Abstract
Objective: To prospectively evaluate the abuse potential of NKTR-181, a novel opioid analgesic, in two phase 3 clinical trials using a newly developed reporting system: the Misuse, Abuse, and Diversion Drug Event Reporting System (MADDERS®).
Methods: SUMMIT-07 was an enriched enrollment randomized withdrawal study that examined the safety and efficacy of NKTR-181 across 12 weeks in opioid-naïve subjects with chronic low back pain. SUMMIT-LTS was a 52 week open-label study in opioid-naïve and experienced subjects with chronic low back pain or noncancer pain rolled over from SUMMIT-07 or enrolled de novo. System evaluations were triggered by adverse events of interest and drug accountability discrepancies signaling potentially abuse-related events. Each event was assigned a primary classification and supplementary classification(s) by investigators and by a blinded, independent committee of substance abuse experts (adjudicators). At the final study visit, investigators administered a survey to subjects to identify overlooked events of interest.
Results: Seventy-nine (6.6%) of 1189 subjects were associated with 86 events in SUMMIT-07 and 51 (8.0%) of 638 subjects were associated with 59 events in SUMMIT-LTS. Most events were attributed to “Withdrawal” and, primarily in SUMMIT-07, “Therapeutic Error” (unintentional overuse) or “Misuse” (intentional overuse for a therapeutic purpose) of study medication. Adjudicators identified five possible “Abuse” events (three NKTR-181, two placebo) in SUMMIT-07 and four possible “Abuse” events (all NKTR-181) in SUMMIT-LTS.
Conclusions: The MADDERS® system discerns potentially abuse-related events and identified low rates of withdrawal and a low risk of abuse potential, diversion or addiction associated with NKTR-181 in phase 3 trials.
Introduction
Prescription drug abuse is a major public health problem in the United StatesCitation1,Citation2. A key strategy for addressing this issue involves the development of new medications with lower abuse potential and the proper assessment of abuse potential during clinical development. The US Food and Drug Administration (FDA) final guidance on the assessment of abuse potential of drugsCitation3 indicates that drugs with central nervous system (CNS) activity and suspected abuse potential based on pre-clinical evidence or pharmacological characteristics require a full assessment of abuse potential in humans. Full assessment includes identifying potentially abuse-related events in randomized, controlled and open-label safety and efficacy (typically phase 2–4) clinical trials and reporting those events to the FDA to assist the agency in approval, labeling and scheduling recommendations.
Historically, no validated tools have been available for assessing drug abuse potential in clinical trials. Traditional methodologies include the retrospective identification of treatment-emergent side effects and adverse events (AEs) as well as the use of patient-reported and clinician-reported instruments; however, these tools have limited reliability and can lead to the misclassification of abuse-related events, resulting in an overestimation or underestimation of a drug’s true abuse potentialCitation4. A recent systematic review by the Analgesic, Anesthetic, and Addiction Clinical Trial Translations, Innovations, Opportunities, and Networks (ACTTION) public–private partnership in conjunction with the FDA concluded that none of the abovementioned approaches represent a standardized, acceptable method for identifying and evaluating events related to misuse or abuse in clinical trialsCitation4,Citation5. Additionally, many current methodologies do not evaluate instances of tampering with medication or the dispensing device or drug use discrepancies (e.g. differences between the expected and actual amount of medication returned by a study subject) and do not implement standardized definitions to categorize and report potentially abuse-related events.
The Misuse, Abuse, and Diversion Drug Event Reporting System (MADDERS®)Citation1 was developed to provide a more complete characterization of potentially abuse-related events as they occur in clinical settings, helping sponsors and the FDA to more accurately evaluate a drug’s abuse potential in patient populations. Specifically, it is a multi-step process that standardizes: (1) the prospective identification of potentially abuse-related events by trained investigators and site staff; (2) the collection of relevant event-related information in real time using standardized forms; (3) formal adjudication of events by third-party substance abuse experts based on the consensus terminology and definitions set forth by ACTTION; and (4) tabulating and reporting events per FDA regulatory requirements for clinical trial dataCitation6,Citation7. To date, the system has been utilized in 17 phase 2–4 clinical trials investigating a range of psychoactive medications for pain and non-pain indications in adult and pediatric populations. The Abuse Liability Evaluation for Research, Treatment, and Training (ALERTT) working group of ACTTION concluded that MADDERS® is “the only system available to comprehensively evaluate inappropriate medication use events prospectively in order to determine the underlying intent”Citation5.
NKTR-181 is a new molecular entity mu-opioid agonist that exhibits a slow rate of entry into the CNS and slow mu-opioid receptor activation, possibly indicating a lower abuse potential relative to conventional opioidsCitation8. In order to prospectively and systematically examine the abuse potential of NKTR-181 in patient populations, the MADDERS® system was recently utilized in two large multicenter phase 3 clinical trials (SUMMIT-07 and SUMMIT-LTS). SUMMIT-07 was a double-blind, randomized, placebo-controlled clinical trial that assessed the efficacy, safety and tolerability of NKTR-181 in subjects with moderate to severe chronic low back pain. SUMMIT-LTS was an open-label study that evaluated the long-term safety and tolerability of NKTR-181 in subjects with moderate to severe chronic low back pain and noncancer pain. Results from SUMMIT-07 indicate that NKTR-181 maintained a statistically significant decrease in weekly pain scores over the course of 12 weeks and was well tolerated by patientsCitation9. Here, we describe the results of the MADDERS® system implementation in the phase 3 clinical program of a novel opioid analgesic and provide an operational analysis of the system.
Methods
Patients
The SUMMIT-07 study enrolled opioid-naïve subjects with moderate to severe chronic low back pain. Key inclusion criteria were: age 18–75 years; a body mass index of 18–39 kg/m2; a clinical diagnosis of moderate to severe chronic low back pain for a minimum of 6 months; a 7-day average pain score of 5–9 points on the 0–10 numerical rating scale (NRS) during the screening period; inadequate pain relief or failed treatment with non-opioid analgesics; and opioid-naïve status defined as taking no more than 10 mg morphine sulfate equivalents (MSEs)/day of a short-acting opioid for 14 days prior to signing informed consent for trial participation. Exclusion criteria included a history of substance, alcohol or opioid abuse within the last year; known history of hypersensitivity, intolerance or allergy to opioids, acetaminophen or hydrocodone; withdrawal symptoms during screening; use of an extended-release/long-acting opioid within 6 months of signing informed consent; untreated back pain or other lower back pathology; and a history of low back surgery or intention to undergo low back surgery during the study period. Screening for past year substance, alcohol or opioid abuse was conducted via patient medical history, urine drug testing and alcohol breathalyzer at the screening visit.
The SUMMIT-LTS study included rollover subjects from the SUMMIT-07 study and de novo subjects who met specific entry criteria. Rollover subjects were included if they completed the end of treatment visit for SUMMIT-07 and had no break in treatment with the study drug. Eligible de novo subjects had a clinical diagnosis of moderate to severe chronic low back pain or chronic noncancer pain for more than 3 months and were either opioid naïve or treating their chronic pain with 10–60 mg MSEs/day for at least 7 days before signing informed consent. In addition to the SUMMIT-07 exclusion criteria, subjects in the SUMMIT-LTS study were excluded if they had a diagnosis of chronic migraine. Both studies were conducted in accordance with the ethical principles outlined in the Declaration of Helsinki and in accordance with Good Clinical Practice guidelines and all applicable federal regulations. All patients received a full explanation of the study procedures and provided written informed consent prior to study participation. Both trials were registered with clinicaltrials.gov (NCT02362672, NCT02367820).
Study procedures
The SUMMIT-07 study was a double-blind enriched enrollment randomized withdrawal design that included a screening period followed by an open-label titration period and subsequent double-blind randomized treatment period. The primary study endpoint was change in weekly pain score relative to baseline. During the titration period, subjects were initially dosed with NKTR-181 at 100 mg twice per day (BID) and subsequently titrated to a final dose of 100 mg, 200 mg, 300 mg or 400 mg BID over a period of 3–7 weeks depending on tolerability and effectiveness. Acetaminophen (500 mg tablets, 1–2 tablets every 6 h as needed, up to 6 tablets per day) was available as rescue medication during the titration period. Subjects were eligible for randomization to treatment if they had a 7 day average pain score of ≤4 points on the NRS with average daily pain of ≤4 on 5 of 7 days; had a decrease in pain from baseline of ≥2 points on the NRS; and did not use rescue medication more than twice in the 7 days preceding randomization. Subjects who did not achieve adequate pain control with a maximum dose of 400 mg BID or those who did not tolerate the study drug were discontinued from the study. Eligible subjects were randomized in a 1:1 ratio to receive either the titrated dose of NKTR-181 or placebo BID for 12 weeks. Those randomized to placebo were not tapered from study drug after randomization; however, 5 mg hydrocodone/300 mg acetaminophen (1 tablet every 6 h as needed, up to 2 tablets per day) was provided as rescue medication during the first 2 weeks of the randomized treatment period to address possible withdrawal symptoms related to discontinuation of the study drug. All subjects completed a 1 week double-blind taper at the end of the 12 week randomized treatment period.
The SUMMIT-LTS study was conducted to evaluate the long-term safety and tolerability of NKTR-181 and was divided into three periods: a screening period of up to 21 days, a 52 week open-label treatment period (51 weeks and a 1 week taper), and a safety follow-up period. Subjects who were rolled over from SUMMIT-07 were screened for SUMMIT-LTS eligibility during regularly scheduled SUMMIT-07 study visits. The end of treatment visit for rollover subjects in SUMMIT-07 was the first visit of the open-label treatment period for SUMMIT-LTS. Rollover subjects from SUMMIT-07 were tapered down to 100 mg BID by the end of treatment visit for that study. During the SUMMIT-LTS treatment period, all subjects were titrated upward from a starting dose of 100 mg BID to a maximum dose of 600 mg BID. Eligible de novo subjects who receiving opioid treatment prior to entering SUMMIT-LTS were tapered to an opioid dose of no more than 30 mg MSE/day over a 1 week period before beginning NKTR-181 treatment. Only over-the-counter analgesics (e.g. aspirin, ibuprofen) were available as rescue medication. The primary study endpoint was assessment of long-term safety. The study also evaluated the maintenance of long-term pain control using the modified Brief Pain Inventory–Short Form at clinic visits.
MADDERS® system
MADDERS®Citation6 was used in the SUMMIT-07 and SUMMIT-LTS studies to identify potentially abuse-related events occurring in association with use of the MADDERS® study drug. As defined by ACTTION, “abuse” is any intentional, non-therapeutic use of a drug for the purpose of achieving a desirable psychological or physiological effect. Per the MADDERS® system, a “potentially abuse-related event” was any event possibly signifying abuse (e.g. an AE of interest or drug accountability discrepancy) that required further information for adjudication as an abuse-related event or otherwise. “Abuse-related events” is a general category that refers to a broad set of behaviors that may represent or indicate abuse or may be confused with abuse (e.g. misuse or suicide-related ingestion of medication), are important consequences of abuse (e.g. overdose), are concomitants of abuse (e.g. medication tampering) or form part of the broader assessment of abuse potential (e.g. withdrawal). All site staff and adjudicators received comprehensive training on the system’s definitions and processes and were required to pass a competency quiz or correctly classify mock cases before each trial, respectively.
MADDERS® system assessment of a potentially abuse-related event was triggered in two ways in the SUMMIT-07 and SUMMIT-LTS studies. Triggering events were identified during scheduled study visits by qualified study staff who were trained prior to the study start and consisted of either: (1) pre-specified triggering AE terms or categories that possibly indicate abuse potential of study medicationCitation6 or (2) drug accountability discrepancy events (DADEs). The AE terms and categories included: drug abuse; drug misuse; thoughts of suicide, attempted suicide or suicide; therapeutic error; drug withdrawal or drug withdrawal syndrome; addiction behavior; diversion; overdose; tampering or altering the route of administration; euphoria or inappropriate elation; and drunkenness, feeling high or intoxication. Co-use of the study medication with licit or illicit CNS-active drugs could trigger a system event; however, staff were trained to use their clinical judgment to determine whether use of other drugs while on study medication would constitute a potentially abuse-related event and, if so, to determine whether the event was related to use of the study medication or another substance. In other words, treatment-emergent AEs that involved co-use of other substances could be considered potentially abuse-related events depending on the type of AEs involved and the context. For example, a subject reporting profoundly altered or euphoric mood after consuming multiple alcoholic beverages at dinner while taking study medication would constitute a triggering event to collect more information from the subject about the event. The DADEs consisted of missing pills exceeding a pre-specified threshold (i.e. ≥20% less than expected amount of study drug returned at a site visit) and tampering (e.g. recovered drug was not intact, not including pierced blisters or loose tablets removed from wallet).
After the identification of a triggering event by trained site staff, additional event-related information was collected using a Supplemental AE or Supplemental DADE Form during a structured interview with the subject. The interview was conducted as soon as possible after the event occurred and the questions were designed to probe for contextual information about the circumstances surrounding the event. Event details and answers provided by study subjects were used by site investigators to assign one of six primary event category classifications and, if applicable, one or more supplemental classifications () using a Site Classification Form. Additionally, at the final study visit (i.e. end of study or early termination), all subjects who took any amount of study drug completed a Medication Use Survey (MUS) in the form of a structured interview administered by site staff. MUS questions were designed to capture any instances of potentially abuse-related events or behaviors that were not identified prospectively by site staff as triggering AEs or DADEs. When a subject who had a triggering event exited the study, a listing of his or her relevant individual clinical data (i.e. a Patient Profile) and all system forms were provided to an independent MADDERS® Adjudication Committee (MAC) for review. The MAC consisted of substance abuse experts with clinical experience in drug abuse, psychiatry, psychology and/or pain management. Committee members were blinded to study treatment assignment information in the SUMMIT-07 study and did not receive information about doses of study drug taken by subjects reporting system events in the SUMMIT-07 and SUMMIT-LTS studies. Two members of the MAC independently assigned classifications and completed a Preliminary Adjudicator Classification Form for each triggering event. If the adjudicators reached consensus, the MAC Administrator generated a Final Event Classification Form; otherwise, a third adjudicator completed a Preliminary Adjudicator Classification Form in an attempt to reach consensus or alternatively the case was discussed at a MAC meeting until a consensus was reached. MAC classifications were compared to site investigator classifications and inter-rater reliability (IRR) values were calculated for quality control.
Table 1. Event classifications in the MADDERS® system.
Statistical analysis
Data are described as the mean and standard deviation for continuous variables and as the number and proportion for categorical variables. IRR between site primary classifications and MAC primary classifications was assessed by calculating the percentage of agreement and Cohen’s kappa statistic. Duplicate primary events for the same subject were excluded from the IRR analysis to eliminate redundancy. All analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC). The threshold for statistical significance was p < .05.
Results
MADDERS® Population characteristics
The MADDERS® population was comprised subjects who had at least one triggering event during the study period. Seventy-nine (6.6%) of 1189 subjects reported 86 events in the SUMMIT-07 study and 51 (8.0%) of 638 subjects reported 59 triggering events in the SUMMIT-LTS study. The average age of subjects in each MADDERS® population was 50.6 ± 12.29 years and 51.5 ± 11.83 years, respectively. A summary of demographic information for each event population relative to the overall study population is provided in and .
Table 2. Demographic characteristics in the SUMMIT-07 study.
Table 3. Demographic characteristics in the SUMMIT-LTS study.
Summary of MADDERS® event classifications
Primary and supplemental classifications in the SUMMIT-07 study
A summary of all primary and supplementary classifications as adjudicated by the MAC in the SUMMIT-07 study is shown in . Of 79 subjects reporting a MADDERS® event, 48 had an event during the titration period and 31 had an event during the randomized double-blind treatment period. The incidence of events in the randomized treatment period was similar between the NKTR-181 and placebo groups (5.5 vs. 4.7%, respectively).
Table 4. MADDERS® event classifications in the SUMMIT-07 study.
Among all 86 MADDERS® events, 57 were triggered by AEs and 29 were triggered by DADEs. Most events were classified by the MAC as “None of the Above”, “Misuse” or “Therapeutic Error”. The most frequent supplemental classification was “Withdrawal”. “Withdrawal” events were similarly distributed between the NKTR-181 and placebo groups during the randomized treatment period, although they tended to occur at different points in the study for each group. For example, “Withdrawal” events in the NKTR-181 group often occurred following abrupt cessation of treatment (e.g. early termination), missing scheduled doses or during tapering at the end of the study. In contrast, “Withdrawal” events in the placebo group most frequently occurred following randomization. Five subjects had events that were classified by the MAC as “Abuse” (three subjects receiving NKTR-181 during the titration period and two subjects receiving placebo during the double-blind randomized treatment period). All five events were DADEs involving missing pills that the adjudicators determined to be part of a pattern of not returning the expected amount of medication (in some cases large amounts) or were accompanied by suspicious stories reported by the subject to site staff and reported on Supplemental DADE Forms.
Primary and supplemental classifications in the SUMMIT-LTS study
A summary of all primary and supplementary MADDERS® classifications as adjudicated by the MAC in the SUMMIT-LTS study is shown in . De novo opioid-naïve subjects had the lowest rate of triggering events among all four subgroups (4.1 vs. 7.5–9.2%, respectively). The highest dose group had the highest number of subjects with triggering events, but no other trends were observed in terms of abuse, misuse or frequency of event type.
Table 5. MADDERS® event classifications in the SUMMIT-LTS study.
Among all 59 events reported by 51 subjects, 48 were triggered by AEs and 11 were triggered by DADEs. Most events were classified by the MAC as “None of the Above” or “Unknown”. The most frequent supplemental classification was “Withdrawal”. Four events were classified by the MAC as “Abuse”; of these, two were triggered by AEs and two were triggered by DADEs.
Investigator–MAC agreement for MADDERS® system classifications
A comparison of primary classifications assigned by investigators and the MAC is shown in . Detailed investigator classifications are available in Supplemental Tables 1 and 2. In the SUMMIT-07 study, investigators and the MAC agreed on the final primary classifications of 38 of 51 (74.5%) events during the titration period and the IRR was moderate and statistically significant (κ = 0.57, p < .001). For the double-blind randomized treatment period, investigators and the MAC agreed on the primary classifications of 23 of 35 events (65.7%) and the simple kappa coefficient was fair and statistically significant (κ = 0.39, p < .001). The highest level of agreement was observed for the classification of “None of the Above”, whereas there was less agreement on classifications of “Misuse” and “Therapeutic Error”. Across both study periods, events that were classified as “Therapeutic Error” by study sites were often classified by the MAC as “Misuse”. A review of these cases indicated that the MAC often believed that subjects who reported unintentionally taking more medication than instructed were doing so intentionally and therefore misusing their assigned treatment. Review of the MAC meeting minutes related to these cases indicated that adjudicators often used contextual information from the patient profiles such as history of missing medication and suspicious stories to make their determination of intent. There was site vs. MAC disagreement on the five DADEs that the MAC classified as “Abuse”; these events were classified by study sites as “Unknown” (1 event), “None of the Above” (2 events), and “Therapeutic Error” (2 events).
Figure 1. Primary classifications of potentially abuse-related events in the SUMMIT-07 and SUMMIT-LTS studies. Triggering events were classified by trained investigators during the trial and by an independent committee of substance abuse experts termed the MAC after trial completion. Although all classifications were based on standardized definitions, the judgments of site staff were informed by face-to-face interactions with study subjects whereas the MAC had access to complete study data for individual subjects after study completion.
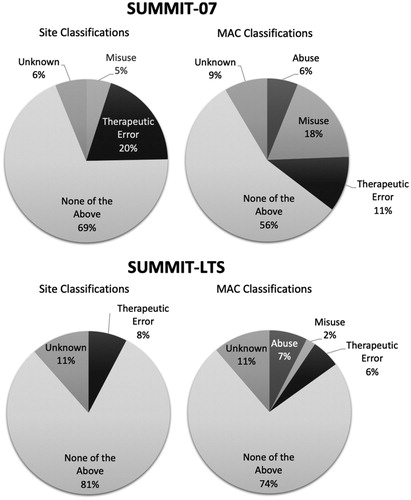
In the SUMMIT-LTS study, there was agreement between investigators and the MAC on the final primary classifications of 50 of 59 (84.7%) events and the IRR was moderate and statistically significant (κ = 0.58, p < .001). The highest level of agreement was observed for the classification of “None of the Above”, and was usually associated with a supplemental designation of “Withdrawal”. There was site vs. MAC disagreement on four events that the MAC classified as “Abuse”; these events were classified by study sites as “None of the Above” (3 events) and “Unknown” (1 event).
Medication Use Survey results
Of 243 MUS respondents in the NKTR-181 group of the SUMMIT-07 study, a majority (94.7%) reported never taking more medication than instructed and most subjects who reported that they took more medication than instructed indicated that they had made an unintentional therapeutic error (). None of the subjects in the NKTR-181 group reported abusing the drug, diverting it, being addicted to it, changing the route of administration or using it to try to commit suicide.
Table 6. Medication use survey (MUS) responses in the SUMMIT-07 study.
Similarly, of 478 MUS respondents in the SUMMIT-LTS study, a majority (95.8%) reported never taking more medication than instructed. Among subjects who did overuse the study medication, more than half indicated that they had made a therapeutic error and seven reported misusing the medication to treat pain or other symptoms. No subjects reported abusing the drug, diverting it, changing the route of administration or using it to try to commit suicide ().
Table 7. Medication use survey (MUS) responses in the SUMMIT-LTS study.
Discussion
Although the FDA mandates that sponsors assess the abuse potential of CNS-active drugs throughout development, historically there were no systematic methods available for identifying, recording and evaluating potentially abuse-related events during clinical trials. Traditional methods for evaluating abuse-related events in clinical trials rely on retrospective approaches that lack contextual information about the event, including the subject’s intent. These deficiencies lead to the misclassification of events and potential false-positive (overestimating abuse potential) and false-negative findings (underestimating abuse potential). The MADDERS® system was developed to address these inadequacies and to help sponsors and the FDA more accurately assess the abuse potential of new drugs. Although it has been implemented in multiple clinical trials evaluating non-opioid medicationsCitation5,Citation10, this is the first report of its application in clinical trials of a mu-opioid analgesic.
Overall, there were relatively few MADDERS® events reported in the SUMMIT-07 and SUMMIT-LTS clinical trials, and most events were related to the emergence of withdrawal signs and symptoms identified by staff as AEs, as well as unintentional (“Therapeutic Error”) or intentional (“Misuse”) overuse of the study medication in the form of DADEs (i.e. missing pills). It is not surprising that the system identified triggering AEs indicating opioid withdrawal since the investigational compound is a mu-opioid agonist that elicits prototypic mu-agonist effectsCitation11. “Withdrawal” events tended to occur when the active drug was discontinued abruptly, when subjects were randomized to placebo after the titration period or when the dose was reduced. However, the overall incidence of withdrawal following cessation of active drug was quite low when considered in the context of an enriched enrollment randomized withdrawal study design, which involves abruptly randomizing one group of study subjects to placebo after several weeks of taking an opioid. Substance abuse experts now widely agree that the presence of a withdrawal syndrome is, in and of itself, not indicative of abuse potential, since many CNS-active drugs devoid of abuse potential such as antidepressants and blood pressure medications can produce physical dependence and withdrawal upon discontinuation. According to the FDA’s finalized guidance on assessing the abuse potential of drugs, “The Agency recognizes that physical dependence does not inherently indicate that a drug has abuse potential. Indeed, some drug classes (such as beta-blockers and monoamine reuptake inhibitors) that are known to produce physical dependence are not abused and are not scheduled under the CSA”Citation3.
With regard to abuse-related events, the MAC classified five events as potential “Abuse” in the SUMMIT-07 study (5 subjects: 3 in the NKTR-181 group during the open-label titration period and 2 in the placebo group during the double-blind randomized treatment period) and four in the SUMMIT-LTS study (4 subjects receiving open-label NKTR-181). All five instances of suspected abuse in the SUMMIT-07 study were triggered by missing medication rather than AEs, whereas the four events in the SUMMIT-LTS study were triggered by AEs of craving and withdrawal as well as DADEs resulting from missing medication. A low rate of MADDERS® events and the identification of very few cases of suspected abuse in both trials are consistent with results from preclinical and human abuse potential studies of NKTR-181 indicating that it may have a lower abuse potential than other mu-opioid agonists such as oxycodoneCitation8,Citation11, likely related to the unique physicochemical properties of NKTR-181 that result in a slow rate of entry into the CNS. However, direct within-study comparisons of the abuse rates of NKTR-181 and a prototypical mu-opioid agonist using the system have not been studied.
In both SUMMIT trials, it was unclear whether events adjudicated as “Abuse” in subjects taking the study drug were actual instances of abuse or false-positives, which are expected for any method that assesses abuse potential in clinical trials. For example, a large study conducted by Adams et al.Citation12 compared the prevalence of tramadol abuse with abuse of nonsteroidal anti-inflammatory drugs (NSAIDs) and hydrocodone-containing analgesics over a 12 month period in >11,000 subjects using a novel interview-based structured questionnaire and scoring algorithm developed for the study. In this study, the percentage of subjects who exhibited behaviors indicative of possible abuse at least once during follow-up was 2.5% for NSAIDs (a drug with no abuse potential), 2.7% for tramadol (an atypical mu agonist with relatively low abuse potential) and 4.9% for hydrocodone (a prototypical mu agonist). The identification of two abuse events in two subjects in the placebo group of the SUMMIT-07 study likely represents “background noise” similar to that reported for subjects taking NSAIDs in the Adams et al. study.
No events led to a supplemental designation of “Addiction-Related Behavior” in the SUMMIT-07 study, while there were four such classifications in the SUMMIT-LTS study. The most compelling evidence for possible addiction-related behavior occurred in one subject from the SUMMIT-LTS study who had multiple adjudicated events. The subject had an initial triggering AE of “euphoria” and later reported that he was craving the study drug (second triggering AE of “craving the drug”) and was having trouble controlling his drug-taking behavior, as corroborated by his responses to the MUS questionnaire administered by study staff at the end of the study. The subject eventually discontinued from the study voluntarily and had a final triggering AE of “Withdrawal” upon cessation of the study drug. Of particular interest in this case is how forthcoming the subject was in response to the structured interview questions administered by site staff, including the retrospective MUS, which relies on self-reporting of past behavior.
Overall, there were three suspected cases of “Diversion” in study subjects taking NKTR-181 (two in SUMMIT-07 and one in SUMMIT-LTS), all triggered by DADEs. These cases tended to be associated with events in which the subject failed to return a large amount of medication and could not provide a credible explanation. Several DADEs in the SUMMIT studies were associated with subjects reporting unintentionally taking more medication than instructed (“Therapeutic Error”) due to confusion regarding the dosing instructions, such as when changes in dosing occurred. Other DADEs occurred when subjects reported intentionally taking more medication than instructed for a therapeutic purpose (i.e. classified as “Misuse” but not “Abuse” or “Therapeutic Error”), such as taking additional tablets to treat the underlying pain syndrome or, as reported in one case, to achieve pain relief for menstrual cramps. DADEs are an important and often overlooked and underreported parameter for evaluating abuse potential in clinical trials and are particularly important as signals of possible drug diversion. Dependence on only a pre-specified list of AEs of interest can result in overlooking cases in which large amounts of medication go missing or cases in which subjects repeatedly return less medication than expected but do not report corresponding AEs.
MADDERS® system implementation in the SUMMIT studies was subject to some limitations. There were some instances of disagreement between site investigators and the MAC with regard to event classification. A certain level of disagreement was expected given that site investigators and adjudicators had access to different types of information (e.g. sites repeatedly met subjects face-to-face, whereas adjudicators and subjects had no in-person interactions) as well as differences in professional training and expertise in substance abuse research and treatment. In general, the MAC exhibited a conservative approach, often selecting a classification of “Abuse” in ambiguous cases. Future efforts to improve the accuracy and consistency of event classifications should include monitoring site and adjudicator performance throughout a trial and providing guidance or retraining when necessary. Second, the SUMMIT studies excluded patients with a history within the past year or current evidence of substance abuse or addiction; thus, the potential event reporting system dataset was limited by lack of exposure to patients with different degrees of risk for developing or exhibiting drug abuse behavior. Third, it is possible that limiting the substance abuse exclusionary criterion to within the past year as opposed to a lifetime history could have potentially confounded the results by allowing some subjects into the studies who were predisposed to substance abuse or addictive behavior. However, this potential confound was addressed by providing the MAC with each subject’s full medical history, which could then be factored into their assessment of the subject’s potentially abuse related event. Fourth, use of the MUS to capture potentially abuse-related events that were not associated with a triggering AE or DADE was limited by a low completion rate, as patients were often lost to follow-up or did not attend a final study visit for completion of the MUS. Finally, the MAC and site investigators were sometimes unable to assign a category for an event (hence classifications of “Unknown”), presumably due to a lack of subject- or event-related information. Steps should be taken in future studies to determine what information can further assist the adjudicators in classifying these events.
Conclusions
The MADDERS® system was successfully implemented to identify and assess potentially abuse-related events in two large multicenter phase 3 clinical trials of NKTR-181 in subjects with moderate to severe chronic low back pain and noncancer pain. Although a small number of events were adjudicated as indicating possible abuse of the study drug, there was no clear evidence of an abuse potential signal or risk of diversion or addiction associated with the use of NKTR-181. There was a low overall rate of MADDERS® events and most events were attributed to either the emergence of withdrawal signs and symptoms upon drug discontinuation or reduced exposure to NKTR-181 or to the unintentional (i.e. mistake in therapeutic regimen) or intentional overuse (i.e. misuse) of medication for a therapeutic purpose. These results are consistent with nonclinical and clinical data suggesting that NKTR-181 has an enhanced safety profile, fewer CNS-related AEs and lower abuse potential compared to other prototypic mu-opioid agonist drugs. With regard to the MADDERS® system, there was generally good agreement between site investigators and the MAC for classifying events. A relatively low overall rate of triggering events suggests that the system functions with good sensitivity and specificity for identifying potentially abuse-related events. Consistent with the opinion of the ALERTT working group of ACTTION, the present findings indicate that MADDERS® is a useful tool for systematically and prospectively identifying and evaluating potentially abuse-related events in phase 2–4 clinical trials.
Transparency
Declaration of funding
This study was funded by Nektar Therapeutics Inc.
Author contributions
All authors contributed to conceptualization of the study and manuscript, interpretation of the data, drafting of the manuscript, critical revision of the article for important intellectual content and approved the final version to be published. All authors agree to be accountable for all aspects of the work presented in this research paper.
Declaration of financial/other relationships
R.K.L., H.E., N.E. and N.P.K. have disclosed that they are employees of Analgesic Solutions, a clinical research and consulting firm with many clients in the pharmaceutical and medical device industries that commercializes a number of products and services, including the MADDERS® system. R.T. has disclosed that he is a former employee of Analgesic Solutions. J.E.H. has disclosed that he is an employee of Pinney Associates, which provides consulting services on the development and regulation of pharmaceutical products for pain, substance use disorders and other CNS disorders contributing the abuse and dependence potential assessment of new drugs and formulations including abuse deterrent opioids. J.Gu. has disclosed that he is a consultant for Nektar Therapeutics. R.R. has disclosed that he received research funding from and is a consultant for Nektar Therapeutics. J.Gi. has no conflicts to disclose. M.T., S.K.D., C.J.D.F., L.L. and S.S. have disclosed that they are employed by and own stock in Nektar Therapeutics.
CMRO peer reviewers on this manuscript have received an honorarium from CMRO for their review work but have no relevant financial or other relationships to disclose.
Previous presentation
MADDERS® system results from the SUMMIT-07 study were presented as an oral communication at the College on Problems of Drug Dependence Annual Scientific Meeting. 2018 Jun 9–14; San Diego, CA, USA.
Acknowledgements
We thank Dr. Ashley Symons for medical writing support and Gillian Sears from Analgesic Solutions for assistance with preparation of the manuscript.
Notes
Notes
1 MADDERS® is a registered trade mark of Analgesic Solutions, LLC, Natick, MA USA.
References
- Denisco RA, Chandler RK, Compton WM. Addressing the intersecting problems of opioid misuse and chronic pain treatment. Exp Clin Psychopharmacol. 2008;16:417–428.
- Hall AJ, Logan JE, Toblin RL, et al. Patterns of abuse among unintentional pharmaceutical overdose fatalities. JAMA. 2008;300:2613–2620.
- Food and Drug Administration Center for Drug Evaluation and Research. Guidance for industry: assessment of abuse potential of drugs. Maryland: FDA; 2017.
- Smith SM, Paillard F, McKeown A, et al. Instruments to identify prescription medication misuse, abuse, and related events in clinical trials: an ACTTION systematic review. J Pain. 2015;16:389–411.
- Smith SM, Jones JK, Katz NP, et al. Measures that identify prescription medication misuse, abuse, and related events in clinical trials: ACTTION critique and recommended considerations. J Pain. 2017;18:1287–1294.
- Treister R, Trudeau JJ, Van Inwegen R, et al. Development and feasibility of the Misuse, Abuse, and Diversion Drug Event Reporting System (MADDERS®). Am J Addict. 2016;25:641–651.
- Smith SM, Dart RC, Katz NP, et al. Analgesic, Anesthetic, and Addiction Clinical Trials, Translations, Innovations, Opportunities, and Networks (ACTTION) public–private partnership. Classification and definition of misuse, abuse, and related events in clinical trials: ACTTION systematic review and recommendations. Pain. 2013;154:2287–2296.
- Miyazaki T, Choi IY, Rubas W, et al. NKTR-181: a novel mu-opioid analgesic with inherently low abuse potential. J Pharmacol Exp Ther. 2017;363:104–113.
- Markman J, Gudin J, Rauck R, et al. Summit-07: a randomized trial of NKTR-181, a new molecular entity, full mu-opioid receptor agonist for chronic low-back pain. Pain. 2019 [cited Mar 1].
- Lanier RK, Kazam I, Elder H, et al. Evaluation of potentially abuse-related events in phase 3 clinical trials of cannabidiol (Epidiolex®) using the MADDERS® prospective system. Poster presented at the 79th annual meeting of the College on Problems of Drug Dependence. 2017 Jun 17–22; Montreal, Canada.
- Webster L, Henningfield J, Buchhalter AR, et al. Human abuse potential of the new opioid analgesic molecule NKTR-181 compared with oxycodone. Pain Med. 2018;19:307–318.
- Adams EH, Breiner S, Cicero TJ, et al. A comparison of the abuse liability of tramadol, NSAIDs, and hydrocodone in patients with chronic pain. J Pain Symptom Manage. 2006;31:465–476.
