Abstract
Background
Pelvic insufficiency fractures (PIFs) are a late complication of radiotherapy for pelvic malignancies. We evaluated the incidence, radiologic findings, clinical course, and outcome of PIFs in patients treated with neoadjuvant (chemo)radiotherapy ((C)RT) for rectal cancer.
Material and methods
Data of patients diagnosed with rectal cancer from a large teaching hospital treated from 2002 to 2012 were extracted from the Dutch Cancer Registry. All hospital records were reviewed for the diagnosis of PIFs or pelvic bone metastases. An expert radiologist reassessed all imaging procedures of the lower back, abdomen, and pelvis.
Results
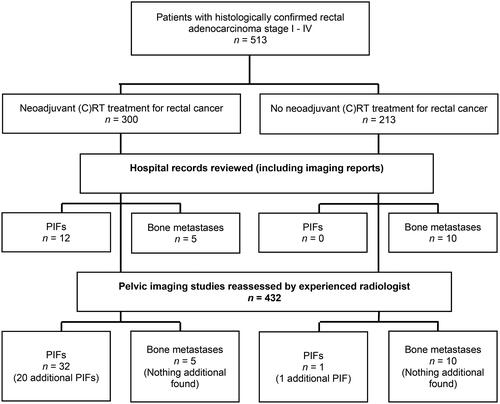
A total of 513 rectal cancer patients were identified of whom 300 patients (58.5%) were treated with neoadjuvant (C)RT (long- vs. short-course radiotherapy: 91 patients [17.7%] vs. 209 [40.7%], respectively). Twelve PIFs were diagnosed initially according to hospital records and imaging reports of all 513 patients. These 12 patients were treated with neoadjuvant (C)RT. After reassessment of all pelvic imaging procedures done in this patient group (432 patients (84.2%)), 20 additional PIFs were detected in patients treated with neoadjuvant (C)RT, resulting in a 10.7% PIF rate in irradiated patients. One PIF was detected in the group of patients not treated with neoadjuvant (C)RT for rectal cancer. This patient had palliative radiotherapy for prostate cancer and is left out of the analysis. Median follow-up time of 32 PIF patients was 49 months. Median time between start of neoadjuvant (C)RT and diagnosis of PIF was 17 months (IQR 9–28). Overall median survival for patients with PIF was 63.5 months (IQR 44–120).
Conclusion
PIFs are a relatively common late complication of neoadjuvant (C)RT for rectal cancer but are often missed or misdiagnosed as pelvic bone metastases. The differentiation of PIFs from pelvic bone metastases is important because of a different treatment and disease outcome.
Background
Neoadjuvant (chemo)radiotherapy ((C)RT) for rectal cancer is nowadays the standard of care in locally advanced disease [Citation1,Citation2]. Treatment with neoadjuvant (C)RT combined with total mesorectal excision (TME) has reduced the recurrence rate but has significant side effects [Citation1,Citation3]. Pelvic insufficiency fractures (PIFs) are known as a late complication of radiotherapy for pelvic malignancies [Citation4–22]. Radiotherapy can cause occlusion of the microvasculature of mature bone, resulting in bone necrosis and altered osteoblastic function and eventually bone fragility [Citation23,Citation24]. Also, bone regeneration and resorption is directly impaired by radiotherapy [Citation4,Citation22]. There are several studies reporting on PIFs after radiotherapy for prostate cancer and gynecological malignancies [Citation9–22,Citation25,Citation26]. However, little is known about PIFs after radiotherapy for rectal cancer [Citation4–8,Citation13].
An important differential consideration of lower back or pelvic pain in patients with a history of rectal cancer is pelvic bone metastases and local recurrence. Local recurrence can be differentiated from PIFs by its extraosseous location, but PIFs can be easily confused with pelvic bone metastases. Differentiating PIFs from pelvic bone metastases is important to avoid inappropriate and potentially hazardous treatment. Moreover, the clinical course and outcome are markedly different for PIFs and pelvic bone metastases.
To enhance our knowledge of PIFs after neoadjuvant (C)RT for rectal cancer, the primary aim of this study was to evaluate the incidence, diagnosis, radiological findings, clinical course, and patient outcome for PIFs in a large cohort of patients treated with neoadjuvant (C)RT for rectal cancer.
Material and methods
Patient selection
Data from the Dutch Comprehensive Cancer Centre (IKNL) were used to identify all patients with pathologically confirmed rectal carcinoma stage I–IV, treated in a large teaching hospital between January 2002 and January 2012. We retrospectively analyzed all hospital records and imaging reports for the presence of PIFs and pelvic bone metastases and recorded prior history, symptoms, treatment, medication usage, disease progression, and survival. The database was updated up to October 2017. The Medical Research Ethics Committees United (MEC-U) declared that the study was not subject to the Medical Research Involving Human Subjects Act (“Wet op Medisch-wetenschappelijk Onderzoek met Mensen” in Dutch). The need for formal ethical review and patient-informed consent was waived accordingly.
Treatment protocol
Patients were treated with neoadjuvant (C)RT in accordance with the Dutch guidelines [Citation27,Citation28]. Neoadjuvant radiotherapy consisted of two different approaches: short-course radiotherapy or long-course radiotherapy combined with oral 5-fluoro-uracil derivate capecitabine, 825 mg/m2 bid. Short-course radiation was delivered in five daily fractions of 5 Gy in 1 week in the supine position, for a total of 25 Gy. Long-course radiation consisted of 50 Gy delivered in 25 daily fractions of 2 Gy five times a week for a period of 5 weeks. Both schedules were delivered using a three-dimensional conformational radiotherapy technique with a beam energy of 10 megavolt. Radical rectal resection was performed with the TME technique by experienced surgeons specialized in colorectal oncology [Citation3].
Clinical follow-up
Follow-up of patients treated with neoadjuvant (C)RT followed by surgery included regular clinical assessments with carcinoembryonic antigen (CEA) testing every 3–6 months, ultrasound of the liver half-yearly and surveillance colonoscopy according to the Dutch guidelines [Citation27,Citation28]. Additional clinical, laboratory, and radiological examinations were performed on symptomatic patients.
Review of all imaging
To detect potentially missed PIFs and pelvic bone metastases, an expert radiologist (Thomas L. Bollen) reassessed all available imaging studies of the abdomen, lower back, and pelvis of all patients (plain radiography, computed tomography [CT], magnetic resonance imaging [MRI], and nuclear studies like a bone scintigraphy or positron emission tomography [PET] scan). Additional imaging was performed for different reasons, for example, an increased CEA, symptoms, or imaging was requested by other specialists in our hospital.
Radiological criteria PIFs and pelvic bone metastases
We differentiated PIFs from pelvic bone metastases based on various radiological findings. First, radiotherapy-induced PIFs are confined to the radiation field. Second, PIFs are frequently bilateral, symmetrical and show fracture lines parallel to the joints; vertically oriented fracture lines parallel to sacroiliac joints and horizontally oriented fracture lines in the acetabulum parallel to the hip joints. Although PIFs can be detected in conventional radiology, they are often diagnosed on follow-up MRI or CT of the pelvis or lumbosacral region. Plain radiography can show a sclerotic band or fracture line, but lacks sensitivity and specificity [Citation29]. In the acute phase, CT shows a cortical disruption and linear fracture line with hazy margins, which are irregular in shape and run parallel to the joints. In the chronic phase, PIFs are seen as irregularly shaped sclerotic linear bands alongside the joints [Citation29]. On MRI, a T1-weighted hypo-intense fracture line combined with surrounding parallel T2-weighted high signal (especially using fat-saturated sequences), indicating bone marrow edema, is virtually diagnostic for PIFs in the acute phase [Citation29,Citation30]. In the chronic phase, PIFs on MRI are detected as T1- and T2-weighted hypo-intense irregularly shaped linear bands or lines parallel to the joints and absence of bone marrow edema. Moreover, a low diffusion signal because of bony sclerosis of a suspected PIF is indicative of a true PIF because malignancy-induced PIFs show a high diffusion signal. Bone scintigraphy can reveal the classical H-shaped (‘Honda’ sign) or ‘butterfly pattern’ in sacral PIFs [Citation29].
Fractures derived from metastatic bone disease may show the same imaging characteristics as insufficiency fractures, although in most cases they are not related to the radiation field. Moreover, these fracture lines are associated with focal mass-like lesions, which are typically rounded or pleomorphic in shape. Also, such a focal lesion may be accompanied by a soft tissue component extending outside the bony contours which is usually not seen in PIFs. Osseous metastases derived from rectal cancer are predominantly osteolytic in nature. Characteristic MRI findings of osseous metastases from rectal cancer are a focal mass lesion with low signal intensity on T1-weighted images, high signal on fat-saturated T2-weighted images, and restricted diffusion on diffusion-weighted images. Radiological characteristics of different imaging modalities are shown in .
Table 1. Radiological characteristics of PIFs and pelvic bone metastases at different imaging modalities.
Statistical analysis
The statistical analysis was performed using IBM SPSS (Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp). Medians with ranges and percentages for categorical or ordinal variables were described. The homogeneity of demographics and clinical data between groups were analyzed using the chi-square or Fisher’s exact tests. Overall survival was calculated using the Kaplan–Meier method.
Results
Incidence of PIF
Between January 2002 and January 2012, 513 patients with pathologically confirmed rectal carcinoma were identified, of whom 300 patients (58.5%) were treated with neoadjuvant (C)RT. The hospital records and radiology reports of all 513 patients were examined and we found 12 patients with 12 PIFs (4%). Of the 513 patients, 432 patients (84.2%) had imaging studies of the abdomen, lower back, or pelvis for several reasons. One dedicated radiologist reassessed all these imaging studies and an additional 20 PIFs were detected. PIFs were only found in patients who had had neoadjuvant (C)RT and no PIFs were found in non-irradiated patients. In the group not treated with (C)RT, one PIF was found but this was a patient who had undergone palliative radiotherapy for prostate cancer and was left out of the analysis. This resulted in a total PIF incidence of 10.7% in patients previously treated with (C)RT. A flowchart of patient selection is shown in .
Baseline characteristics
The baseline characteristics of all patients treated with neoadjuvant (C)RT are shown in . Of these, 182 patients (60.7%) were male and 118 female (39.3%). The mean age was 63 years (range 36–86). A total of 91 patients (30.3%) were treated with long-course chemoradiotherapy and 209 patients (69.7%) with short-course radiotherapy. The distribution of patients across the clinical stages was as follows: stage I, 21.3%; stage II, 24.3%; stage III, 40.7% and stage IV, 8.7%. In 5% of patients, no data regarding clinical stage were available. The median follow-up time was 49 months (interquartile range [IQR] 16–74).
Table 2. Baseline characteristics of rectal cancer patients treated with neoadjuvant (C)RT.
Pelvic insufficiency fractures
We found 32 PIFs in irradiated patients for rectal cancer, resulting in a PIF rate of 10.7%. Of these, 20 PIFs were found after reassessment of the available imaging studies. Four of them were misdiagnosed as bone metastases, one as coxarthrosis, two PIFs were ultimately diagnosed 6 months later and the remaining 14 PIFs were missed. PIFs were diagnosed in 18 patients (56.3%) by CT, in 11 patients (34.4%) by MRI, in two patients (6.3%) by PET scan, and in one patient (3.1%) by conventional radiography.
PIFs were observed in the sacroiliac joints in 12 patients (37.5%; of whom four patients with additional fractures at another site of the pelvis), pubic bone in nine patients (28.1%; of whom five patients also in the sacrum), sacrum in seven patients (21.9%; of whom three patients with additional fractures); acetabulum in two patients (6.3%), lumbar spinal vertebra in one patient (3.1%) and femoral head in one patient (3.1%). PIFs were diagnosed more frequently in women than in men (resp. 71.9% vs. 28.1%, p < .01). No significant difference in incidence of PIFs between short- and long-course radiotherapy was found (11.5% vs. 8.8%). The median time between the start of neoadjuvant (C)RT and diagnosis of PIF was 17 months (IQR 9–28). At the time of PIF diagnosis, eight patients (25%) had lower back pain, two patients (6.3%) had pubic area pain, one patient (3.1%) had pain in the legs, and seven patients (21.9%) had hip pain. The median interval between onset of symptoms and diagnosis of PIF was 4 weeks (IQR 0–7), with two patients with a delay of 23 and 46 weeks, respectively. Thirteen patients had no symptoms related to PIFs and in two patients no documentation was available. In these patients, imaging of the pelvis was performed because of an increased CEA level or abdominal pain. None of the patients underwent a biopsy to confirm the diagnosis.
All symptomatic patients were treated conservatively, consisting of bed rest and mild pain medication (paracetamol, non-steroidal anti-inflammatory drugs [NSAIDs] or opioids) followed by rehabilitation. In three patients, no documentation about the effect of treatment was available in the hospital records. Good relief of symptoms was seen after a treatment period of a median of 16 weeks (IQR 7–31). All medication could be eventually terminated. One patient was also treated with hyperbaric oxygen therapy. No patient required hospitalization. The 5-year overall survival rate was 63.5%; median 86 months (IQR 44–120). Data regarding the diagnosis and clinical course of patients with PIFs treated with neoadjuvant (C)RT are listed in .
Table 3. Clinical course and treatment of PIFs in patients treated with neoadjuvant (C)RT.
Pelvic bone metastases
According to the hospital records and radiology reports, 15 patients (2.9%) were diagnosed with pelvic bone metastases. Of them, five patients were treated with neoadjuvant (C)RT, and 10 were not irradiated. Reassessment of imaging studies did not reveal additional pelvic bone metastases ().
Discussion
In this study, we report the incidence, diagnosis, radiological findings, clinical course, and outcome of PIFs in a large cohort of patients treated with neoadjuvant (C)RT for rectal cancer. Even though PIFs are a relatively common (10.7%) and serious side effect of radiotherapy, it is our impression that clinicians are not always aware of it and patients are often not informed that this complication may occur. Also, radiologists are largely unaware and unfamiliar with the imaging findings because most of the PIFs were missed or misdiagnosed initially. The incidence rate of PIF in previous studies varies from our data. A recent Danish study by Jorgensen et al. of 890 rectal cancer patients treated with surgery with or without neoadjuvant (C)RT, showed PIF in 12.2% of all patients [Citation4]. They found an incidence rate of 33.6% in patients specifically treated with neoadjuvant (C)RT. A difference between our study and the Danish study is that in that study patients were included in a prospective MRI study with a standard pelvic MRI 3 years after treatment. This could have led to the inclusion of symptomatic and asymptomatic patients. In our retrospective study, scans were only obtained when patients had symptoms, an increased CEA, or these scans were requested by other specialists in our hospital. Therefore, it is likely that the PIF incidence of 10.7% in our study is an underestimation. In another group of 562 patients with non-metastatic rectal cancer who were treated with neoadjuvant (C)RT an incidence rate of 3.1% was found [Citation5]. Five-year cumulative incidence rates of PIFs after radiotherapy for other malignancies have been reported to be 13%–19.7% for gynecological cancers and 6.8% for prostate cancer, respectively [Citation15,Citation16,Citation18,Citation20,Citation31–33].
All patients who developed PIFs were previously treated with radiotherapy. Our results showed no significant difference in incidence of PIFs between short- and long-course radiotherapy. Only one previous study reported a higher radiation dose (>50.4 Gy) as predisposing factor for developing PIFs after radiotherapy, in patients with cervical cancer [Citation16]. PIFs occurred significantly more frequently in women than in men in this study, resp. 71.9% vs. 28.1%. Previous studies have reported that female sex, history of osteoporosis, and increasing age are independent risk factors for the development of PIFs [Citation4–6].
Patients with PIFs were all treated conservatively, consisting of rest and pain medication (paracetamol, NSAIDs, or opioids) followed by rehabilitation. Good relief of symptoms was seen after a treatment period of a median of 16 weeks (IQR 7–31) and no patient required hospitalization. This is comparable with other studies, that showed that most patients can be treated with analgesics and bed rest [Citation5,Citation9,Citation18,Citation20]. Some studies suggest the use of pentoxifylline or CT-guided sacroplasty in PIFs resistant to conservative management [Citation34,Citation35].
The following limitations of our study should be considered. Although all imaging studies were reviewed by an expert radiologist, the incidence of PIFs was likely underestimated. Lower back pain is very common and imaging studies are often not performed. In these patients, a PIF could have been missed. Due to the retrospective nature of this study, some data were missing. Another limitation of this study is that the radiotherapy treatment techniques used to treat patients in this study are outdated. Currently, (image-guided) intensity-modulated treatment techniques lower the dose for most pelvic structures compared to the older techniques. But, even with these treatment techniques, a significant part of the sacral bone still receives a significant radiotherapy dose. Therefore, the problem probably still exists with current techniques. A strength of our study is that an expert radiologist reassessed all imaging studies for both PIFs and pelvic bone metastases, which was not done in previous studies.
In conclusion, PIFs are a relatively common late complication of neoadjuvant (C)RT for rectal cancer, and we recommend that patients are informed about this complication before start of treatment. It is important to differentiate PIFs from pelvic bone metastases because of a different treatment and disease outcome.
| Abbreviations | ||
| CEA | = | carcinoembryonic antigen |
| (C)RT | = | (chemo)radiotherapy |
| CT | = | computed tomography |
| Gy | = | gray |
| IKNL | = | Dutch Comprehensive Cancer Centre |
| IQR | = | interquartile range |
| MDCT | = | multidimensional CT |
| MEC-U | = | Medical Research Ethics Committees United |
| MRI | = | magnetic resonance imaging |
| NSAID | = | non-steroidal anti-inflammatory drugs |
| PIF | = | pelvic insufficiency fracture |
| TLB | = | T. L. Bollen |
| TME | = | total mesorectal excision |
Disclosure statement
No potential conflict of interest was reported by the author(s).
Data availability statement
All data underlying the results are available as part of the article and no additional source data are required.
Additional information
Funding
References
- Bosset J-F, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–1123. doi: 10.1056/NEJMoa060829.
- Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl_4):iv22–iv40. doi: 10.1093/annonc/mdx224.
- Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345(9):638–646. doi: 10.1056/NEJMoa010580.
- Jørgensen JB, Bondeven P, Iversen LH, et al. Pelvic insufficiency fractures frequently occur following preoperative chemo-radiotherapy for rectal cancer – a nationwide MRI study. Colorectal Dis. 2018;20(10):873–880. doi: 10.1111/codi.14224.
- Herman MP, Kopetz S, Bhosale PR, et al. Sacral insufficiency fractures after preoperative chemoradiation for rectal cancer: incidence, risk factors, and clinical course. Int J Radiat Oncol Biol Phys. 2009;74(3):818–823. doi: 10.1016/j.ijrobp.2008.08.054.
- Kim HJ, Boland PJ, Meredith DS, et al. Fractures of the sacrum after chemoradiation for rectal carcinoma: incidence, risk factors, and radiographic evaluation. Int J Radiat Oncol Biol Phys. 2012;84(3):694–699. doi: 10.1016/j.ijrobp.2012.01.021.
- Inoue Y, Miki C, Ojima E, et al. Pelvic insufficiency fractures after preoperative radiotherapy for rectal carcinoma. Int J Clin Oncol. 2003;8(5):336–339. doi: 10.1007/s10147-003-0340-x.
- Kang Y-M, Chao T-F, Wang T-H, et al. Increased risk of pelvic fracture after radiotherapy in rectal cancer survivors: a propensity matched study. Cancer Med. 2019;8(8):3639–3647. doi: 10.1002/cam4.2030.
- Huh SJ, Kim B, Kang MK, et al. Pelvic insufficiency fracture after pelvic irradiation in uterine cervix cancer. Gynecol Oncol. 2002;86(3):264–268. doi: 10.1006/gyno.2002.6756.
- Grasland A, Pouchot J, Mathieu A, et al. Sacral insufficiency fractures: an easily overlooked cause of back pain in elderly women. Arch Intern Med. 1996;156(6):668–674. doi: 10.1001/archinte.1996.00440060096012.
- Bliss P, Parsons CA, Blake PR. Incidence and possible aetiological factors in the development of pelvic insufficiency fractures following radical radiotherapy. Br J Radiol. 1996;69(822):548–554. doi: 10.1259/0007-1285-69-822-548.
- Shih KK, Folkert MR, Kollmeier MA, et al. Pelvic insufficiency fractures in patients with cervical and endometrial cancer treated with postoperative pelvic radiation. Gynecol Oncol. 2013;128(3):540–543. doi: 10.1016/j.ygyno.2012.12.021.
- Baxter NN, Habermann EB, Tepper JE, et al. Risk of pelvic fractures in older women following pelvic irradiation. JAMA. 2005;294(20):2587–2593. doi: 10.1001/jama.294.20.2587.
- Peh WC, Khong PL, Sham JS, et al. Sacral and pubic insufficiency fractures after irradiation of gynaecological malignancies. Clin Oncol (R Coll Radiol). 1995;7(2):117–122. doi: 10.1016/s0936-6555(05)80814-3.
- Iğdem S, Alço G, Ercan T, et al. Insufficiency fractures after pelvic radiotherapy in patients with prostate cancer. Int J Radiat Oncol Biol Phys. 2010;77(3):818–823. doi: 10.1016/j.ijrobp.2009.05.059.
- Oh D, Huh SJ, Nam H, et al. Pelvic insufficiency fracture after pelvic radiotherapy for cervical cancer: analysis of risk factors. Int J Radiat Oncol Biol Phys. 2008;70(4):1183–1188. doi: 10.1016/j.ijrobp.2007.08.005.
- Oh D, Huh SJ. Insufficiency fracture after radiation therapy. Radiat Oncol J. 2014;32(4):213–220. doi: 10.3857/roj.2014.32.4.213.
- Ogino I, Okamoto N, Ono Y, et al. Pelvic insufficiency fractures in postmenopausal woman with advanced cervical cancer treated by radiotherapy. Radiother Oncol. 2003;68(1):61–67. doi: 10.1016/s0167-8140(03)00128-2.
- Tokumaru S, Toita T, Oguchi M, et al. Insufficiency fractures after pelvic radiation therapy for uterine cervical cancer: an analysis of subjects in a prospective multi-institutional trial, and cooperative study of the Japan Radiation Oncology Group (JAROG) and Japanese Radiation Oncology Study Group (JROSG). Int J Radiat Oncol Biol Phys. 2012;84(2):e195–e200. doi: 10.1016/j.ijrobp.2012.03.042.
- Ikushima H, Osaki K, Furutani S, et al. Pelvic bone complications following radiation therapy of gynecologic malignancies: clinical evaluation of radiation-induced pelvic insufficiency fractures. Gynecol Oncol. 2006;103(3):1100–1104. doi: 10.1016/j.ygyno.2006.06.038.
- Uezono H, Tsujino K, Moriki K, et al. Pelvic insufficiency fracture after definitive radiotherapy for uterine cervical cancer: retrospective analysis of risk factors. J Radiat Res. 2013;54(6):1102–1109. doi: 10.1093/jrr/rrt055.
- Kwon JW, Huh SJ, Yoon YC, et al. Pelvic bone complications after radiation therapy of uterine cervical cancer: evaluation with MRI. AJR Am J Roentgenol. 2008;191(4):987–994. doi: 10.2214/AJR.07.3634.
- van den Blink QU, Garcez K, Henson CC, et al. Pharmacological interventions for the prevention of insufficiency fractures and avascular necrosis associated with pelvic radiotherapy in adults. Cochrane Database Syst Rev. 2018;4:CD010604.
- Hopewell JW. Radiation-therapy effects on bone density. Med Pediatr Oncol. 2003;41(3):208–211. doi: 10.1002/mpo.10338.
- Saraux A, Valls I, Guedes C, et al. Insufficiency fractures of the sacrum in elderly subjects. Rev Rhum Engl Ed. 1995;62(9):582–586.
- Park J-W, Park S-M, Lee HJ, et al. Mortality following benign sacral insufficiency fracture and associated risk factors. Arch Osteoporos. 2017;12(1):100. doi: 10.1007/s11657-017-0395-3.
- Landelijke Werkgroep Gastro-intestinale Tumoren. Rectumcarcinoom; Landelijke richtlijn, Versie: 1.2. 2005;1–23.
- Landelijke Werkgroep Gastro-intestinale Tumoren. Rectumcarcinoom; Landelijke richtlijn, Versie: 2.1. 2008.
- Krestan C, Hojreh A. Imaging of insufficiency fractures. Eur J Radiol. 2009;71(3):398–405. doi: 10.1016/j.ejrad.2008.04.059.
- Zhong X, Li J, Zhang L, et al. Characterization of insufficiency fracture and bone metastasis after radiotherapy in patients with cervical cancer detected by bone scan: role of magnetic resonance imaging. Front Oncol. 2019;9:183. doi: 10.3389/fonc.2019.00183.
- Lei S, Ge Y, Tian S, et al. Colorectal cancer metastases to brain or bone and the relationship to primary tumor location: a population-based study. J Gastrointest Surg. 2020;24(8):1833–1842. doi: 10.1007/s11605-019-04308-8.
- Gaitanidis A, Alevizakos M, Tsaroucha A, et al. Predictive nomograms for synchronous distant metastasis in rectal cancer. J Gastrointest Surg. 2018;22(7):1268–1276. doi: 10.1007/s11605-018-3767-0.
- Qiu M, Hu J, Yang D, et al. Pattern of distant metastases in colorectal cancer: a SEER based study. Oncotarget. 2015;6(36):38658–38666. doi: 10.18632/oncotarget.6130.
- Beşe NS, Ozgüroğlu M, Kamberoğlu K, et al. Pentoxifylline in the treatment of radiation-related pelvic insufficiency fractures of bone. Radiat Med. 2003;21:223–227.
- Andresen R, Radmer S, Wollny M, et al. CT-guided cement sacroplasty (CSP) as pain therapy in non-dislocated insufficiency fractures. Eur J Orthop Surg Traumatol. 2017;27(8):1045–1050. doi: 10.1007/s00590-017-2001-1.