Abstract
Objective: To explore persistence and adherence with once-daily, twice-daily, or once-weekly DPP-4 inhibitors (DPP-4i) in Japanese patients with type 2 diabetes.
Methods: This retrospective, longitudinal, observational cohort study used data from the Japanese nationwide hospital-based Medical Data Vision (MDV) administrative claims database. Data were collected for patients given a new DPP-4i prescription between May 2015 and June 2017 with 1-year follow-up until May 2018. Treatment persistence was defined as the total duration of continuous prescription. Adherence to treatment was measured as the proportion of days covered (PDC).
Results: A total of 598,419 patients with a prescription for DPP-4i treatment were identified in the MDV database. Of the 39,826 patients who met the inclusion criteria, 82.4% were receiving once-daily DPP-4i, 15.6% twice-daily DPP-4i, and 2.0% once-weekly DPP-4i. Twelve-month persistence rates with once-daily regimens were 66.3% versus 64.7% with twice-daily (p = .1187), and versus 38.8% with once-weekly, regimens (p < .0001) in the overall population (including untreated [UT] and previously treated [PT] patients); 62.8% with once-daily versus 58.3% with twice-daily (p = .0309), and versus 12.3% with once-weekly regimens (p < .0001) in the UT cohort; and 68.6% with once-daily versus 67.9% with twice-daily (p = .5471), and versus 49.1% with once-weekly regimens (p < .0001) in the PT cohort. In the overall population, 97.8% of patients had a mean PDC of 0.97 with once- and twice-daily, and 65.8% of patients had a mean PDC of 0.74 with once-weekly, DPP-4i regimens.
Conclusions: Overall, persistence at 12 months was highest in patients receiving once-daily DPP-4i regimens.
Introduction
Diabetes is a chronic metabolic disease that requires continuous medical careCitation1. In 2017, 425 million people worldwide were reported to have diabetesCitation2. By 2045, the number of people with diabetes in the Western Pacific region is expected to rise from 159 million to 183 millionCitation2. In Japan, the prevalence of type 2 diabetes mellitus (T2DM) continues to rise, largely owing to the aging populationCitation3 and lifestyle factors, such as sedentary behavior and obesity associated with westernizationCitation4,Citation5. Left untreated, T2DM can have life-threatening consequences, placing a substantial cost burden on healthcare systemsCitation6–10.
Treatment adherence (also referred to as medication compliance in terms of drug schedule and dosage) and treatment persistence (i.e. continuing to take medication for the prescribed duration) to antidiabetes therapy are both critical for the achievement of optimal outcomes in patients with diabetesCitation11. Measures of adherence and persistence are both recognized by the ISPOR (International Society for Pharmacoeconomics and Outcomes Research) Medication Compliance and Persistence Work GroupCitation12,Citation13. Cramer et al. reported that clinical outcomes of treatment are affected by how well, and how long, patients take their medication, and therefore adherence and persistence should be defined and measured separately to comprehensively characterize medication-taking behaviorCitation13.
Improving adherence to antidiabetes medication is thus essential, as this has been shown to result in improved glycemic control and consequently lead to a reduction in disease-related events, hospitalizations, mortality and healthcare costsCitation14–17. For example, an increase in patient adherence to medication has been shown to reduce the risk of hospitalization or emergency room visits by 13%, equating to an annual cost saving of approximately $4.68 billion. Further improvement in treatment adherence would lead to an additional saving of $3.61 billionCitation18. A possible linear association between treatment adherence and hospitalization has also been reported: improvements in adherence may help to reduce economic burden by decreasing total annual healthcare costs (8.6–28.9% decrease in annual costs with every 10% increase in adherence)Citation19. Outcomes from studies suggest that adherence to treatment remains a challenge for patients with T2DM, with reported adherence rates of 50% or lessCitation15,Citation16,Citation20. Persistence (time from the first prescription to discontinuation of treatment in patients with T2DM), has also been shown to be suboptimal in observational and retrospective studies analyzing persistence with oral antidiabetes drugs (OADs) or insulinCitation21,Citation22. Factors affecting adherence and persistence rates include age, patient–provider interaction, safety, efficacy, and complexity of the treatment regimenCitation16,Citation20,Citation23. Patients taking twice-daily antidiabetes medications had an almost five-fold greater risk of non-adherence versus a once-daily regimen, while those taking antidiabetes medications three times a day had a > 8-fold greater risk of non-adherence versus once-daily regimensCitation24. Of note, the American Diabetes Association advocates a patient-centered approach that gives consideration to patients’ values and preferences when prescribing pharmacologic agentsCitation1.
Dipeptidyl peptidase-4 inhibitors (DPP-4i) are a relatively new class of antidiabetes agents that inhibit the enzyme DPP-4 and in turn prolong the activity of endogenous glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide, resulting in improved glycemic controlCitation25. Evidence suggests that a substantial number of treatment-naïve patients receive DPP-4i as their first antidiabetes medication, indicating that DPP-4i is becoming a first-line treatment in JapanCitation26.
A recently published real-world study, based on Japanese health insurance claims data, found that the use of, and persistence and adherence to, DPP-4i-containing regimens was high among previously treated and previously untreated Japanese patients with T2DMCitation27. The availability of new treatment regimens in Japan, particularly once-weekly dosing, coupled with universal health insurance coverage and unrestricted access to almost any healthcare provider means that patients are able to select physicians or medical facilities of their choice. This means that patients can and often visit multiple healthcare institutionsCitation28. Since prescribing patterns may vary, it is necessary to compare persistence and adherence of current treatment regimens using real-world data in order to aid decision making. Moreover, assessing both persistence and adherence provides a richer understanding of medication-taking behavior, and thus both were assessed in our study. Of note, treatment persistence and adherence are affected by several factors, including the mode of administration, administration frequency, and patient expectationsCitation11. Data regarding the effect of administration frequency (once daily, twice daily, or once weekly) of antidiabetic drugs within the DPP-4i class, on persistence and adherence, are limited in Japan, and would help physicians to make more informed treatment decisions in clinical practice. Here, we present the findings from an observational study that used data from a large Japanese health insurance claims database to investigate real-world trends in previously untreated or previously treated Japanese patients with T2DM in terms of treatment persistence and adherence to assigned DPP-4i treatments that have differing administration frequencies (once daily, twice daily, or once weekly).
Methods
Study design and data source
The objectives of this study were to compare treatment persistence rates and the proportion of patients adherent to treatment receiving different DPP-4i administration frequencies (once daily, twice daily, or once weekly) following 12 months’ treatment, in Japanese patients with T2DM aged ≥18 years who were treatment-naïve or previously treated and who were listed in the MDV database. Outcomes were measured in treatment-naïve, and previously treated patients because patterns may differ in these patient groups. We conducted a retrospective, longitudinal, observational cohort study, using data extracted from the Japanese nationwide hospital-based Medical Data Vision (MDV) administrative claims database (Medical Data Vision Co., Ltd; Tokyo, Japan). As of July 2018, the MDV database contained almost 24 million patients accumulated since April 2008 who had been treated as inpatients or outpatients at approximately 360 hospitals (21% of all hospitals participating in the Diagnostic Procedure Combination (DPC)/Per-Diem payment system) in Japan. Of these patients, approximately three million (12%) were diagnosed with diabetes mellitus. The MDV holds anonymized information about patient characteristics, diagnoses, medical expenses, medical procedures and drug prescriptions. All patient data were encrypted before entry into the MDV database. Administrative claims made between 1 May 2015 and 31 May 2018 were extracted.
Study population
The study population consisted of all patients who had been prescribed a DPP-4i within the selection period. Patients were included if they had a diagnosis of T2DM (International Classification of Diseases, 10th revision [ICD-10] code E11 or E14), and were aged ≥18 years. Patients were required to have ≥6 months’ continuous enrollment in the MDV database before (referred to as the window period) and ≥12 months after (referred to as the follow-up period) the index date (defined as the first prescription date of DPP-4i during the selection period). Patients could be either naïve to OAD treatment (UT) or previously treated (PT; received at least one OAD prescription, with the exception of a DPP-4i prescription in the window period). Patients who had been prescribed a DPP-4i in the window period and those who had a diagnosis of type 1 diabetes mellitus (T1DM), or were without a diagnosis of T2DM in the window period, were excluded from the analysis. Where follow-up/enrollment information was missing from the MDV database, patients had to have at least one medical claim in each quarter within the window and follow-up periods in order to confirm that continuous hospital visits were made.
Classification of DPP-4i administration frequencies and definitions of outcomes
Patients included in this study had been prescribed one of nine DPP-4i, given at three different administration frequencies: once-daily alogliptin, sitagliptin, linagliptin, teneligliptin, and saxagliptin; twice-daily anagliptin and vildagliptin; once-weekly omarigliptin and trelagliptin. In line with the ISPOR Medication Compliance and Persistence Work GroupCitation12 persistence to treatment was defined as the total duration of continuous prescription (i.e. time from first prescription of DPP-4i to discontinuation). A switch from DPP-4i to another OAD class was considered discontinuation of DPP-4i, whereas an add-on to a DPP-4i or a change in DPP-4i regimen within the same OAD class was not considered discontinuation. A patient was considered to have discontinued treatment if an antidiabetic drug category was not prescribed within the grace period. The grace period was based on an allowed minimum gap between two consecutive prescriptions of the same antidiabetic drug category. Persistence rate was defined as the number of patients who continued the drug over 12 months divided by the number of patients who were administered the drug. In line with the ISPOR Medication Compliance and Persistence Work GroupCitation12 adherence to treatment was measured as the proportion of days covered (PDC) calculated within a 12-month period as the total number of days the DPP-4i was taken divided by the total number of days in the treatment period in which treatment was expected to be taken. Only one time point (12 months after the index-date) was considered in the adherence calculation. Although patients with a PDC ≥0.8 have been considered to have good adherence to treatmentCitation27,Citation29, this cut-off should be interpreted with caution. Adherence rate was defined as PDC divided by the number of days.
Statistical analysis
A continuous variable is presented as the mean and standard deviation (SD). Categorical variables are shown as numbers and percentages.
Persistence to DPP-4i treatment in UT and PT patients according to administration frequency was calculated at 12 months using the Kaplan–Meier (KM) method, and hazard ratios (HR) and 95% confidence intervals (CI) were estimated using the Cox proportional hazard model including the following covariates for comparisons among administration frequency groups and between the UT and PT cohorts: age (18–34, 35–44, 45–54, 55–64, 65–74, and ≥75 years), sex, number of medications (0, 1–3, 4–5, 6–8, or >8), comorbidities (hypertension, hyperlipidemia, dementia, diabetic nephropathy, or moderate decline of renal function, and liver disease, Charlson Comorbidity Index [CCI] score), and prescription of oral steroids at baseline. The confounding factors were selected based on the variables recorded in the database. The first discontinuation of the index DPP-4i treatment was considered as the survival event and patients were censored if they reached the end of follow-up without discontinuation. The Cox proportional hazard assumption was assessed by plotting KM curves. The KM curves were parallel during the observation period, which suggested that the proportional hazard assumption was satisfied.
Patient adherence to DPP-4i treatment in UT and PT patients according to administration frequency was measured as PDC. Adherence was analyzed for all patients who had been prescribed the same DPP-4i more than twice during the data period, and was compared by administration frequency and stratified by prior treatment history (UT and PT patients). Odds ratios (OR) were estimated from multivariate logistic regression analysis adjusted for the same aforementioned covariates, to compare adherence by administration frequency. For all analyses, a two-sided p < .05 was considered statistically significant.
Detecting statistical differences in adherence and persistence between UT and PT patients was beyond the scope of the current analysis. Data management and statistical analyses were handled by Creativ-Ceutical K.K., Japan. Data analyses were performed using SAS v.9.3 (SAS Institute; Cary, NC, USA).
Statement of ethics
Based on ethical guidelines for epidemiological research issued by the Japanese Ministry of Health, Labor and Welfare, ethics approval and informed consent were not applicable for this study. The study complied with the International Society of Pharmacoepidemiology guidelines for good pharmacoepidemiology practices. All authors had full access to all the data, and take responsibility for its integrity and the data analysis.
Results
Patient disposition
Between May 2015 and May 2018, about 598,419 patients in the MDV database who received a prescription for DPP-4i treatment were identified. Of these, 39,826 patients (6.7%) met the inclusion criteria and were included in the study cohort; 32,800 patients (82.4%) were receiving a once-daily DPP-4i regimen, 6212 (15.6%) were receiving a twice-daily DPP-4i regimen, and 814 (2.0%) were receiving a once-weekly DPP-4i regimen ().
Figure 1. Patient selection and cohorts. Abbreviations: DPP-4i: dipeptidyl peptidase 4 inhibitor; T1DM: type 1 diabetes mellitus; T2DM: type 2 diabetes mellitus.
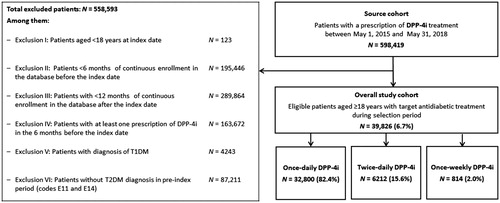
Of all patients in the UT population (n = 15,435), 85%, 13%, and 1% received once-daily, twice-daily, and once-weekly DPP-4i regimens at the index date, respectively. Of all patients in the PT population (n = 24,391), 81%, 17%, and 2% received once-daily, twice-daily, and once-weekly DPP-4i regimens, respectively.
Patient characteristics
Patient demographics and characteristics of treatment according to DPP-4i administration frequency group are presented in . In the overall study cohort, 60.9% of patients were male (60.9%, 61.0%, and 58.0% in the once-daily, twice-daily, and once-weekly groups, respectively) and the mean age was 68.4 years (SD: 12.0) (68.8 years [SD: 11.8], 66.6 years [SD: 12.6], and 64.9 years [SD: 12.5] for the once-daily, twice-daily, and once-weekly groups, respectively).
Table 1. Baseline patient characteristics and clinical characteristics in the overall study cohort and in the UT and PT cohorts, among all patients and according to DPP-4i administration frequency.
Persistence
In the overall study cohorts, 12-month persistence rate in patients receiving once-daily DPP-4i (66.3%) was comparable to patients receiving twice-daily DPP-4i (64.7%) (HR for discontinuation = 1.022 [95% CI: 0.994–1.050]; p = .1187, and significantly higher versus patients receiving once-weekly DPP-4i (38.8%) (HR for discontinuation = 1.699 [95% CI: 1.585–1.822]; p < .0001) ().
Table 2. Persistence with DPP-4i treatment in the overall study cohort and in the UT and PT cohorts according to administration frequency.
In the UT cohort, 12-month persistence rate was significantly higher in patients receiving once-daily DPP-4i (62.8%) versus patients receiving twice-daily DPP-4i (58.3%) (HR for discontinuation = 1.053 [95% CI: 1.005–1.103]; p = .0309; median time to discontinuation log-rank p < .0001) and versus patients receiving once-weekly DPP-4i (12.3%) (HR for discontinuation = 3.802 [95% CI: 3.331–4.339]; p < .0001; median time to discontinuation log-rank p < .0001) ().
In the PT cohort, 12-month persistence rate in patients receiving once-daily DPP-4i (68.6%) was comparable to patients receiving twice-daily DPP-4i (67.9%) (HR for discontinuation = 1.010 [95% CI: 0.977–1.045]; p = .5472), and significantly higher versus patients receiving once-weekly DPP-4i (49.1%) (HR for discontinuation = 1.420 [95% CI: 1.308–1.542]; p < .0001; median time to discontinuation log-rank p < .0001) ().
Adherence
The proportion of patients with mean PDC 0.97 was similar between once-daily and twice-daily regimens in the overall study cohort (97.8% for both; OR = 0.945 [95% CI: 0.780–1.145]; p = .5636), and both the UT (96.4% vs 95.6%; OR = 0.874 [95% CI: 0.686–1.115]; p = .2789) and PT (98.8% for both; OR = 0.979 [95% CI: 0.710–1.350; p = .8979) cohorts. In the overall cohort, UT cohort, and PT cohort, 65.8%, 28.5%, and 76.2% of patients had a mean PDC of 0.74, 0.46, and 0.81, respectively, with once-weekly DPP-4i treatment, which were significantly lower than once-daily or twice-daily regimens (p < .0001) ().
Table 3. Adherence to DPP-4i therapy in the overall study cohort and in the UT and PT cohorts, among all patients and according to administration frequency.
Discussion
This is the first study to provide real-world evidence on 12-month persistence rates and describe patient adherence to treatment in patients with T2DM prescribed once-daily, twice-daily, or once-weekly DPP-4i dosing regimens in Japan. We investigated the persistence and adherence of once-daily versus twice-daily DPP-4i, and once-weekly administration, in UT and PT patients using administrative claims data from the MDV database, which revealed that 85%, 13%, and 1% of patients in the UT cohort received once-daily, twice-daily, and once-weekly DPP-4i regimens at the index date, respectively. Of all patients in the PT population, 81%, 17%, and 2% received once-daily, twice-daily, and once-weekly DPP-4i regimens, respectively. The results of this study showed that persistence to the once-daily regimen was highest in the overall, PT, and UT cohorts. Across all cohorts, patients receiving once-daily DPP-4i tended to have similar (though numerically higher) rates of persistence to those receiving twice-daily regimens, but significantly higher rates than those receiving once-weekly treatment. A similar pattern was observed for patient adherence to treatment. These findings are important as they indicate that not only do patients who are prescribed once-daily DPP-4i perform best in terms of following their physician’s instructions to take their medication as prescribed within the designated observation period (i.e. they are most adherent), they are also more likely to continue to take their prescribed medication (i.e. they are most persistent). While both are important surrogate measures of treatment success and satisfaction and are necessary for the management of T2DMCitation30, persistence is particularly important in chronic conditions, such as T2DM, where extended treatment is required to prevent complications and mortalityCitation11.
It has been reported that only 58% of patients take oral antidiabetic medication for more than 80% of their days ‘on therapy’ in the year averageCitation31. Moreover, the reported inverse association between medication adherence and poor glycemic control has suggested that patients less than 50% compliant with their medication are almost three times more likely to have poor glycemic control than patients who are adherent to their medication more than 80% of the timeCitation32. Barriers to treatment adherence and persistence in patients with T2DM may include dosing frequency, patient expectations, and inadequate follow-up or supportCitation1,Citation11,Citation20. The number of medications and the complexity of the regimen may also affect adherence to treatmentCitation11,Citation20,Citation33. For example, fixed-dose combinations are associated with greater persistence and adherence than two-pill combinationsCitation34.
The results of our study with respect to once-daily dosing corroborate findings of published studies that report patient preferences for once-daily dosing, including a randomized trial reporting a greater preference for once-daily versus once-weekly DPP4i treatmentCitation35,Citation36. Once-daily dosing has been reported as a preferred regimen, as patients were less likely to forget taking their medicationCitation37. Patients have reported improved adherence with once-daily than twice-daily therapiesCitation38,Citation39. Interestingly, patients consider the reduction in their medication dosing frequency an important factor, expressing preference for once-weekly dosing; a finding that was more prevalent in patients naïve to antidiabetes treatment compared with those who had been previously treatedCitation40. Results of a study in patients with osteoporosis reported that switching patients from once-daily bisphosphonates to weekly dosing improved treatment adherence compared with patients who were newly prescribed a once-weekly regimenCitation41. Moreover, a questionnaire-based study by Suzuki et al. reported an improvement in patient satisfaction and compliance after patients switched from a once-daily DPP4i regimen to a once-weekly regimenCitation42,Citation43. This raises the possibility that a reduction in the frequency of dosing may improve medication adherence among patients with diabetesCitation41. With respect to once-weekly dosing, findings of the current study reported lower adherence and persistence rates with once-weekly DPP-4i regimens than with once-daily DPP-4i regimens. In the overall, UT, and PT cohorts, median times to discontinuation, and rates of persistence and adherence were lowest with once-weekly DPP-4i. It has been suggested that adherence rates may not necessarily be related to the simplicity of a regimen, the severity of the disorder, or the possible consequences of missed dosesCitation44. One potential reason for the short times to discontinuation observed with the once-weekly DPP-4i regimen in the current study might have been due to the limited 2-week prescription period with the once-weekly DPP-4i regimen (In Japan, only 2 weeks’ worth of new drug can be prescribed the first year after its approval), and thus frequent patient visits may have impaired patient adherence and persistence to treatment. This restriction was lifted in June 2016, however, it could have placed a burden on patients; and it could be inferred that some patients may have discontinued in order to switch to an alternative long-term daily prescription. Additionally, consideration should be given to the fact that once-weekly DPP-4i regimens had only been introduced to the Japanese market in 2015, and therefore lack of physician and patient knowledge of this new therapeutic option may have impacted their uptake. The 12-month follow-up period may also have affected adherence data, since any disruption in scheduled appointments – such as a reduction in the number of visits by the patient to the hospital due to individual patient circumstances or a lack of availability of hospital appointments – would have a larger impact in a shorter observation period. Although less frequent medication administration may have the potential to improve persistence and adherence, there remains a need for further investigationCitation45–47. Hence, future studies in environments that are not affected by long-term prescription restrictions are necessary. Further studies are warranted in larger numbers of patients before definitive conclusions can be drawn regarding the effects of once-weekly DPP-4i on treatment persistence and adherence.
Limitations
A key strength of this analysis is that it provides valuable real-world information from a large number of patients with T2DM receiving treatment with a DPP-4i with continuous enrollment in the MDV claims database, which is one of the largest medical databases in Japan. However, the study was not randomized, and therefore limited by its observational nature. It is important to recognize that the MDV database comprised patients treated at large acute care hospitals in Japan, and therefore the population may not be representative of patients outside of DPC hospitals. However, the proportion of T2DM patients in the MDV database (12%) is similar to that reported in the 2017 Japanese Health and Nutrition Survey (14%)Citation48, which suggests that the MDV dataset may be at least somewhat representative of the Japanese population. Additional study limitations include the assumption that all patients who filled their prescriptions actually took their medication, and that information such as reasons for discontinuing treatment, whether medication was taken correctly and at the correct time of day, incidence of pill dumping or stockpiling, and details of non-reimbursed treatments, are not recorded in the MDV database. Data on education, marital status, income, out-of-pocket medication expenses and patient-physician interactions were also not recorded in the MDV database. Thus, the adjusted regression analyses were limited to the confounders that were available in the MDV database, which included age, sex, multiple medications and co-morbidities.
Another limitation associated with the use of the MDV database is the absence of data linkage between medical care facilities. Therefore, if a patient received care in different medical facilities, their data will be incomplete. For example, receipt of a prescription at another medical facility could result in a missing medication history and misclassification of the patient in our analysis. Moreover, the MDV database only includes data on claims from DPC hospitals without clinics; data on patients transferred to a different hospital are not traceable from the MDV database after transfer; and the electronic medical records of patients in MDV are extracted from only a select number of hospitals, which may result in bias. Long-term once-weekly DPP-4i treatment only became available in Japan in 2016, and therefore a limited number of patients were receiving once weekly DPP-4i. The low proportion of once-weekly DPP-4i received by patients in the MDV database may also be explained by the fact that once-weekly trelagliptin was first approved in Japan in 2015, whereas once-daily and twice-daily DPP-4i have been approved for use for many years.
Despite these limitations, the findings of this study will give physicians an insight into real-world DPP-4i prescribing patterns, treatment continuation rate and adherence to DPP-4i treatments in previously treated, and untreated patient populations in Japan.
Conclusion
Once-daily administration within the DPP-4i class was the most common administration frequency used in Japan. Twelve-month persistence rates were greater in patients receiving once-daily DPP-4i than with twice-daily DPP-4i in UT patients, and greater than with once-weekly DPP-4i in UT and PT patients. The proportion of adherent patients to once-daily and twice-daily DPP-4i were comparable in both UT and PT patients; however, the proportion of adherent patients to once-weekly DPP-4i was significantly lower compared to other regimens in both UT and PT patients. Long-term prescription of once-weekly DPP-4i only became available in Japan in 2016; therefore, further analysis is warranted for once-weekly DPP-4i based on accumulated data.
Transparency
Declaration of funding
This work was supported by Takeda Pharmaceutical Company Limited, Tokyo, Japan.
Declaration of financial/other relationships
KS has received lecturer’s fees from Novartis Pharma K.K., Takeda Pharmaceutical Co. Ltd, Nippon Boehringer Ingelheim Co. Ltd, Eli Lilly Japan K.K., Mitsubishi Tanabe Pharma Corporation, MSD K.K., and ONO Pharmaceutical Co. LTD. TM has received a research grant from Nexis; lecturer’s fees from AbbVie, AstraZeneca, Daiichi Sankyo, Kyorin, Mitsubishi Tanabe, and Pfizer; manuscript fees from Pfizer; and has served on advisory boards for Asahi Kasei, Boston Scientific, and Bristol-Myers Squibb. AO, KK, NN, and YS are employees of Takeda Pharmaceutical Co. Ltd. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors are responsible for the work described in this paper, including planning the study. AO contributed to the conception and design of the study. AO, KS, and TM were involved in the interpretation of results, and contributed substantially to drafting the manuscript. All authors provided critical revisions to the manuscript for intellectual content and approved the final version for publication.
Disclaimer and data sharing
The study made use of de-identified data from the MDV database. The opinions, results and conclusions reported are those of the authors. No endorsement by MDV or any of its funders or partners is intended or should be inferred.
Given the administrative nature of the data, patients did not provide informed consent for data sharing; however, all data are fully anonymized and the risk of patient identification is low.
Acknowledgements
We thank Laura Vergoz and Sabah Farooq of FireKite, an Ashfield company, part of UDG Healthcare plc, for writing support during the development of this manuscript, which was funded by Takeda Pharmaceutical Co. Ltd. (Tokyo, Japan) in compliance with Good Publication Practice 3 ethical guidelines (Battisti et al. Ann Intern Med. 2015;163:461–464). We would also like to thank Yoshie Onishi, PhD, employee of Creativ-Ceutical K.K., for data analysis support.
References
- American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2018. Diabetes Care. 2018;41(Suppl 1):S13–S27.
- International Diabetes Federation. IDF diabetes atlas - 8th edition. 2017. [cited 2019 14 March]. Available from: http://diabetesatlas.org/resources/2017-atlas.html.Epublished.
- Goto A, Noda M, Inoue M, et al. Increasing number of people with diabetes in Japan: is this trend real? Intern Med. 2016;55(14):1827–1830.
- Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus–present and future perspectives. Nat Rev Endocrinol. 2012;8(4):228–236.
- Zimmet P, Alberti KG, Shaw J. Global and societal implications of the diabetes epidemic. Nature. 2001;414(6865):782–787.
- Aljuaid MO, Almutairi AM, Assiri MA, et al. Diabetes-related distress assessment among type 2 diabetes patients. J Diabetes Res. 2018;2018:7328128.
- Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389(10085):2239–2251.
- Nanayakkara N, Pease A, Ranasinha S, et al. Depression and diabetes distress in adults with type 2 diabetes: results from the Australian National Diabetes Audit (ANDA) 2016. Sci Rep. 2018;8(1):7846.
- Seuring T, Archangelidi O, Suhrcke M. The economic costs of type 2 diabetes: a global systematic review. Pharmacoeconomics. 2015;33(8):811–831.
- Trikkalinou A, Papazafiropoulou AK, Melidonis A. Type 2 diabetes and quality of life. WJD. 2017;8(4):120–129.
- Guerci B, Chanan N, Kaur S, et al. Lack of treatment persistence and treatment nonadherence as barriers to glycaemic control in patients with type 2 diabetes. Diabetes Ther. 2019;10(2):437–449.
- Burrell A, Wong P, Ollendorf D, et al. PHP46 defining compliance/adherence and persistence: ISPOR Special Interest Working Group. Value Health. 2005;8(6):A194–A95.
- Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47.
- Asche C, LaFleur J, Conner C. A review of diabetes treatment adherence and the association with clinical and economic outcomes. Clin Ther. 2011;33(1):74–109.
- Bell KF, Cappell K, Liang M, et al. Comparing medication adherence and persistence among patients with type 2 diabetes using sodium-glucose cotransporter 2 inhibitors or sulfonylureas. Am Health Drug Benefits. 2017;10(4):165–174.
- Kennedy-Martin T, Boye KS, Peng X. Cost of medication adherence and persistence in type 2 diabetes mellitus: a literature review. PPA. 2017;11:1103–1117.
- Kirkman MS, Rowan-Martin MT, Levin R, et al. Determinants of adherence to diabetes medications: findings from a large pharmacy claims database. Diabetes Care. 2015;38(4):604–609.
- Jha AK, Aubert RE, Yao J, et al. Greater adherence to diabetes drugs is linked to less hospital use and could save nearly $5 billion annually. Health Aff (Millwood). 2012;31(8):1836–1846.
- Balkrishnan R, Rajagopalan R, Camacho FT, et al. Predictors of medication adherence and associated health care costs in an older population with type 2 diabetes mellitus: a longitudinal cohort study. Clin Ther. 2003;25(11):2958–2971.
- Garcia-Perez LE, Alvarez M, Dilla T, et al. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4(2):175–194.
- Ascher-Svanum H, Lage MJ, Perez-Nieves M, et al. Early discontinuation and restart of insulin in the treatment of type 2 diabetes mellitus. Diabetes Ther. 2014;5(1):225–242.
- Iglay K, Cartier SE, Rosen VM, et al. Meta-analysis of studies examining medication adherence, persistence, and discontinuation of oral antihyperglycemic agents in type 2 diabetes. Curr Med Res Opin. 2015;31(7):1283–1296.
- Spain CV, Wright JJ, Hahn RM, et al. Self-reported barriers to adherence and persistence to treatment with injectable medications for type 2 diabetes. Clin Ther. 2016;38(7):1653–1664.e1.
- Koyanagi K, Kubota T, Kobayashi D, et al. Prescription factors associated with medication non-adherence in Japan assessed from leftover drugs in the SETSUYAKU-BAG campaign: focus on oral antidiabetic drugs. Front Pharmacol. 2016;7:212.
- Patel BD, Ghate MD. Recent approaches to medicinal chemistry and therapeutic potential of dipeptidyl peptidase-4 (DPP-4) inhibitors. Eur J Med Chem. 2014;74:574–605.
- Seino Y, Kuwata H, Yabe D. Incretin-based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig. 2016;7(Suppl 1):102–109.
- Nishimura R, Kato H, Kisanuki K, et al. Treatment patterns, persistence and adherence rates in patients with type 2 diabetes mellitus in Japan: a claims-based cohort study. BMJ Open. 2019;9(3):e025806.
- Kato D, Ryu H, Matsumoto T, et al. Building primary care in Japan: literature review. J Gen Fam Med. 2019;20(5):170–179.
- Wake M, Oh A, Onishi Y, et al. Adherence and persistence to hyperlipidemia medications in patients with atherosclerotic cardiovascular disease and those with diabetes mellitus based on administrative claims data in Japan. Atherosclerosis. 2019;282:19–28.
- Fukuda H, Mizobe M. Impact of nonadherence on complication risks and healthcare costs in patients newly-diagnosed with diabetes. Diabetes Res Clin Pract. 2017;123:55–62.
- Cramer JA, Benedict A, Muszbek N, et al. The significance of compliance and persistence in the treatment of diabetes, hypertension and dyslipidaemia: a review. Int J Clin Pract. 2008;62(1):76–87.
- Feldman BS, Cohen-Stavi CJ, Leibowitz M, et al. Defining the role of medication adherence in poor glycemic control among a general adult population with diabetes. PLoS One. 2014;9(9):e108145.
- Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Dia Care. 2018;41(12):2669–2701.
- Nishimura R, Kato H, Kisanuki K, et al. Comparison of persistence and adherence between fixed-dose combinations and two-pill combinations in Japanese patients with type 2 diabetes. Curr Med Res Opin. 2019;35(5):869–878.
- Witticke D, Seidling HM, Klimm HD, et al. Do we prescribe what patients prefer? Pilot study to assess patient preferences for medication regimen characteristics. Patient Prefer Adherence. 2012;6:679–684.
- Meguro S, Matsui S, Itoh H. Treatment preference for weekly versus daily DPP-4 inhibitors in patients with type 2 diabetes mellitus: outcomes from the TRINITY trial. Curr Med Res Opin. 2019;35:2071–2078.
- Keil R, Wasserbauer M, Zadorova Z, et al. Adherence, risk factors of non-adherence and patient’s preferred treatment strategy of mesalazine in ulcerative colitis: multicentric observational study. Scand J Gastroenterol. 2018;53(4):459–465.
- Goette A, Hammwöhner M. How important it is for therapy adherence to be once a day? Eur Heart J Suppl. 2016;18(suppl I):I7–I12.
- Dezii CM, Kawabata H, Tran M. Effects of once-daily and twice-daily dosing on adherence with prescribed glipizide oral therapy for type 2 diabetes. South Med J. 2002;95(1):68–71.
- Hauber AB, Tunceli K, Yang JC, et al. A survey of patient preferences for oral antihyperglycemic therapy in patients with type 2 diabetes mellitus. Diabetes Ther. 2015;6(1):75–84.
- Tanaka R, Hayakawa K, Oshima H. [Drug compliance of bisphosphonate in Japan: cohort study of bisphosphonate in a private clinic]. Osteoporosis Japan. 2009;17:252–255.
- Suzuki K, Hasegawa K, Watanabe M. Efficacy and patient satisfaction of dipeptidyl peptidase-4 inhibitor after switching from once-daily DPP-4 inhibitor to once-weekly regimen. J Clin Med Res. 2018;10(8):641–647.
- Ito H, Ando S, Tsugami E, et al. Changes in medication adherence and unused drugs after switching from daily dipeptidyl peptidase-4 inhibitors to once-weekly trelagliptin in patients with type 2 diabetes. Diabetes Res Clin Pract. 2019;153:41–48.
- Cramer JA. A systematic review of adherence with medications for diabetes. Diabetes Care. 2004;27(5):1218–1224.
- Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–1310.
- Lloyd CE, Mughal S, Roy T, et al. What factors influence concordance with medications? Findings from the UK Asian Diabetes study. Diabet Med. 2014;31(12):1600–1609.
- Miccoli R, Penno G, Del Prato S. Multidrug treatment of type 2 diabetes: a challenge for compliance. Diabetes Care. 2011;34(Supplement_2):S231–S5.
- Ministry of Health, Labour and Welfare. [National Health and Nutrition Survey]. 2017. [cited 2019 Nov 4]. Available from: https://www.mhlw.go.jp/bunya/kenkou/kenkou_eiyou_chousa.html.
