Abstract
Objectives: To assess glycemic effectiveness, adherence and persistence within 6 months of treatment initiation with dulaglutide, a once weekly GLP-1 receptor agonist, in a US real-world setting.
Methods: This retrospective claims analysis included adults (≥18 years) with T2DM from the HealthCore Integrated Research Database, who had HbA1c laboratory results around initiation and within 6 months after initiation. Glycemic control was assessed by change in HbA1c from pre-initiation to post-initiation. Patients were considered adherent if their proportion of days covered (PDC) was ≥0.80; persistence was measured as days of continuous therapy from initiation to 6 months after initiation with no gaps >45 days between fills.
Results: Of the 308 analyzed patients, the majority (n = 188; 61%) were adherent to dulaglutide (mean PDC 0.76; SD 0.26), with 115 patients (37%) discontinuing treatment. Mean persistence was 152 days/5 months. Mean HbA1c decreased from 8.49% (SD 1.70, median 8.20%) at baseline to 7.59% (SD 1.51, median 7.30%) at follow-up, corresponding to a mean HbA1c change of −0.90% (95% confidence interval [CI] −1.08 to −0.73; p < .01; median −0.70%). Patients who were adherent to or persistent with dulaglutide experienced larger reductions (−1.14% and −1.12% respectively), as did those without prior GLP-1 RA use (−1.03%). The proportion of patients with HbA1c <7% increased from 18% to 40%.
Conclusions: Dulaglutide was associated with a significant decrease in HbA1c levels 6 months after treatment initiation. Patients who adhered to or persisted with dulaglutide therapy, or were naïve to GLP-1 RA use, experienced greater decreases in HbA1c levels.
Introduction
Type 2 diabetes mellitus (T2DM) is considered a global epidemic despite a wide variety of available treatment options to improve glycemic controlCitation1. Treatment guidelines published by the American Diabetes Association and the European Association for the Study of Diabetes recommend initial treatment with metformin plus lifestyle changes consisting of diet and exercise. If patients do not achieve their hemoglobin A1c (HbA1c) targets after 3 months of treatment, the guidelines call for metformin to be combined with another oral antihyperglycemic drug, basal insulin or a glucagon-like peptide-1 receptor agonist (GLP-1 RA)Citation2,Citation3. Six GLP-1 RA drugs are approved for use by the US Food and Drug Administration (exenatide BID, exenatide QW, liraglutide, albiglutide, dulaglutide, and lixisenatide). While all GLP-1 RAs are effective in reducing HbA1c levels, they differ regarding the magnitude of HbA1c reduction, weight loss, and the frequency and severity of adverse eventsCitation4. Dulaglutide demonstrated either superiority or noninferiority in reducing HbA1c compared with metformin, sitagliptin, exenatide BID, liraglutide or insulin glargine in six clinical trialsCitation5.
One challenge in treating T2DM is suboptimal patient adherence to antihyperglycemic treatment. As many as 55% of patients do not adhere to their prescribed diabetes treatment regimenCitation6, compromising the effectiveness of therapy and raising associated costs, healthcare utilization and even mortality riskCitation7–13. Patient adherence to a given medication can be affected by many factors, including dosing regimens, medication costs and general patient preferencesCitation14. In patients with T2DM, adherence has been shown to increase with the use of prefilled pens compared with vials and syringes for administration of basal insulinCitation11,Citation15–20, and through simplified dosing regimensCitation11,Citation21.
All currently available GLP-1 RAs are injectable medications, but the dosing frequency varies from once or twice daily to once weekly and some require reconstitution prior to use. Dulaglutide is administered once weekly via a single-dose pen that is an autoinjector with a hidden pre-attached needleCitation22. In a 28 week study, 95% of the patients found the single-dose pen easy or very easy to use and 90% were overall satisfied with the injection experienceCitation23.
Dulaglutide first gained marketing approval in the US in late 2014 and its increasing time on the market now allows the conduct of studies examining treatment adherence, persistence and effectiveness in a real-world setting. One prior study using administrative claims found that adherence to dulaglutide over 6 months was higher than adherence to exenatide QW and to liraglutideCitation24. However, glycemic control with dulaglutide and the relationship between adherence and glycemic control have not yet been evaluated. There is also little information available about the demographic and clinical characteristics of patients who have been prescribed dulaglutide. To address this evidence gap, we conducted a retrospective, observational single-cohort study to assess glycemic control and its association with patient treatment adherence and persistence in commercially insured patients with T2DM initiating dulaglutide in the US. Additionally, the study also describes the characteristics of patients initiating dulaglutide in this real-world setting.
Methods
Data source
This retrospective analysis used data compiled from the HealthCore Integrated Research Database (HIRD). The HIRD is a single-payer health insurance database containing claims integrated across data sources and types (i.e. professional claims, facility claims, outpatient pharmacy claims, outpatient laboratory results and health plan enrollment information) as well as across years (from 2006 through the most recent calendar quarter). Data is geographically diverse and obtained from 14 health plans in the Northeastern, Mid-Atlantic, Southeastern, Midwest, Central and Western regions of the US, representing members in each of the 50 states. As a non-interventional retrospective study using a HIPAA-compliant de-identified research database with no contact at all with the study subjects, review and approval by an Institutional Review Board and informed consent were not necessary. Formulary requirements for the plans represented in the HIRD state that a patient must have had a trial of metformin or a contraindication for metformin to receive approval for any preferred GLP-1 RA treatment, including dulaglutide (with a quantity limit of four prefilled pens per 28 days)Citation25.
Patient selection
Patients with at least one pharmacy claim for dulaglutide between 1 November 2014 (when the product was first available) and 30 November 2015 were identified from the HIRD, with the earliest dulaglutide claim date set as the index date. All patients were adults (aged 18 years or older) with at least 6 months’ continuous medical and pharmacy enrollment in commercial or Medicare Advantage plans prior to and after the index date. Patients were required to have at least one medical claim with an International Classification of Diseases, Ninth or Tenth Revision, Clinical Modification (ICD-9/10-CM) diagnosis code for T2DM during the 6 month pre-initiation period. Patients with one or more claims with diagnosis codes for type 1 diabetes or for secondary or gestational diabetes during the pre-initiation period were excluded from the study.
All patients identified in the HIRD who met the study criteria constituted the full population. Of this full population, a subset (the “HbA1c sample”) was defined by the availability of at least one pre-initiation and one post-initiation HbA1c laboratory result to examine change in HbA1c levels. This subset was used for the main study analysis and evaluation of outcomes measures. Pre-initiation HbA1c was determined between 183 days before and 14 days after the index date. If multiple HbA1c results were available for a patient, the one closest to the index date was chosen. Post-initiation HbA1c was determined between 93 and 213 days after the index date, with a preference for the result closest to and prior to 183 days after the index date.
Glycemic control outcome measures
Glycemic control was assessed in three ways: (1) mean change in HbA1c from pre-initiation to post-initiation; (2) percentage of patients who achieved post-initiation HbA1c <7%, 7 to <8%, 8 to <9%, and ≥9%; (3) percentage of patients showing improvement in clinical scoring post-initiation. Our metric of clinical scoring improvement (success vs. failure to achieve HbA1c target levels) was influenced by the Healthcare Effectiveness Data and Information Set (HEDIS) metric commonly used by health plansCitation26. In particular, patients were assigned to a risk level according to their pre- and post-initiation HbA1c levels: HbA1c ≤8.0% was defined as mild risk; 8.1% to ≤9.0% as moderate risk; and >9.0% as severe risk. “Success” was defined as a patient either remaining at the mild risk level, or moving from severe to moderate or mild risk, or moving from moderate to mild risk. All other outcomes were considered “failure”.
Adherence and persistence outcome measures
Adherence was defined by the proportion of days covered (PDC), calculated as the sum of the number of days with available supply of dulaglutide divided by the number of days in the post-initiation observation period (i.e. 183). The start date of each new prescription fill was adjusted to begin after the previous fill ran out whenever the patient had overlapping days’ supply from the previous fillCitation27. A patient with PDC of 0.80 or greater was considered adherent. Persistence was calculated as the number of days of continuous therapy from initiation until discontinuation or the end of the 183 day post-initiation period. To determine the duration of continuous therapy, dates of early refills were adjusted to the day after finishing the supply from the previous fill. A patient with a gap between the run-out date of the previous fill and the next fill of more than 45 days was considered to have discontinued treatment.
Other patient characteristics
Demographic characteristics of patients as well as the prescribing physicians’ specialties were assessed on the index date; pre-initiation comorbidities were captured using ICD-9/10-CM codes from medical claims, including the Quan–Charlson Comorbidity Index (QCI)Citation28 and adapted Diabetes Complications Severity Index (aDCSI)Citation29; other selected comorbidities of interest were also reported. Antihyperglycemic medication usage was evaluated over the pre- and post-initiation periods and included insulin (any type), oral antihyperglycemic drugs (OADs; 7 classes), and other GLP-1 RAs.
Statistical analysis
All variables were summarized using descriptive statistics, with mean, standard deviation (SD) and medians for continuous variables, and absolute and relative frequencies for categorical variables. McNemar’s test was used to compare the proportions of patients using selected antihyperglycemic medications between the pre- and post-initiation periods.
Continuous glycemic control metrics (pre- and post-initiation HbA1c and HbA1c change) were stratified by selected patient characteristics, and paired t-tests were used to test change in HbA1c from pre- to post-initiation within the whole sample and within each subgroup. Two-sample t-tests were used to test for differences in means across these subgroups. Categorical glycemic control metrics (pre- and post-initiation HbA1c <7%, and clinical scoring [success vs. failure]) were also stratified across patient characteristics; differences in proportions between subgroups were tested using χ2 or Fisher’s exact test.
Change in HbA1c from pre-initiation was analyzed using a linear regression model with specific baseline characteristics (see Supplementary Online Appendix Table 6) as well as adherence and persistence as independent variables. Patients reaching HbA1c <7% post-initiation and clinical scoring success were analyzed using logistic regression with similar independent variables. Independent variables were chosen for these models based on clinical importance while avoiding multicollinearity.
Statistical significance was set at an alpha level of .05. No adjustments for multiple comparisons were made in this study. Data was analyzed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
Results
Patient selection
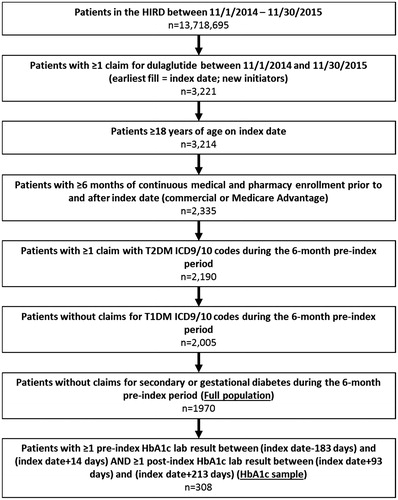
The full population contained 1970 patients, of whom 308 had pre- and post-initiation HbA1c laboratory results and were included in the main analysis (“HbA1c sample”; ). Our description of the results focuses on the HbA1c sample, unless otherwise noted.
Demographic and clinical characteristics
Among the 308 patients with pre- and post-initiation HbA1c laboratory results, the mean (SD) age was 53 (9.4) years, 51% were male and the majority (55%) resided in the Southern US (). Most patients had a diabetes-associated QCI score of 1, suggesting a low prevalence of other mortality-predicting diseases. Additionally, the majority of patients had no claims for diabetes complications such as nephropathy. A high proportion of patients had dyslipidemia (81%) and hypertension (74%). Other prevalent complications included obesity (32%), neuropathy (15%) and cardiovascular complications (13%). Approximately 89% were using an OAD at baseline and 51% used more than one OAD class. At baseline, 33% of patients were using insulin and 21% used a GLP-1 RA (). Similar results were observed in the full population with the exception of patient residence, where a higher proportion of patients lived in the Midwest and lower proportions lived in the South and West ( and ).
Figure 2. Antihyperglycemic medication utilization before and after dulaglutide initiation: (A) HbA1c sample (n = 308); (B) full population (N = 1970). *p < .05 using McNemar’s test comparing proportions of patients with medication before and after dulaglutide initiation.
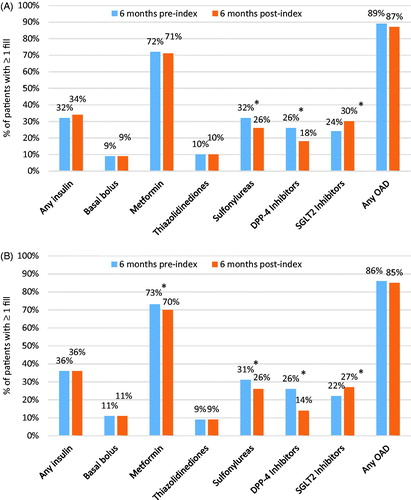
Table 1. Patient demographic and clinical characteristics.
Treatment patterns
Prescribing specialty for the index dulaglutide prescription was approximately 40% each for endocrinologists and primary care physicians (PCPs; ). Slightly more than half of the HbA1c sample (56%) initiated on the lower dose of dulaglutide (0.75 mg). Of these, 37% increased to 1.5 mg during follow-up. Of the 137 patients (45%) who initiated on the higher dose of dulaglutide, only two patients decreased to the lower dose during follow-up.
During the 6 month post-initiation period, the proportions of patients using insulin and any OAD remained constant (p > .05); however, use of sulfonylureas and DPP-4 inhibitors decreased while use of SGLT-2 inhibitors increased (p < .05; ). Of the 208 patients not using insulin at baseline, 14 patients (6.3%) initiated insulin during follow-up.
Similar patterns were observed in the full population ( and Supplementary Online Appendix Table 1) with the exception of metformin use, which decreased slightly but statistically significantly during follow-up (from 73% to 70%, p < .05).
Dulaglutide adherence and persistence
The majority of patients (73%) filled four or more prescriptions (median six prescription fills). Sixty-one percent of patients were adherent to dulaglutide, as measured by PDC ≥0.80. The mean PDC was 0.76 (SD 0.26, median 0.89). During the post-initiation period, 115 patients (37%) discontinued treatment (i.e. had a gap of more than 45 days between prescription fills). The mean number of days of persistent use of dulaglutide was 152 days (SD 53.1, median 183). Approximately 90% of patients started with monthly fills, with the remaining patients receiving a 3 month supply of medication with their index. Similar results were obtained in the full population (Supplementary Online Appendix Table 1).
Glycemic control
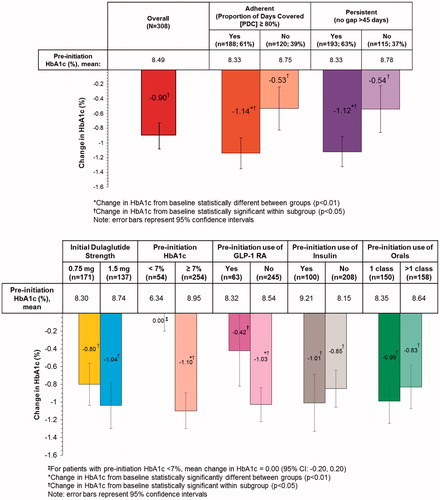
At baseline, mean HbA1c was 8.49% (SD 1.70, median 8.20%), which decreased to 7.59% (SD 1.51, median 7.30%) at follow-up. This corresponded to a mean change in HbA1c of −0.90% (95% confidence interval [CI]: −1.08 to −0.73; p < .01; median −0.70%, ). For patients not at goal (HbA1c ≥7%) at pre-initiation (n = 254), the mean change in HbA1c was −1.10% (95% CI: −1.30 to −0.89); p < .01; median −0.90). The mean change in HbA1c was also significantly larger for patients with no prior GLP-1 RA use (−1.03%), those who were adherent (PDC ≥0.80; −1.14%), and those who were persistent (−1.12%; all p < .01). There were no significant differences in mean HbA1c change by dulaglutide index strength, prior insulin use or prior OAD use ( and Supplementary Online Appendix Table 2).
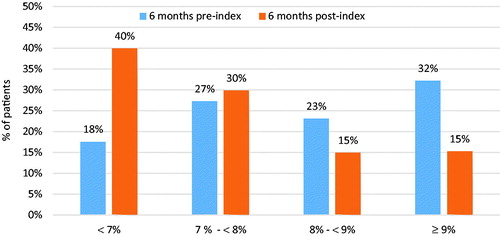
The percentage of patients with HbA1c <7% increased from 18% to 40% from baseline to follow-up (). In the clinical scoring outcome measure, 79% of patients achieved success (42% remained at mild risk [HbA1c ≤8%], 22% moved from severe [HbA1c >9%] to moderate [8%< HbA1c ≤9%] or mild risk; 15% moved from moderate to mild risk; Supplementary Online Appendix Table 3). Achievement of HbA1c <7% and clinical scoring success occurred more frequently in subgroups without prior insulin use, receiving no more than one OAD, or who were adherent to or persistent with dulaglutide (Supplementary Online Appendix Tables 4 and 5).
Multivariable analysis
The regression model for change in HbA1c included the following independent variables: gender, pre-initiation insulin use, pre-initiation SGLT-2 inhibitor use, pre-initiation GLP-1 RA use, pre-initiation OAD use, index dose of dulaglutide (categorical), age, pre-initiation QCI score, pre-initiation HbA1c and adherence (continuous PDC). Persistence, as measured by days of continuous therapy, was not included in the regression model since it was highly correlated with adherence (Pearson r = .92). The results suggested that no previous insulin use, no previous GLP-1 RA use, higher pre-initiation HbA1c, using no more than one class of OAD and high adherence to dulaglutide are associated with a reduction in HbA1c (all p < .05, Supplementary Online Appendix Table 6).
The logistic regression models for HbA1c <7% and the clinical scoring outcome included the same independent variables used in the linear regression. The results suggested that no previous insulin use, lower pre-initiation HbA1c, using no more than one class of OAD and higher adherence to dulaglutide were significantly associated with a higher likelihood of achieving HbA1c <7% post-initiation (Supplementary Online Appendix Figure 1). The logistic regression results for the clinical scoring outcome metric are summarized in Supplementary Online Appendix Figure 2; older age, no prior GLP-1 RA use, lower pre-initiation HbA1c, using no more than 1 class of OAD and higher adherence to dulaglutide were similarly significantly associated with a higher likelihood of achieving success.
Discussion
Results of this retrospective, real-world analysis showed significant improvement in all selected glycemic control metrics following initiation of dulaglutide. The mean change in HbA1c was −0.90%; the percentage of patients with HbA1c <7% increased from 18% pre-initiation to 40% post-initiation; and 79% of patients achieved success as determined by the clinical scoring metric. Both strengths of dulaglutide showed significant reductions in HbA1c 6 months after initiation of dulaglutide. These results are consistent with the clinical trials of dulaglutide: in the Assessment of Weekly AdministRation of LY2189265 (Dulaglutide) in Diabetes (AWARD) trials, the 0.75 mg strength of dulaglutide changed HbA1c by −0.71% to −1.59%, while the 1.5 mg strength changed HbA1c by −0.78% to −1.64%Citation5,Citation30,Citation31.
Mean HbA1c change was greater for the 80% of patients who did not use a GLP-1 RA before initiating dulaglutide. These patients experienced a significant change in HbA1c of −1.03%, while those who did use a GLP-1 RA in the 6 months before initiating dulaglutide experienced a change of −0.42%. This suggests that both new initiators and previous users of GLP-1 RAs experience improvements in glycemic control on dulaglutide. This reduction in HbA1c among previous GLP-1 RA users is similar to results from a randomized clinical trial evaluating the HbA1c change among patients switching from twice daily exenatide to liraglutide (additional HbA1c reduction of 0.32%)Citation32. Additionally, in our study, the previous GLP-1 RA users had a lower mean baseline HbA1c compared to those who did not use GLP-1 RA previously (8.3 vs. 8.5%) and a higher proportion were already at HbA1c goal of ≤7% (29% vs. 15%). We could not assess the reasons for switching to dulaglutide among patients with previous exposure to GLP-1 RA as it was not captured in the dataset.
Patients who were adherent (PDC ≥80%) experienced a greater change in HbA1c compared with those who were not (−1.14% vs. −0.53%). A similar result was observed for persistent vs. non-persistent patients (−1.12% vs. −0.54%). Adherence remained strongly associated with HbA1c change after adjusting for potential confounders (adjusted mean change of −0.18% for each 10% PDC increase; 95% CI: −0.23 to −0.13; p < .01).
A study by Alatorre et al. described the characteristics of patients initiating treatment with dulaglutide. Their findings were largely similar to the population in our current study in terms of age, region of residency and comorbidities, although lower proportions of patients in the current study used insulin, GLP-1 RAs and SGLT-2 inhibitors prior to initiation. In this same retrospective study using administrative claims, patients initiating dulaglutide had a mean PDC of 0.72, with 54% of patients being adherent and 27% discontinuing dulaglutide treatment, which is comparable to our findingsCitation24. Both Alatorre et al. and our study also showed that after dulaglutide initiation the use of sulfonylureas and DPP-4 inhibitors decreased while use of SGLT-2 inhibitors increased; the latter may be due to additional weight loss advantages.
Our adherence results for patients initiating dulaglutide can also be compared with studies of other GLP-1 RAs. Previous claims analyses reported mean PDC values of patients treated with GLP-1 RAs ranging from 0.56 to 0.69Citation33–35 with the percentage of patients having good adherence (PDC ≥0.80) ranging from 30% to 54%Citation24,Citation33–36. In comparison, the mean PDC in our study was 0.76 with 61% of patients having good adherence. As expected, the evidence indicates that the highest adherence rates were associated with once weekly GLP-1 RA dosing regimens and the lowest were associated with a twice daily dosing regimen. With once weekly dosing, dulaglutide would be expected to fall at the higher end of the range of adherence rates, which is supported by the findings from Alatorre et al. (percentage of patients with good adherence: 54% vs. 38% for matched dulaglutide vs. exenatide QW, and 54% vs. 44% for matched dulaglutide vs. liraglutide)Citation24.
Regardless of the dosing regimen, the literature consistently reports larger improvements in HbA1c control among patients who adhere and persist with treatmentCitation33–39. For example, Buysman et al. found that the change in mean HbA1c from 6 months pre-initiation of liraglutide to 12 months’ follow-up was greater in adherent patients compared with non-adherent patients (unadjusted −0.81% vs. −0.42%, p < .001) and in persistent patients than in non-persistent patients (unadjusted −0.78% vs. −0.21%, p < .001); results were similar after regression adjustmentCitation33. In our study, every 10% increase in PDC was associated with a change in HbA1c of approximately −0.2%; it is possible that the higher adherence observed with dulaglutide has an indirect, positive impact on glycemic control (however, our study was not designed to test this causal pathway).
While a multitude of factors can affect treatment adherence, treatment-related issues (e.g. treatment complexity, convenience, cost) are among those considered especially importantCitation11,Citation14. Adherence improves as dosing frequency decreases, for example, from once daily to once weeklyCitation11,Citation21. The mode of medication administration – an important consideration for T2DM treatments that include injectable drugs using syringes versus prefilled pens – also influences adherence, which increases with simpler delivery systemsCitation11,Citation15–18,Citation20. In this regard, a patient-reported outcomes study showed high satisfaction with the dulaglutide single-dose penCitation23. Not surprisingly, as patient out-of-pocket costs increase, adherence decreasesCitation6,Citation11,Citation40–42. Restrictions put in place by commercial health plans limiting quantities or requiring prior authorization for specific classes of drugs, including GLP-1 RAs, may also affect adherenceCitation43,Citation44.
Limitations
Our analysis has some limitations. Glycemic control was evaluated in a subsample of patients who had HbA1c laboratory results available, which may limit generalizability. This concern is partially addressed by our comparison of the smaller sample with HbA1c results to the full study population, which was found to be similar in demographic and clinical characteristics as well as treatment patterns and dulaglutide adherence/persistence. However, the results might not be fully generalizable due to the possibility of other unmeasured differences. Because claims information is collected for billing purposes rather than research, certain patient characteristics such as weight, education and provider preferences that may be associated with outcomes of interest were not available for this study; our estimates of factors associated with glycemic control were not designed to explore causality. In addition, due to the nature of claims data, a filled pharmacy prescription claim does not necessarily indicate that the medication was consumed or taken as prescribed. The patients in this study were all members of a large commercial health plan in the US; the results may not be generalizable to patients with other types of health insurance, the uninsured or those living outside of the US. Although the study population was geographically dispersed, the sample size was limited due to the short time dulaglutide was available on the market at the time the study was conducted.
Conclusions
Initiation of dulaglutide in this real-world setting was associated with a significant improvement in glycemic control 6 months after treatment initiation, as measured by mean changes in HbA1c, proportion of patients with HbA1c < 7%, and clinical scoring success. Patients who adhered to and persisted with dulaglutide therapy experienced better glycemic control 6 months after treatment initiation, compared with those who were not adherent or persistent with therapy. This underlines the importance of medication adherence in improving patient outcomes. Further research is required to determine the real-world effectiveness of dulaglutide compared with other GLP-1 RAs in terms of glycemic control, healthcare resource utilization and costs, and long-term outcomes.
Transparency
Declaration of funding
Funding for this study was provided to HealthCore Inc. by Eli Lilly and Company. Eli Lilly and Company was involved in the study design, data collection and interpretation, the decision to publish, and manuscript preparation.
Author contributions: R.M.: study concept and design; data interpretation; manuscript preparation and critical review; final approval of the manuscript. M.G.: study concept and design; data collection; data interpretation; manuscript preparation and critical review; final approval of the manuscript. M.Y.: study concept and design; data interpretation; manuscript preparation and critical review; final approval of the manuscript. R.T.: study concept and design; data collection; data interpretation; manuscript preparation and critical review; final approval of the manuscript. A.Y.M.K.: study concept and design; data interpretation; manuscript preparation and critical review; final approval of the manuscript. W.Y.: study concept and design; data collection; data interpretation; manuscript preparation and critical review; final approval of the manuscript. L.F.L.: study concept and design; data interpretation; manuscript preparation and critical review; final approval of the manuscript.
Declaration of financial/other relationships
R.M., M.Y., A.Y.M.K and L.F.L. have disclosed that they are employees and stockholders of Eli Lilly and Company. M.G., R.T. and W.Y. have disclosed that they are employees of HealthCore Inc., a wholly owned subsidiary of Anthem Inc. HealthCore, Inc. was under contract with Eli Lilly and Company for the conduct of the study.
Supplemental Material
Download MS Word (187.2 KB)Acknowledgements
Cheryl Jones, an employee of HealthCore Inc., provided writing and editorial support for this manuscript.
References
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Assiciation for the Study of Diabetes (EASD) [published correction appears in Diabetes Care 2013;36:490]. Diabetes Care 2012;35:1364-79
- American Diabetes Association. Standards of Medical Care in Diabetes – 2018. Diabetes Care 2018 Jan;41(Suppl 1):S73-S85
- Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach. Update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2015;38:140-9
- Trujillo JM, Nuffer W, Ellis SL. GLP-1 receptor agonists: a review of head-to-head clinical studies [published correction appears in Ther Adv Endocrinol 2015;6:135-6]. Ther Adv Endocrinol 2015;6:19-28
- Jendle J, Grunberger G, Blevins T, et al. Efficacy and safety of dulaglutide in the treatment of type 2 diabetes: a comprehensive review of the dulaglutide clinical data focusing on the AWARD phase 3 clinical trial program. Diabetes Metab Res Rev 2016;32:776-90
- Curkendall SM, Thomas N, Bell KF, et al. Predictors of medication adherence in patients with type 2 diabetes mellitus. Curr Med Res Opin 2013;29:1275-86
- DiBonaventura M, Wintfeld N, Huang J, et al. The association between nonadherence and glycated hemoglobin among type 2 diabetes patients using basal insulin analogs. Patient Prefer Adherence 2014;8:873-82
- Egede LE, Gebregziabher M, Dismuke CE, et al. Medication nonadherence in diabetes: longitudinal effects on costs and potential cost savings from improvement. Diabetes Care 2012;35:2533-9
- Egede LE, Gebregziabher M, Echols C, et al. Longitudinal effects of medication nonadherence on glycemic control. Ann Pharmacother 2014;48:562-70
- Huber CA, Rapold R, Brüngger B, et al. One-year adherence to oral antihyperglycemic medication and risk prediction of patient outcomes for adults with diabetes mellitus: an observational study. Medicine (Baltimore) 2016;95:e9994
- Polonsky WH, Henry RR. Poor medication adherence in type 2 diabetes: recognizing the scope of the problem and its key contributors. Patient Prefer Adherence 2016;10:1299-307
- Rozenfeld Y, Hunt JS, Plauschinat C, et al. Oral antidiabetic medication adherence and glycemic control in managed care. Am J Manag Care 2008;14:71-5
- Simpson H, Eurich DT, Majumdar SR, et al. A meta-analysis of the association between adherence to drug therapy and mortality. BMJ 2006;333:15
- Qin L, Chen S, Flood E, et al. Glucagon-like peptide-1 receptor agonist treatment attributes important to injection-naive patients with type 2 diabetes mellitus: a multinational preference study. Diabetes Ther 2017;8:321-34
- Buysman E, Conner C, Aagren M, et al. Adherence and persistence to a regimen of basal insulin in a pre-filled pen compared to vial/syringe in insulin-naïve patients with type 2 diabetes. Curr Med Res Opin 2011;27:1709-17
- Davis SN, Wei W, Garg S. Clinical impact of initiating insulin glargine therapy with disposable pen versus vial in patients with type 2 diabetes mellitus in a managed care setting. Endocr Pract 2011;17:845-52
- de Vries ST, Keers JC, Visser R, et al. Medication beliefs, treatment complexity, and non-adherence to different drug classes in patients with type 2 diabetes. J Psychosom Res 2014;76:134-8
- Xie L, Zhou S, Pinsky BW, et al. Impact of initiating insulin glargine disposable pen versus vial/syringe on real-world glycemic outcomes and persistence among patients with type 2 diabetes mellitus in a large managed care plan: a claims database analysis. Diabetes Technol Ther 2014;16:567-75
- Xie L, Zhou S, Wei W, et al. Does pen help? A real-world outcomes study of switching from vial to disposable pen among insulin glargine-treated patients with type 2 diabetes mellitus. Diabetes Technol Ther 2013;15:230-6
- Grabner M, Chu J, Raparla S, et al. Clinical and economic outcomes among patients with diabetes mellitus initiating insulin glargine pen versus vial. Postgrad Med 2013;125:204-13
- Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther 2001;23:1296-310
- Eli Lilly and Company. Trulicity prescribing information. Indianapolis, IN, 2017. Available from: http://uspl.lilly.com/trulicity/trulicity.html#pi [Last accessed 30 March 2017]
- Yu M, Van Brunt K, Milicevic Z, et al. Patient-reported outcomes with once weekly dulaglutide versus placebo, both in combination with once daily insulin glargine (+/- metformin) in type 2 diabetes (AWARD-9). Paper presented at: European Association for the Study of Diabetes 52nd Annual Meeting, Munich, Germany, 12–16 September 2016
- Alatorre C, Fernández Landó L, Yu M, et al. Treatment patterns in patients with type 2 diabetes mellitus treated with glucagon-like peptide-1 receptor agonists: Higher adherence and persistence with dulaglutide compared with once-weekly exenatide and liraglutide. Diabetes Obes Metab 2017;19:953-61
- Anthem. Drug lists: the prescription drugs your plan covers. Available at: https://www11.anthem.com/pharmacyinformation/home.html [Last accessed 6 April 2017]
- National Committee for Quality Assurance. HEDIS measures. Available at: http://www.ncqa.org/hedis-quality-measurement/hedis-measures [Last accessed 3 April 2017]
- Nau DP. Proportion of days covered (PDC) as a preferred method of measuring medication adherence. Pharmacy Quality Alliance, 2011. Available at: http://www.pqaalliance.org/images/uploads/files/PQA%20PDC%20vs%20%20MPR.pdf [Last accessed 3 April 2017]
- Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care 2005;43:1130-9
- Chang HY, Weiner JP, Richards TM, et al. Validating the adapted Diabetes Complications Severity Index in claims data. Am J Manag Care 2012;18:721-6
- Dungan KM, Weitgasser R, Perez Manghi F, et al. A 24-week study to evaluate the efficacy and safety of once-weekly dulaglutide added on to glimepiride in type 2 diabetes (AWARD-8). Diabetes Obes Metab 2016;18:475-82
- Pozzilli P, Norwood P, Jódar E, et al. Placebo-controlled, randomized trial of the addition of once-weekly glucagon-like peptide-1 receptor agonist dulaglutide to titrated daily insulin glargine in patients with type 2 diabetes (AWARD-9). Diabetes Obes Metab 2017;19:1024-31
- Buse JB, Sesti G, Schmidt WE, et al. Switching to once-daily liraglutide from twice-daily exenatide further improves glycemic control in patients with type 2 diabetes using oral agents. Diabetes Care 2010;33:1300-3
- Buysman EK, Liu F, Hammer M, et al. Impact of medication adherence and persistence on clinical and economic outcomes in patients with type 2 diabetes treated with liraglutide: a retrospective cohort study. Adv Ther 2015;32:341-55
- Johnston SS, Nguyen H, Felber E, et al. Retrospective study of adherence to glucagon-like peptide-1 receptor agonist therapy in patients with type 2 diabetes mellitus in the United States. Adv Ther 2014;31:1119-33
- Yu M, Xie J, Fernandez Lando L, et al. Liraglutide versus exenatide once weekly: persistence, adherence, and early discontinuation. Clin Ther 2016;38:149-60
- Nguyen H, Dufour R, Caldwell-Tarr A. Glucagon-like peptide-1 receptor agonist (GLP-1RA) therapy adherence for patients with type 2 diabetes in a Medicare population. Adv Ther 2017;34:658-73
- Durden E, Lenhart G, Lopez-Gonzalez L, et al. Predictors of glycemic control and diabetes-related costs among type 2 diabetes patients initiating therapy with liraglutide in the United States. J Med Econ 2016;19:403-13
- Lin J, Lingohr-Smith M, Fan T. Real-world medication persistence and outcomes associated with basal insulin and glucagon-like peptide 1 receptor agonist free-dose combination therapy in patients with type 2 diabetes in the US. Clinicoecon Outcomes Res 2016;9:19-29
- Wilke T, Mueller S, Groth A, et al. Non-persistence and non-adherence of patients with type 2 diabetes mellitus in therapy with GLP-1 receptor agonists: a retrospective analysis. Diabetes Ther 2016;7:105-24
- Eaddy MT, Cook CL, O’Day K, et al. How patient cost-sharing trends affect adherence and outcomes: a literature review. PT 2012;37:45-55
- Kirkman MS, Rowan-Martin MT, Levin R, et al. Determinants of adherence to diabetes medications: findings from a large pharmacy claims database. Diabetes Care 2015;38:604-9
- Piette JD, Heisler M, Wagner TH. Problems paying out-of-pocket medication costs among older adults with diabetes. Diabetes Care 2004;27:384-91
- Happe LE, Clark D, Holliday E, et al. A systematic literature review assessing the directional impact of managed care formulary restrictions on medication adherence, clinical outcomes, economic outcomes, and health care resource utilization. J Manag Care Spec Pharm 2014;20:677-84
- Shirneshan E, Kyrychenko P, Matlin OS, et al. Impact of a transition to more restrictive drug formulary on therapy discontinuation and medication adherence. J Clin Pharm Ther 2016;41:64-9