Abstract
Objective: This study compared the efficacy, safety, and pharmacokinetics of a preservative-free latanoprost formulation to an established, benzalkonium chloride (BAK) containing formulation for the treatment of open-angle glaucoma or ocular hypertension.
Methods: This was a phase II, randomized, cross-over, investigator-masked, multi-center, pilot study (NCT01494753). A total of 30 untreated adult patients (aged ≥18 years) with primary open angle glaucoma, pseudo-exfoliative glaucoma, pigmentary glaucoma, or ocular hypertension received either preservative-free or preserved latanoprost once daily in both eyes for 6 weeks, before crossing over to receive the other treatment. Efficacy (intraocular pressure [IOP] at 8 am, midday, 4 pm and 8 pm, and global efficacy assessment by investigator), safety (adverse events, ocular symptoms and global tolerance, slit lamp examination, funduscopy, visual field examination, visual acuity, and heart rate), and pharmacokinetics were assessed at Days 0, 42, and 84.
Results: Both treatments resulted in a reduction in IOP that was similar for the preservative-free and the preserved formulation at all time points. Similarly, the overall diurnal reduction was similar in both groups (6.3 mmHg [27.9% reduction] and 6.4 mmHg [28.1% reduction] for preserved and preservative-free latanoprost, respectively). There were no differences in global efficacy assessment or in the safety and tolerance of each treatment. Systemic concentrations of latanoprost were very low; AUC0–30 and Cmax were lower and tmax was longer for preservative-free latanoprost.
Conclusions: Preservative-free latanoprost showed similar efficacy at all time points compared to BAK preservative containing formulation, with no difference in tolerance, allowing progression to phase III clinical development.
Introduction
Glaucoma is one of the most common causes of irreversible blindness and is associated with significant health care costsCitation1,Citation2. Topical prostaglandin analogs, which decrease the intraocular pressure (IOP) and reduce the rate of visual field progressionCitation3, consequently reducing the rates of glaucoma surgeryCitation4,Citation5, are usually the first choice for medical management of primary open-angle glaucoma and ocular hypertensionCitation6,Citation7. Of the common topical prostaglandin analogues used for glaucoma treatment, latanoprost is one of the most widely prescribed due to its favorable benefit–risk ratioCitation8–10 and compares favorably in terms of IOP reduction with other widely used therapeutic classes such as beta-blockersCitation11.
Many ophthalmic solutions, including prostaglandin-containing eye drops contain benzalkonium chloride (BAK), the most commonly used ocular preservative. Benzalkonium chloride is a cationic surfactant (quaternary ammonium compound) that has a detrimental effect on ocular surface cell membranes which, when used chronically, can result in instability of the lacrimal film, reduced tear break-up time and irritation of the ocular surface due to toxicity and inflammationCitation12. Furthermore, increased exposure to BAK-preserved eye drops prior to filtering surgery can have an adverse effect on surgical outcomeCitation13. The use of prostaglandin-containing eye drops without BAK has been shown to decrease epithelial permeability and preserve cell viability and membrane integrityCitation14,Citation15.
Glaucoma requires long-term, chronic treatment. As such, preservative-free treatments which are better tolerated than preserved treatments could lead to better therapeutic adherenceCitation16,Citation17 due to the lower incidence of adverse eventsCitation18.
This phase II, pilot study was conducted prior to further clinical development of a preservative-free latanoprost formulation to compare the diurnal efficacy, safety, and pharmacokinetics of the preservative-free formulation versus a preserved latanoprost formulation in newly diagnosed patients with open-angle glaucoma or ocular hypertension.
Patients and methods
Study design and patients
This was a phase II, randomized, cross-over, investigator-masked, study conducted in three centers in India (ClinicalTrials.gov identifier: NCT01494753). The study was conducted in accordance with Good Clinical Practice, applicable International Conference on Harmonization and the European directive 2001/20/CE guidance documents and practices, and in compliance with the ethical principles that have their origins in the Declaration of Helsinki (2000) and local regulations (including independent ethics committee approvals before starting the study in each center). Prior to enrolment, written informed consent was obtained from each patient. The study took place between June 2008 and December 2008.
Male or female newly diagnosed patients aged ≥18 years with primary open angle glaucoma, pseudo-exfoliative glaucoma, pigmentary glaucoma, or ocular hypertension who had not received any anti-glaucoma treatment previously were eligible for inclusion. Intraocular pressure [IOP] had to be between 22 and 30 mmHg in at least one eye for at least one baseline diurnal measurement (i.e. 08:00, 12:00, 16:00, or 20:00 on Day 0). The main exclusion criteria were: secondary (including pseudo-exfoliative or pigmentary) glaucomas; advanced cupping, severe visual field loss, risk of visual field worsening due to trial participation (according to the judgment of the investigator), absolute defect in the central 10 degrees area; best far-corrected visual acuity ≤20/200; aphakia; history of ocular allergy, blepharitis, or uveitis; dry eye syndrome (e.g. clinically relevant superficial punctate keratitis, epithelial erosions of the cornea, or use of tear substitutes); ocular trauma, infection, inflammation, or surgery within 3 months prior to the study.
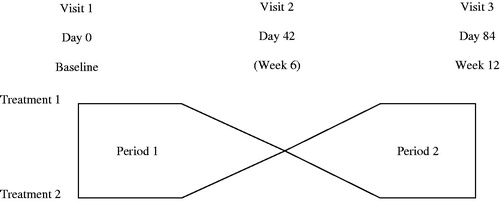
At the baseline visit (Day 0), eligible patients were randomly assigned to receive either preserved (active control group; multi-dose XalatanFootnote1) or preservative-free (test product group; single-dose MonoprostFootnote2 [T2345]) latanoprost 0.005% formulation at 20:00 once daily for 6 weeks into both eyes in the first treatment period. After 6 weeks, patients crossed over to receive the other medication (with no washout period) in the second treatment period (). Each patient was to attend the study center at the inclusion visit (Day 0), and at Day 42 ± 3 days (the end of the first treatment period) and Day 84 ± 3 days (the end of the second treatment period). Patients were questioned at each visit to check compliance with the treatment regimen and the time of instillation; additionally, the number of used and unused single dose units and vials were counted at the end of the study.
Efficacy assessments
Tonometry IOP assessment
On Day 0, Day 42, and Day 84, IOP in each eye was assessed using a Goldmann applanation tonometer at 08:00, 12:00, 16:00, and 20:00 (prior to instillation) (each time point was ±30 minutes). Two initial measurements were made. If these differed by more than 2 mmHg, a third measurement was made. The average measurement was used in all analyses.
Global efficacy assessment by investigator
On Day 42 and Day 84, the investigator evaluated the efficacy of the treatment as very satisfactory, satisfactory, not very satisfactory, or unsatisfactory.
Safety assessments
Adverse events
Adverse event (AE) data was collected at Day 42 and Day 84. All AEs observed by the investigator or reported by patients were recorded, along with their severity and potential relationship to study treatment.
Ocular symptoms and global tolerance
Ocular symptoms that occurred upon instillation (Day 42 and Day 84: ocular discomfort [pruritus, burning/stinging], blurred vision, sticky eye sensation, and foreign body sensation) and those that occurred but not upon instillation (Days 0, 42, and 84: eye dryness sensation, irritation/burning/stinging, itching, tearing, foreign body sensation, and photophobia) were assessed by the patient as none, present but not disturbing, disturbing, or very disturbing (for 1, 2, and 3 the duration and frequency was recorded). Additionally, global tolerance was assessed by each patient at Day 42 and Day 84 as very satisfactory, satisfactory, not very satisfactory, or unsatisfactory.
Slit lamp examination
A slit lamp examination and fluorescein test was done at each visit to assess conjunctival hyperemia (none, conjunctival vessels moderately dilated, conjunctival and episcleral vessels dilated, or conjunctival and episcleral vessels very dilated); folliculo-papillary conjunctivitis (absent or present); palpebral abnormality (absent or present); punctate corneal staining (absent, some punctates <10%, diffuse punctates ≤50% corneal area, or punctates >50% corneal area); anterior chamber flare (Tyndall effect) (absent, mild, moderate, or severe); and other ocular abnormality (absent or present).
Other safety parameters
Funduscopy with dilation was done at each visit and the cup/disc ratio was recorded for each eye. A visual field examination was done at the same time of day (±2 hours) at each visit (Days 0, 42, and 84) with a Humphrey field analyzer using the 30° sita-standard program. Best far-corrected visual acuity was assessed at each visit (Days 0, 42, and 84) using a Snellen chart. Heart rate (radial pulse, measured manually) and concomitant medication were assessed at each visit (Days 0, 42, and 84).
Pharmacokinetic assessments
Blood samples were taken for the pharmacokinetic assessment pre-instillation and at 5, 10, 15 and 30 minutes after instillation in each patient on Day 42 and Day 84. Blood was immediately centrifuged and plasma was frozen at −80 °C; at the end of the study, the frozen samples were shipped to at SGS Cephac Europe for the measurement of plasma concentrations of latanoprost by a validated liquid chromatography–mass spectrometry (LC-MS/MS) method (solid phase extraction followed by reversed phase [Zorbax SB phenyl] LC analysis of the extract with tandem mass spectrometric detection). The lower limit of quantification (LOQ) of the assay was 40.0 pg/mL.
Statistical analyses
As this was a pilot study, no primary criteria were defined. For quantitative variables descriptive statistics were calculated (mean, median, standard deviation [SD], minimum, maximum, and number of observations [n]), and for categorical variables the frequency distribution was calculated (frequencies and percentages).
For the IOP data the difference between treatments was calculated with the 95% CIs for the difference between treatments (preservative-free minus preserved). An analysis of period, sequence, and treatment effects on IOP at 8:00 and on AUC0–30, Cmax was performed using the non-parametric approach of Hills and ArmitageCitation19; tests were two-sided using the 5% level of significance.
The pharmacokinetic parameters AUC0–30, Cmax, t½, and tmax were calculated using Statistical Analysis Software (SAS) Version 9.2. For each patient, where possible: AUC0–30 was determined from the plasma concentration–time curve using the linear/log trapezoidal rule; Cmax and tmax were calculated directly from the concentration–time data; and t½ was calculated by ln(2)/λz, where λz is the terminal elimination rate for the plasma concentration–time curve. Geometric means were calculated for AUC0–30, Cmax, and t½ (due to their log-normal distribution). For plasma concentrations below the LOQ of 40 pg/mL, separate calculations were done using below the limit of quantification (BLQ) values of 0 pg/mL, 20 pg/mL, and 39 pg/mL in the calculation of AUC0–30 and Cmax.
For data recorded in both eyes, the analysis was performed separately for each eye and the worse eye was defined as the eligible eye with the highest average IOP on Day 0 (if Day 0 IOP was the same in both eyes, the right eye was considered to be the worse eye).
As this study comprised exploratory analyses, no formal sample size calculation was performed and 30 patients per group were considered to be sufficient to meet the objectives of the study.
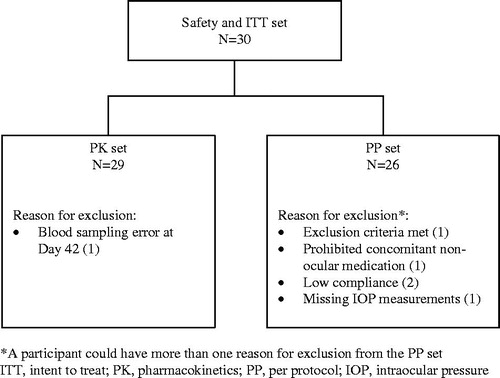
The safety set (used for safety evaluations) was defined as all patients enrolled in the study in whom the study medication had been used and for whom any follow-up information was available. The intention-to-treat (ITT) set comprised enrolled patients for whom any follow-up efficacy information was available, and the per protocol (PP) set comprised ITT patients who did not have any major protocol violation that could have affected the efficacy assessments. Efficacy assessments were performed using the PP set and confirmed using the ITT set. Pharmacokinetic analyses were performed using the PK set, which comprised all ITT patients who did not have any major protocol violation that could have affected the pharmacokinetic assessments.
Results
Patients studied
A total of 30 patients were included in the study; 15 patients were randomized to receive the preservative-free latanoprost formulation first and then the preserved formulation, the other 15 patients received the treatments in the reverse sequence. All patients completed the study and were present at each visit (Day 0, Day 42, and Day 84). A total of 29 and 26 patients were included in the PK and PP sets, respectively (), with a similar age and sex distribution to the overall group; 2 patients were excluded from the PP set due to low treatment compliance, 1 patient was excluded for missing IOP measurements, 1 patient was excluded for not meeting certain exclusion criteria, and 1 patient was excluded for receiving prohibited concomitant non-ocular medication (). Overall treatment compliance was very good (99.7% for the preserved formulation and 98.0% for the preservative-free formulation).
Demographic and other baseline characteristics are presented in . The overall mean ± SD age was 50.7 ± 12.8 years (range: 31-81 years), with 70% males and 30% females. There were no unexpected findings in the baseline characteristics.
Table 1. Demographic and other baseline characteristics.
Efficacy
IOPs for the worse eye at baseline and following treatment for each group (PP set) are shown in , together with the difference between treatments (preservative-free minus preserved) and the associated 95% CIs for the difference for each time point. There was no significant difference in the reduction of IOP between treatments at any time point, with the upper 95% CI being less than 1.5 mmHg for each comparison (this being an accepted limit for non-inferiority used in similar studiesCitation20) and all 95% CIs of the difference containing the 0 value. The biggest difference was at 08:00, with absolute IOP values of 16.2 ± 2.9 mmHg (preserved) versus 16.6 ± 2.2 mmHg (preservative-free) and a difference of 0.5 ± 2.5 mmHg between treatments, but the 95% CI contained the 0 value and the upper 95% CI for the difference remained <1.5 mmHg (1.49 mmHg)Citation20. Overall, diurnal IOP was similar in both groups (absolute values of 16.4 ± 2.6 [preserved latanoprost] and 16.3 ± 2.4 [preservative-free latanoprost] mmHg). Compared to baseline values, these absolute diurnal data represented reductions of 6.3 mmHg (27.9%) and 6.4 mmHg (28.1%) for preserved and preservative-free latanoprost, respectively. The IOP data for each treatment period showed no differences between groups.
Table 2. Mean ± SD IOP in the worse eye at baseline and following treatment in each group (PP set).
Overall global efficacy assessment by the investigator for preserved and preservative-free latanoprost (PP set) was very satisfactory for 38.5% and 46.2% of patients, satisfactory for 50.0% and 34.6% of patients, and not very satisfactory for 11.5% and 19.2% of patients, respectively. No investigator considered either treatment to be unsatisfactory.
This data was confirmed by a repeat analysis using the ITT set.
Safety and tolerability
The overall incidence of AEs was very low. One patient (preserved latanoprost) experienced an ocular AE (chalazion) and 5 patients experienced 6 systemic AEs (diarrhea and ear infection in the preserved latanoprost group, as well as pyrexia, nasopharyngitis, forearm fracture, and status asthmaticus in the preservative-free latanoprost group). No ocular or systemic AE was considered to be related to the study product. The AE of status asthmaticus in the preservative-free latanoprost group was classified as an SAE as this required treatment after occurring during the Day 42 visit, but was considered unlikely to be related to the study drug. No AE or SAE caused premature discontinuation of the study drug.
The frequencies of ocular symptoms that occurred upon instillation and of those that occurred but not upon instillation are presented in . Although there were small differences for some symptoms between groups (e.g. the incidence of sticky eye was higher in the preservative-free latanoprost group), the overall incidence of each symptom was low and any group differences were not considered to be of clinical importance.
Table 3. Ocular symptoms that occurred upon instillation and that occurred but not upon instillation (safety set).
Overall, the global assessment of tolerance by the patients was good, with all but one patient being satisfied or very satisfied with both treatments. The one patient who assessed the tolerance as not very satisfactory (preservative-free latanoprost) did not experience an AE, ocular sign or symptom upon instillation.
Slit lamp examination data for the worse eye is presented in . The incidence of conjunctival hyperemia, punctate corneal staining, folliculo-papillary conjunctivitis, and palpebral abnormality was low in each group and small differences between groups were not considered to be clinically relevant. No patient in either group experienced anterior chamber flare.
Table 4. Slit lamp examination for the worse eye (safety set).
At the end of treatment there was no difference from baseline for either group with regard to retinal status or visual acuity. Heart rate decreased slightly in the preserved latanoprost group (mean ± SD -2.0 ± 9.2 bpm) and increased slightly in the preservative-free latanoprost group (1.9 ± 8.5 bpm) but these differences were not considered to be of clinical significance.
Pharmacokinetics
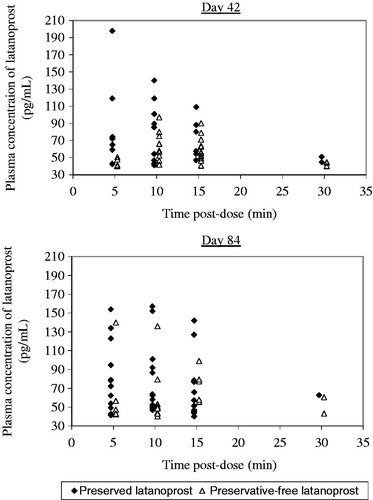
Systemic concentrations of latanoprost were very low, with much data not quantifiable meaning that t½ was not calculable. AUC0–30, Cmax, and tmax data is presented in and individual concentrations of latanoprost in each group are presented in . For AUC0–30 and Cmax there was no sequence or period effect, irrespective of the BLQ used (p > 0.05 in each case). However, there was a treatment effect for both AUC0–30 and Cmax: AUC0–30 was significantly lower (p < 0.05) for preservative-free latanoprost than for preserved latanoprost for BLQs of 20 and 39 pg/mL (for BLQ of 0 pg/mL AUC0–30 was visually lower but not statistically significant [p > 0.05]), and Cmax was significantly lower (p < 0.05) for preservative-free latanoprost than for preserved latanoprost for each BLQ. For AUC0–30 and Cmax, respectively, the geometric mean ratio (preservative-free/preserved) ranged from 0.615 to 0.880 and 0.748 to 0.783. For preservative-free latanoprost, tmax was slightly longer than for preserved latanoprost (10.7 minutes versus 7.3 minutes).
Table 5. Summary of AUC0–30, Cmax, and tmax (PK set).
Discussion
This phase II study, conducted to evaluate the diurnal efficacy, safety, and pharmacokinetics of a preservative-free latanoprost eye drop formulation, adds to the existing body of data describing this formulation, notably the phase III dataCitation20. The present study evaluated IOP efficacy data throughout the day compared to the single time point analysis in the phase III study. The data in the present study show the diurnal IOP-lowering behavior of the preservative-free formulation, which was similar to that for the preserved formulation, and supported the optimization of the timing for the IOP assessment in the confirmatory phase III studyCitation20. Since the early morning time point in the present study was shown to be the most challenging time to demonstrate non-inferiority of the preservative-free to the preserved formulation, it was decided to conduct the IOP assessment during that time in the phase III study, this time point being strongly representative of the IOP-lowering effect in the afternoon. Moreover, this phase II study also adds new pharmacokinetic data for the preservative-free latanoprost formulation and provides further safety analyses. As a small-scale pilot study in 30 patients, the findings are mainly descriptive and have limited statistical power, but allowed progression to phase III clinical development.
At each time point IOP was reduced following the administration of both treatments, and there was no difference in overall diurnal efficacy in terms of the reduction in IOP. Despite the small sample size in the present study, this data is aligned with the demonstration of non-inferiority in the pivotal phase III studyCitation20. In addition, the data from the present study demonstrate excellent diurnal control of IOP by both preservative-free and preserved latanoprost. The global efficacy assessment by the investigator was also similar between groups, and there were no differences in safety or patient-based tolerance between groups.
Decreased tolerance due to the presence of BAK usually only manifests after long periods of chronic use, and is often related to BAK-induced increased incidence of ocular surface diseaseCitation18. In this study, however, there was no difference in tolerance between the groups, which may reflect the short treatment period (42 days) in this study and the small number of patients. In the phase III study, a 3 month treatment period was investigated and clear differences in tolerance were evident – moderate to severe conjunctival hyperemia was less frequent for the preservative-free formulation and upon instillation the global subjective ocular symptom score was lower for the preservative-free formulationCitation20. In an overall assessment of the benefit of the preservative-free formulation this improved tolerance in chronic use with maintained efficacy is a key issue.
Overall, AUC0–30 and Cmax were slightly lower and tmax was slightly higher for preservative-free latanoprost. However, there was a high incidence of BLQ plasma values, which is not unexpected for the systemic absorption of topically administered eye drops, and these differences, while statistically significant for AUC0–30 and Cmax, did not translate into differences in either efficacy or tolerance.
Limitations of the study included the small sample size, the rather short treatment period that does not reflect long-term use in a real-world situation, and the lack of a washout period between the treatment periods. However, no residual influence of the previous treatment to lower IOP would be expected after a 6 week treatment periodCitation21 and there was no treatment period or sequence effect in this study. Also, the pharmacokinetic data analysis was hampered by the large number of BLQ plasma concentrations.
In conclusion, the preservative-free prostaglandin-analogue eye drop formulation showed good diurnal efficacy and tolerability. This is in keeping with the data from the recently published phase III trialCitation20. Extending this data, the present study shows that adequate diurnal IOP control can be obtained with the novel preservative-free prostaglandin formulation.
Transparency
Declaration of funding
Editorial support for this study was funded by Laboratoires Théa, Clermont-Ferrand, France. No author received payment for their contribution to this article.
Declaration of financial/other relationships
F.A. has disclosed that he is a paid consultant of Laboratoires Théa. R.C. and I.S. have disclosed that they have no significant relationships with or financial interests in any commercial companies related to this study or article.
CMRO peer reviewers on this manuscript have received an honorarium from CMRO for their review work, but have no relevant financial or other relationships to disclose.
Acknowledgments
The study personnel and patients are acknowledged for their participation.
The authors would also like to thank Dr Andrew Lane (Lane Medical Writing) who provided professional medical writing assistance, funded by Laboratoires Théa, in the preparation and development of this manuscript in accordance with the European Medical Writers Association guidelines and GPP3 (Good Publication Practice) guidelines.
Notes
Notes
1 Xalatan is a registered trade name of Pfizer, New York, USA
2 Monoprost is a registered trade name of Laboratoires Théa, Clermont-Ferrand, France
References
- Alward WL. Medical management of glaucoma. N Engl J Med 1998;339:1298-307
- Thygesen J, Aagren M, Arnavielle S, et al. Late-stage, primary open-angle glaucoma in Europe: social and health care maintenance costs and quality of life of patients from 4 countries. Curr Med Res Opin 2008;24:1763-70
- Garway-Heath DF, Crabb DP, Bunce C, et al. Latanoprost for open-angle glaucoma (UKGTS): a randomised, multicentre, placebo-controlled trial. Lancet. 2015;385:1295-304
- Kenigsberg PA. Changes in medical and surgical treatments of glaucoma between 1997 and 2003 in France. Eur J Ophthalmol 2007;17:521-7
- Lee CW, Buckley F, Costello S, Kelly S. A systematic review of the characteristics of randomised control trials featuring prostaglandins for the treatment of glaucoma. Curr Med Res Opin 2008;24:2265-70
- Prum BE Jr, Lim MC, Mansberger SL, et al. Primary open-angle glaucoma suspect preferred practice pattern guidelines. Ophthalmology 2016;123:P112-51
- Prum BE Jr, Rosenberg LF, Gedde SJ, et al. Primary open-angle glaucoma preferred practice pattern guidelines. Ophthalmology 2016;123:P41-111
- Konstas AG, Hollo G, Irkec M, et al. Diurnal IOP control with bimatoprost versus latanoprost in exfoliative glaucoma: a crossover, observer-masked, three-centre study. Br J Ophthalmol 2007;91:757-60
- Parrish RK, Palmberg P, Sheu WP, Group XLTS. A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol 2003;135:688-703
- Simmons ST, Dirks MS, Noecker RJ. Bimatoprost versus latanoprost in lowering intraocular pressure in glaucoma and ocular hypertension: results from parallel-group comparison trials. Adv Ther 2004;21:247-62
- Lou H, Zong Y, Ge YR, et al. Efficacy and tolerability of latanoprost compared with timolol in the treatment of patients with chronic angle-closure glaucoma. Curr Med Res Opin 2014;30:1367-73
- Guenoun JM, Baudouin C, Rat P, et al. In vitro study of inflammatory potential and toxicity profile of latanoprost, travoprost, and bimatoprost in conjunctiva-derived epithelial cells. Investig Ophthalmol Vis Sci 2005;46:2444-50
- Boimer C, Birt CM. Preservative exposure and surgical outcomes in glaucoma patients: the PESO study. J Glaucoma 2013;22:730-5
- Baudouin C, Riancho L, Warnet JM, Brignole F. In vitro studies of antiglaucomatous prostaglandin analogues: travoprost with and without benzalkonium chloride and preserved latanoprost. Investig Ophthalmol Vis Sci 2007;48:4123-8
- McCarey B, Edelhauser H. In vivo corneal epithelial permeability following treatment with prostaglandin analogs [correction of analoges] with or without benzalkonium chloride. J Ocul Pharmacol Therapeut 2007;23:445-51
- Hahn SR, Kotak S, Tan J, Kim E. Physicians’ treatment decisions, patient persistence, and interruptions in the continuous use of prostaglandin therapy in glaucoma. Curr Med Res Opin 2010;26:957-63
- Wilensky J, Fiscella RG, Carlson AM, et al. Measurement of persistence and adherence to regimens of IOP-lowering glaucoma medications using pharmacy claims data. Am J Ophthalmol 2006;141(1 Suppl):S28-S33
- Jaenen N, Baudouin C, Pouliquen P, et al. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur J Ophthalmol 2007;17:341-9
- Hills M, Armitage P. The two-period cross-over clinical trial. Br J Clin Pharmacol 1979;8:7-20
- Rouland JF, Traverso CE, Stalmans I, et al. Efficacy and safety of preservative-free latanoprost eyedrops, compared with BAK-preserved latanoprost in patients with ocular hypertension or glaucoma. Br J Ophthalmol 2013;97:196-200
- European Glaucoma Society. Terminology and Guidelines for Glaucoma, 3rd Edn, 2008. PubliComm, Savona, Italy.