L, et al. Preoperative chemotherapy for operable colon cancer: mature results of an international randomized controlled trial. J Clin Oncol. 2023;41(8):1541–1552. doi: 10.1200/JCO.22.
- Sargent D, Sobrero A, Grothey A, et al. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2009;27(6):872–877. doi: 10.1200/JCO.2008.19.5362.
- Sargent DJ, Goldberg RM, Jacobson SD, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345(15):1091–1097. doi: 10.1056/NEJMOA010957.
- Teufel A, Gerken M, Hartl J, et al. Benefit of adjuvant chemotherapy in patients with T4 UICC II colon cancer. BMC Cancer. 2015;15(1):419. doi: 10.1186/S12885-015-1404-9.
- Gunderson LL, Jessup JM, Sargent DJ, et al. Revised TN categorization for colon cancer based on national survival outcomes data. J Clin Oncol. 2010;28(2):264–271. doi: 10.1200/JCO.2009.24.0952.
- Belt EJT, Stockmann HBAC, Abis GSA, et al. Peri-operative bowel perforation in early stage colon cancer is associated with an adverse oncological outcome. J Gastrointest Surg. 2012;16(12):2260–2266. doi: 10.1007/S11605-012-2053-9.
- Krarup PM, Nordholm-Carstensen A, Jorgensen LN, et al. Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann Surg. 2014;259(5):930–938. doi: 10.1097/SLA.0B013E3182A6F2FC.
- Nitsche U, Zimmermann A, Späth C, et al. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann Surg. 2013;258(5):775–783. doi: 10.1097/SLA.0B013E3182A69F7E.
- Ronnow CF, Arthursson V, Toth E, et al. Lymphovascular infiltration, not depth of invasion, is the critical risk factor of metastases in early colorectal cancer: retrospective population-based cohort study on prospectively collected data, including validation. Ann Surg. 2022;275(1):E148–E154. doi: 10.1097/SLA.0000000000003854.
- Iveson TJ, Sobrero AF, Yoshino T, et al. Duration of adjuvant doublet chemotherapy (3 or 6 months) in patients with high-risk stage II colorectal cancer. J Clin Oncol. 2021;39(6):631–641. doi: 10.1200/JCO.20.01330.
- Tie J, Cohen JD, Lahouel K, et al. Circulating tumor DNA analysis guiding adjuvant therapy in stage II colon cancer. N Engl J Med. 2022;386(24):2261–2272. doi: 10.1056/NEJMOA2200075.