Abstract
Background: Among the numerous therapeutic approaches used in the treatment of interstitial cystitis/bladder pain syndrome (IC/BPS) few have been assessed with a sufficient level of evidence. The safety and efficacy of pentosan polysulfate sodium (PPS) has been shown in several open-label and comparative clinical trials with different populations including two meta-analyses. In the context of the approval procedure of PPS for the treatment of IC/BPS by the European Medicines Agency we updated the findings of the previous analyses by incorporating the results of the latest studies.
Method: Relevant studies based on a systematic review of PubMed/Medline and the Cochrane Library in June 2018 were identified. For completeness control, clinical trial registries were also searched. Only randomized, placebo-controlled clinical trials providing sufficient information to estimate at least one relevant effect size measure to compare the efficacy of PPS versus placebo were included in the analysis.
Results: Of the studies identified in the literature search, six randomized placebo-controlled studies met the pre-defined eligibility criteria. Analyses showed no indication of heterogeneity or publication bias. Treatment with PPS led to a statistically significant improvement in the patient’s overall response assessment (p < .001), pain (p = .009) and urgency (p = .005).
Conclusions: Our meta-analyses confirmed the results of preceding meta-analyses showing that PPS is efficacious compared to placebo in the treatment of bladder pain, urinary urgency and frequency of micturition and thus an evident option for the treatment of IC/BPS symptoms.
Introduction
Interstitial cystitis/bladder pain syndrome (IC/BPS) is a poorly defined clinical condition characterized by the symptoms pelvic pain, urinary urgency and urinary frequency. The symptoms overlap with those of other common conditions and are not associated with specific pathognomonic changes.
Currently, IC/BPS is diagnosed via a process of exclusion using the clinical symptoms and occasionally cystoscopic findings since many patients have no cystoscopic findings. It’s a severe and significantly debilitating disease which is often preceded by a long history of suffering before proper diagnosis. Many patients with mild disease are often misdiagnosed with conditions such as recurrent urinary tract infections (UITs), overactive bladder or gynecologic chronic pelvic painCitation1.
For quite some time the term BPS has been used to describe a broader spectrum of symptoms that meet a more inclusive, symptom-based definition, without cystoscopic and histologic features. The change in terminology was put forward by the European Society for the Study of IC/BPS (ESSIC)Citation2 and has been adopted by the European Association of Urology (EAU) in their respective guidelinesCitation3. Nowadays both terms are commonly used, predominantly IC/BPS. The clinical characteristics shared by all conditions falling under the IC/BPS description are rather non-specific and, in order to differentiate diagnostically between these very heterogeneous forms of IC/BPS, a classification scheme was introduced by the ESSIC group and implemented in the 2015 EAU guideline. This reflects the diagnostic usage of biopsy and cystoscopy with hydrodistension including their respective findings. On the other hand the American Urologic Association guidelines for the diagnosis of IC do not require cystoscopy for diagnosis and it may be diagnosed based on symptoms only.
Pharmacological treatments for IC/BPS may be administered either orally or intravesically. Within the US, two treatments are authorized for use in IC/BPS, oral pentosan polysulfate sodium (PPS) or intravesical dimethyl sulfoxide (DMSO).
PPS has become established as an efficacious treatment for IC/BPS, which is characterized by either glomerulations or Hunner’s lesions. The very first randomized placebo-controlled clinical trial investigating the efficacy of PPS in treating IC/BPS was published in 1987. Subsequently several randomized, controlled clinical trials and two meta-analyses have been published which evaluate the efficacy of PPS in the treatment of IC/BPS. Hwang et al. conducted a first meta-analysis covering the period 1966–1994Citation4. Based on a literature search four randomized, controlled trials (RCTs) including 398 patients were identified showing benefit differences for pain, urgency and frequency in favor of PPS. In 2007, Dimitrakov et al.Citation5 published a systematic review of the pharmacologic management of IC. They identified five placebo-controlled RCTs out of six RCTs with a total of 633 patients, whereby a meta-analysis of effects suggests a statistically significant benefit for the treatment with PPS over placebo.
While PPS capsules for the treatment of IC/BPS were approved in 1993 in Canada, 1994 in Australia and 1996 in the US, it was only in June 2017 that PPS capsules (Elmiron1) gained a marketing authorisation by the European Commission as the first drug treatment for IC/BPS characterized by either glomerulations or Hunner’s lesions in adults with moderate to severe pain, urgency and frequency of micturition.
In relation to the approval procedure by the European Commission, the findings of the comprehensive analyses by Hwang et al. and Dimitrakov et al.Citation4,Citation5 have been updated using a more conservative intent-to-treat (ITT) approach instead of the original “as reported” approach and extended by incorporating the results of the latest studyCitation6.
There was upfront analysis planning but the statistical analyses were also influenced by the submission process with regulatory advice implemented at the time when received. As a consequence no final and complete planning document was generated and registered on PROSPERO a priori. Current methodological guidance for reporting items for systematic reviews and meta-analysesCitation7 has been applied in order to compare and critically evaluate all data available from randomized, placebo-controlled trials testing PPS for the treatment of IC/BPS.
Evidence acquisition
Search strategy
Relevant studies based on a systematic review of PubMed/Medline and the Cochrane Library in June 2018 were identified using the free-text search control terms “interstitial cystitis” or “bladder pain syndrome” and “clinical trial”. Simultaneously a search for additional potentially unpublished studies on PPS was conducted on ClinicalTrials.gov as provided by the US National Library of Medicine and the European Union Clinical Trials Register for the active drug “pentosan polysulfate”. After a first review of abstracts, full text was studied in more detail to extract the required information.
Eligibility criteria
Only randomized, placebo-controlled clinical trials providing sufficient information to estimate at least one relevant effect size measure to compare the efficacy of oral PPS versus placebo were included in the meta-analyses following the reporting items for systematic reviews and meta-analysesCitation7.
To be included in the meta-analyses, studies had to: (a) evaluate the therapeutic effect of oral PPS on treating interstitial cystitis; (b) be placebo-controlled clinical trials; (c) involve patients with a clinical and cystoscopically verified diagnosis of IC; and (d) provide sufficient information to estimate at least one relevant effect size measure to compare the efficacy of PPS versus placebo.
Evidence synthesis
The search identified 268 manuscripts, 1 Cochrane review and 22 records from clinical trial databases. Six manuscripts were included in the meta-analysis (, ).
Figure 1. Evidence acquisition in a systematic review on pentosan polysulfate in the treatment of bladder pain syndrome characterized by either glomerulations or Hunner’s lesions in adults with moderate to severe pain, urgency and frequency of micturition.
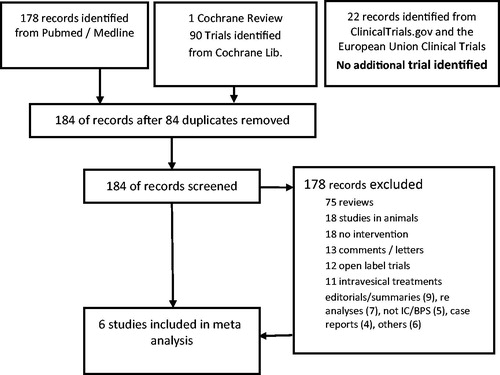
Table 1. Relevant characteristics of studies included into the meta-analysis.
Data extraction
Assessment of the impact of the different symptoms of BPS is very subjective and differs between patients. If patients assess “global response” to treatment, the measured effect size will reflect the significance of the single symptoms for the individual patient. Accordingly, the main focus of the meta-analysis was patient global response assessment (GRA), which was assessed in most of the identified studies. A responder definition of at least moderate or 50% improvement in patient GRA has become established in the medical community as a clinically relevant and meaningful improvement in BPSCitation8. Further correlations with increased patient satisfaction, improved sleep scores and improved sexual functioning show the clinical relevance of the chosen responder definition for an improvement in GRA. For studies that did not evaluate any form of global improvement versus baseline, data was imputed based on the closest information provided in the respective publication. In addition, the effect of PPS on pain and urgency was assessed. No imputations for results on pain or urgency were conducted.
Effect size data for the meta-analyses of target variables was extracted from the original publications as well as from the meta-analyses identified in the literature searchCitation4,Citation5. Reasonable efforts were made to clarify any discrepancies, including contacting authors of the publications, as far as possible.
Methods of statistical analysis
As the individual studies mostly analyzed and compared success rate differences, this approach was also followed for the meta-analyses. Success rate was defined as the proportion of responders per group and differences in success rates (PPS - placebo) were denoted as benefit differences as a greater difference in rates indicated higher benefit with PPS. The intent-to-treat (ITT) principle including all randomized patients was used for the meta-analyses. Patients with missing data were considered as failure.
Heterogeneity was assessed using Q and I2 statistics. Publication bias was tested using various statistical methods (classic fail-safe N; Orwin’s fail-safe N; Begg and Mazumdar rank correlation test; Egger’s regression test); the funnel plot was used to assess the symmetry of the distribution of studies. To estimate the between-study variance, the method of moments by DerSimonian and LairdCitation9 was used in all meta-analyses.
To assess the treatment effect on symptom scores, mean changes from baseline and standard deviations were used. In most studies these results were immediately available, in other studies standard deviations were estimated from other measures e.g. from p values. The standardized mean difference was used as the effect measure. This was calculated by dividing the raw mean difference between groups by the pooled standard deviation of the two treatment groups. Comprehensive Meta-Analysis Software from Biostat2 and JMP3 were used for conducting the meta-analyses.
Results
Of the studies identified in the literature search, and in accordance with the search results of two previous meta-analysesCitation4,Citation5, six randomized placebo-controlled studies met the pre-defined eligibility criteria and were included in the meta-analyses conducted. The study characteristics are summarized in .
Overall, the patient population enrolled was comparable across studies for the proportion of female patients and age distribution, and is reflective of the general population suffering from BPS. In four studies, the majority of patients had their diagnosis confirmed by cystoscopyCitation10–13, while Sant et al. and Nickel et al.Citation6,Citation14 enrolled a more heterogeneous patient population, in whom the diagnosis was mainly based on clinical symptoms.
The last two studies were conducted in the US at a time, when PPS was commercially available for the treatment of IC. Both studies faced severe recruitment problems, which might have led to enrollment of patients who had previously been treated with PPS.
Two dosing schemes (3 × 100 mg PPS per day and 2 × 200 mg PPS per day) were evaluated in the six studies, which were considered comparable. Furthermore, the treatment periods of the studies covered 3–6 months and were considered comparable, although longer treatment with PPS is expected to lead to better effects.
All studies focused on patient-reported response to treatment. However, only three studies (Mulholland et al., Parsons et al., Sant et al.)Citation11–13 evaluated comparable primary endpoints (the proportion of patients with ≥50% [moderate] global improvement from baseline). A fourth study by Nickel et al.Citation6 used a responder analysis based on a 30% improvement in the ICSI (Interstitial Cystitis Symptom Index); a responder analysis based on a 50% improvement in the GRA scale was evaluated as secondary endpoint.
The study reported by Parsons and MulhollandCitation10 did not evaluate any form of global assessment but separately evaluated patient-reported improvement for four distinct subjective symptoms (urgency, frequency, nocturia and pain). A patient reaching a 50% improvement compared to baseline was considered a responder for the specific symptom. The mean response rates by treatment group across the individual response rates of pain, urgency and frequency were imputed for this study as response rates for the primary meta-analysis.
Holm-Bentzen et al.Citation14 evaluated improvement of the patients under therapy by assessing the mean total symptom score values with regard to the symptoms pain, frequency, nocturia, dysuria and urgency. A decrease in the total symptom score values was classified as at least four steps (with a total of 1.00 or 0.25 for each step). A decrease of at least 1.00 in mean total symptom score was considered to be a clinically significant improvement. Only absolute improvement was evaluated; no relative comparison to baseline made. The responder analysis based on an improvement of at least 1.00 in mean total symptom score was therefore imputed in the primary meta-analysis in order to approach the responder definition (at least moderate or 50% improvement) used in all other studies as close as possible.
While Holm-Bentzen et al. and Nickel et al.Citation6,Citation14 did not show a statistical difference between PPS and placebo, four studies detected a benefit of PPS over placebo, of which the studies by Parsons and Mulholland, Mulholland et al. and Parsons et al.Citation10–12 revealed a clearly statistically significant difference. The Sant et al.Citation13 study for the primary efficacy analysis used a rather conservative analysis method (exact conditional test version of the Mantel–Haenszel method to control for clinical center clustering) resulting in a p value of .064; this was in slight contrast to our testing result for this study (p = .042) which was based on the simple chi-square test as for all individual studies included in this meta-analysis.
The efficacy endpoints evaluated in the placebo-controlled studies were all based on a patient-reported outcome of subjective symptoms. In such analyses, placebo effects are often high. Furthermore, each of the studies enrolled a very limited number of patients due to the rarity of the disease. These two aspects made it difficult to consistently detect statistically significant differences in the individual studies. Accordingly, meta-analysis of these studies is appropriate to assess and estimate the overall difference in efficacy between PPS and placebo (see also ).
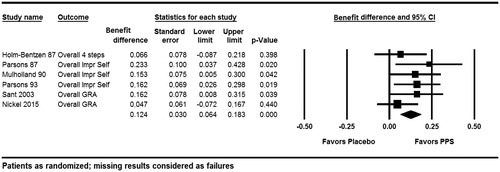
Figure 2. Global response assessment (Holm-BentzenCitation14, ParsonsCitation10, MulhollandCitation11, ParsonsCitation12, SantCitation13, NickelCitation6).
Results of meta-analysis
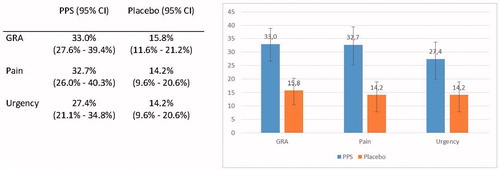
In order to evaluate the improvement in individual patients’ burden of disease, the primary meta-analysis evaluated the percentage of patients that experienced a clinically relevant improvement in GRA compared to baseline (). A 12.4% difference in the responder rates between PPS-treated patients and placebo-treated patients was estimated (95% CI: 6.4%–18.3%). The combined difference between the two groups was highly statistically significant (p < .001). This primary meta-analysis showed no indication of heterogeneity in standard measures (Q-value = 4.019, p = .547, I2 = 0).
The meta-analysis of the primary endpoints from the publications did not show any indication of heterogeneity (Q-value = 5.098, p = .404, I2 = 1.924%); the estimated benefit difference was 11.9% (95% CI: 5.8%–18.0%) and thus very close to that of the GRA-based analysis presented in .
In addition, the efficacy of PPS on pain () and urgency () was evaluated across all identified studies as far as respective results were reported. An improvement in pain and urgency was considered to be a ≥50% reduction compared to baseline.
Figure 3. Improvement in pain (Holm-BentzenCitation14, ParsonsCitation10, MulhollandCitation11, ParsonsCitation12, NickelCitation6).
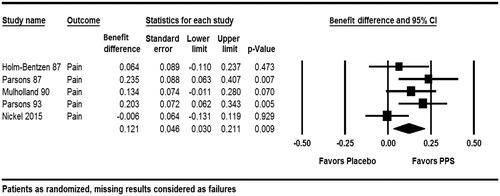
Figure 4. Improvement in urgency (ParsonsCitation10, MulhollandCitation11, ParsonsCitation12, NickelCitation6).
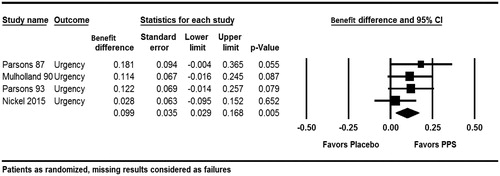
For an improvement in pain, the estimated combined responder rate difference between PPS and placebo was 12.1% (95% CI: 3.0–21.1%; p = .009). Moderate heterogeneity was detected for the efficacy of PPS on pain observed across the studies (Q = 7.35, p = .119, I2 = 45.6%). The results for an improvement in urgency showed a statistically significant difference of 9.9% (95% CI: 2.9–16.8%) in the responder rates in favor of PPS (p = .005). The meta-analysis revealed no indication of heterogeneity (Q = 2.163, p = .539, I2 = 0%).
Efficacy of pentosan polysulfate in patients with interstitial cystitis/bladder pain syndrome diagnosed based on cystoscopic findings
Focusing on the four studies that limited enrolment to those patients diagnosed based on cystoscopic examinations, the respective outcome results were even clearer. This was not unexpected given the more homogeneous patient population. This patient population represents the patient population for whom PPS is currently indicated in the EU.
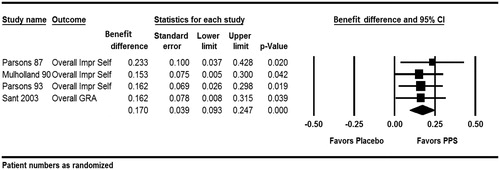
The primary meta-analysis on these four studies showed a benefit difference of 17.0% (95% CI: 9.3–24.7%) in favor of PPS (). The difference was highly statistically significant (p < .001) without any indication of heterogeneity (Q = 0.470, p = .925, I2 = 0).
Figure 5. Global response assessment, cystoscopically diagnosed patients only (ParsonsCitation10, MulhollandCitation11, ParsonsCitation12, SantCitation13).
A more appropriate assessment of the clinical relevance of the PPS effect size is a pooled analysis of results per treatment group (PPS treatment versus no PPS treatment) as the pooled results allow a comparison between the magnitudes of treatment effects in each treatment group. Results of this analysis revealed an approximate duplication of response rates between PPS and placebo for GRA, pain and urgency ().
The duplication of the treatment effect was confirmed by an adequate meta-analysis which resulted in a benefit ratio of GRA of 2.085 (95% CI: 1.464–2.967) ().
Figure 7. Global response assessment, benefit ratio (ParsonsCitation10, MulhollandCitation11, ParsonsCitation12, SantCitation13).
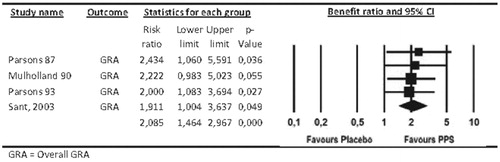
Additionally, there was no indication of heterogeneity for this meta-analysis (Q = 0.245, p = .970, I2 = 0). This confirms that the benefit ratio is an appropriate measure to describe the superiority of PPS over placebo.
Publication bias
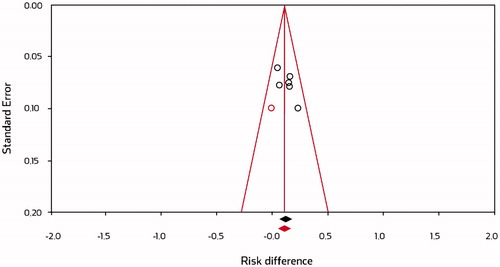
Six methods were applied to investigate publication bias; three of them (including visual inspection of the funnel plot) identified potential trends for publication bias. This finding is best quantified by Duval and Tweedie’s Trim and Fill approach () which identified one study as possibly missing and this missing study was imputed to achieve approximate symmetry. Based on this conservative imputation (red circle in ), the meta-analysis still showed a clear superiority of PPS over placebo: 0.113 (0.056, 0.170) (difference in GRA response rates with 95% CI).
Discussion
Four studies (Parsons and Mulholland; Mulholland et al.; Parsons et al.; Sant et al.)Citation10–13 and meta-analyses by Hwang et al. and Dimitrakov et al.Citation4,Citation5 have demonstrated the efficacy of PPS in the treatment of BPS. However, this clear and homogeneous picture has been challenged in a more recent publication by Nickel et al.Citation6 in 2015 which has raised questions.
Although the Nickel et al.Citation6 study was a large study enrolling an appropriate number of patients and was planned with good intent in accordance with best practices, it did present several challenges. The relevant meta-analyses did not show heterogeneity in standard measures used, but it was rather obvious for us that the study deviated from the remaining studies and a lot of effort was spent to explore and explain this deviation. The authors themselves acknowledged considerable differences in the enrolled patient population compared to earlier studies. Patients were enrolled with milder symptoms, without applying cut-off criteria for pain or urgency, without determination of flare status, without an entry criterion based on cystoscopy findings, and without the exclusion of conditions commonly associated with BPS such as irritable bowel disease, depression or pelvic floor dysfunction disease. In addition, the commercial availability of PPS for the treatment of IC/BPS some time before the study was conducted may have impacted patient recruitment, leading to a very slow recruitment rate and ultimately to early termination of the study. Furthermore, by potentially including patients who were not PPS-naive, the study may have inadvertently selected patients who were non-responders to previous PPS therapy or potential non-responders based on disease phenotype. All these factors could have severely biased the treatment and placebo responses; a response was achieved in 51% of patients treated with placebo. If analysis is limited to only patients completing the study, the placebo response rate is 76%. This high placebo response rate was likely enhanced by the described multiple factors of patient selection, including patients with milder IC entering during a symptom flare, regression to the mean, introduction (inadvertent or not) of conservative therapy, which accentuated the benefits of placebo in another recent trial, and failure of clinical sites to keep patients in the trial which resulted in a high dropout rate.
The forest plot in illustrates the high placebo response rate in the Nickel et al. study compared to the placebo response rates of all other studies found in the literature. The response rate in the PPS group of the Nickel study corresponds well with the overall picture of success rates for PPS, but the particularly high response rate in the placebo group is a point of concern. Overall, there are severe limitations to the Nickel study with regard to early termination, study design, enrolment criteria and high dropout rates.
Figure 9. Success rates under placebo - comparison of placebo response rates (ParsonsCitation10, MulhollandCitation11, ParsonsCitation12, SantCitation13, van OphovenCitation16, WarrenCitation17).
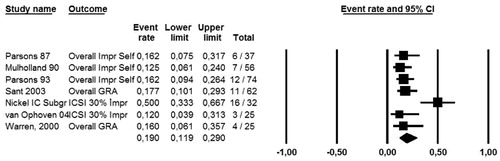
To gain a clear indication of the efficacy of PPS based on all placebo-controlled, randomized clinical studies conducted to date, the meta-analyses described above were conducted. Based on the large number of patients (n = 368) enrolled in the Nickel study, the study results were assessed with relevant weight in these meta-analyses (as demonstrated by the size of the square describing the results per study in ).
The overall evaluation of all available data resulted in a statistically significant benefit of PPS over placebo in terms of patients reaching at least a moderate or ≥50% improvement in their GRA compared to baseline. Furthermore, statistically significantly more patients reached a ≥50% improvement in pain and urgency compared to baseline. These effects were even more pronounced when the meta-analysis focused on the more homogeneous patient population that was diagnosed based on cystoscopic findings, which is not an unexpected finding.
The pooled response rate on GRA improvement following PPS treatment indicated that 33.0% of patients experienced a clinically relevant reduction in disease burden while the same effect was only observed in 15.8% treated with placebo. The relative difference of 109% in responder rates between PPS and placebo is clearly clinically relevant. This is supported further by the effects on the symptoms pain and urgency following PPS treatment. For GRA the percentage of responders experiencing ≥50% improvement in pain and urgency was approximately two-fold higher following PPS compared to placebo.
As stated in more detail before, in 2008 the ESSIC classification was established accompanied with a change in terminology, reflecting the diagnostic value of biopsy and cystoscopy. This classification became established as a de-facto standard to differentiate diagnostically between distinct forms of IC/BPS. Recognizing this, evidence regarding differences in the therapeutic benefit of PPS in relation to biopsy results and cystoscopic findings would be valuable. However, the available results of the early studies included do not provide further information in this regard. While most studies were conducted long before the ESSIC criteria became established, this also applies in particular for the latest study.
Conclusion
Our current meta-analysis confirms the results of a preceding meta-analysis and comprehensive review showing that PPS is more efficacious than placebo in the treatment of pain, urgency and frequency, and beneficial for symptoms of IC/BPS. The response rates of the studies included correspond well with the overall picture of success rates for PPS, but limitations like the particular high drop-out rate and response rate in the placebo group in the latest studies are to be considered.
In summary, treatment with PPS led to a statistically significant and clinically relevant improvement in patients’ overall response assessment (at least moderate or ≥50% improvement from baseline) in addition to clinically relevant improvements in the main symptoms of IC/BPS, i.e. pain and urgency (≥50% improvement from baseline). Thus PPS is an evident option for the treatment of IC/BPS symptoms as stipulated in the clinical guidelines of the relevant professional associationsCitation3,Citation15.
Transparency
Declaration of funding
All work performed was financially supported by bene-Arzneimittel GmbH and bene pharmaChem GmbH & Co.KG.
Author contributions
Study concept and design: A.v.O., G.A., K.P.M., K.V., W.K.; data acquisition: K.P.M., K.V., W.K.; analysis and interpretation of data: A.v.O., G.A., K.P.M., K.V., W.K.; manuscript: A.v.O., K.P.M., K.V.; revision: A.v.O., G.A., K.P.M., K.V., W.K.; supervision: A.v.O., K.P.M.
Declaration of financial/other relationships
A.v.O., K.V., W.K., G.A. and K.P.M. have disclosed that they are employees, former employees or consultants of bene-Arzneimittel GmbH and bene pharmaChem GmbH & Co. KG. Pfleger Arzneimittel GmbH markets Elmiron in Germany; bene pharmaChem GmbH & Co. KG is the manufacturer of pentosan polysulfate. CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
No assistance in the preparation of this article is to be declared.
Notes
Notes
1 Elmiron is a registered trade mark of bene-Arzneimittel GmbH, Munich, Germany.
2 Comprehensive Meta-Analysis (CMA) software for meta-analysis Version 3, Biostat, Englewood, USA.
3 JMP (Version 11.2.1) is a registered trade mark of SAS Institute Inc., Cary, NC, USA.
References
- Parsons CL. The role of a leaky epithelium and potassium in the generation of bladder symptoms in interstitial cystitis/overactive bladder, urethral syndrome, prostatitis and gynaecological chronic pelvic pain. BJUI Int. 2011;107:370–375.
- van de Merwe JP, Nordling J, Bouchelouche P, et al. Diagnostic criteria, classification and nomenclature for painful bladder syndrome/interstitial cystitis: an ESSIC Proposal. Eur Urol. 2008;53:60–67.
- Engeler D, Baranowski AP, Borovicka J, et al. Guidelines on Chronic Pelvic Pain. 2015. Available from: https://uroweb.org/wp-content/uploads/EAU-Guidelines-Chronic-Pelvic-Pain-2015.pdf
- Hwang P, Auclair B, Beechinor D, et al. Efficacy of pentosan polysulfate in the treatment of interstitial cystitis: a meta-analysis. Urology. 1997;50:39–43.
- Dimitrakov J, Kroenke K, Steers WD, et al. Pharmacologic management of painful bladder syndrome/interstitial cystitis: a systematic review. Arch Intern Med. 2007;167:1922–1929.
- Nickel JC, Herschorn S, Whitmore KE, et al. Pentosan polysulfate sodium for treatment of interstitial cystitis/bladder pain syndrome: insights from a randomized, double-blind, placebo-controlled study. J Urol. 2015;193:857–862.
- Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Clin Epidemiol. 2009;62:e1–e34.
- Lubeck DP, Whitmore K, Sant GR, et al. Psychometric validation of the O’leary–Sant interstitial cystitis symptom index in a clinical trial of pentosan polysulfate sodium. Urology. 2001;57:62–66.
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188.
- Parsons CL, Mulholland SG. Successful therapy of interstitial cystitis with pentosanpolysulfate. J Urol. 1987;138(3):513–516.
- Mulholland SG, Hanno P, Parsons CL, et al. Pentosan polysulfate sodium for therapy of interstitial cystitis. A double-blind placebo-controlled clinical study. Urology. 1990;35(6):552–558.
- Parsons CL, Benson G, Childs SJ, et al. A quantitatively controlled method to study prospectively interstitial cystitis and demonstrate the efficacy of pentosanpolysulfate. J Urol. 1993;150(3):845–848.
- Sant GR, Propert KJ, Hanno PM, et al.; Interstitial Cystitis Clinical Trials Group. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol. 2003;170(3):810–815.
- Holm-Bentzen M, Jacobsen F, Nerstrøm B, et al. A prospective double-blind clinically controlled multicenter trial of sodium pentosanpolysulfate in the treatment of interstitial cystitis and related painful bladder disease. J Urol. 1987;138(3):503–507.
- Hanno P. International Journal of Urology supplement. 3rd International Consultation Interstitial Cystitis Japan (ICICJ) and International Society for the Study of Bladder Pain Syndrome (ESSIC) Joint Meeting 21–23 March 2013 Kyoto, Japan. Int J Urol. 2014;21:3.
- van Ophoven A, Pokupic S, Heinecke A, et al. A prospective, randomized, placebo controlled, double-blind study of amitriptyline for the treatment of interstitial cystitis. J Urol. 2004;172(2):533–536.
- Warren JW, Horne LM, Hebel JR, et al. Pilot study of sequential oral antibiotics for the treatment of interstitial cystitis. J Urol. 2000;163(6):1685–1688.