Abstract
Filgrastim prophylaxis, both primary and secondary, was rapidly incorporated into clinical practice in the 1990s. When pegfilgrastim became available in 2002, it quickly replaced filgrastim as the colony-stimulating factor (CSF) of choice for prophylaxis. Use of prophylaxis increased markedly in the first decade of this century and has stabilized during the present decade. Data concerning real-world CSF prophylactic practice patterns are limited but suggest that both primary and secondary prophylaxis are common, and that use is frequently inappropriate according to guidelines. The extent of inappropriate use is controversial, as are issues concerning the cost-effectiveness of prophylaxis versus no prophylaxis and the cost-effectiveness of primary prophylaxis versus secondary prophylaxis. Nevertheless, CSF prophylaxis is firmly established as a valuable adjunct to chemotherapy and will almost certainly continue to be widely used for the foreseeable future. In this article, we chronicle the use and impact of CSF prophylaxis in US patients receiving myelosuppressive chemotherapy for non-myeloid malignancies. We emphasize the interplay of expert opinion, clinical evidence, and economic factors in shaping the use of CSFs in clinical practice over time, and, with the recent introduction of new CSF agents and options, we aim to provide useful clinical and economic information for healthcare decision makers.
Introduction
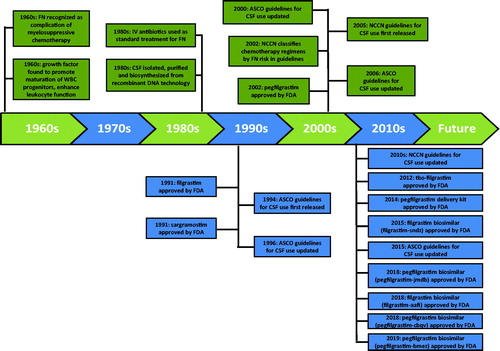
Febrile neutropenia (FN) has been recognized as a severe complication of myelosuppressive chemotherapy since the 1960s and by the 1980s, standard treatment of FN was hospitalization and intravenous antibiotics; mortality – approximately 10% – was substantialCitation1. Myeloid growth factors that promote the proliferation and maturation of white blood cell progenitors and enhance the function of mature leukocytes were first identified in the 1960sCitation2. In the 1980s, extensive research on these growth factors – called colony-stimulating factors (CSFs), because they induce the formation of colonies of cells from single progenitor cells – resulted in their isolation, purification, and biosynthesis via recombinant DNA methodologyCitation3.
Recognition of their potential benefits in hematology and oncology, especially the prevention and treatment of FN, led to the development of granulocyte CSF (G-CSF), also known as filgrastim, and the granulocyte-macrophage CSF (GM-CSF), sargramostim. In phase III trials, use of filgrastim in the first and all subsequent chemotherapy cycles (i.e. as primary prophylaxis) reduced the risk of FN by 50%, which led to FDA approval of filgrastim in 1991 “to decrease the incidence of infection, as manifested by FN, in patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with a clinically significant incidence of febrile neutropenia”Citation4–6. In the same year, GM-CSF was approved with a different indication: acceleration of neutrophil recovery in patients following high-dose cytotoxic therapy and autologous bone marrow transplantation in patients with lymphoid malignancies.
Since the early 1990s, CSFs have been rapidly and widely incorporated into oncology practice for the approved indications (). By far, the most common and best-studied use of CSFs has been for primary prophylaxis of FN and secondary prophylaxis of FN (i.e. administration of prophylaxis after starting chemotherapy, in response to FN or severe neutropenia in a prior cycle). In this article, we limit our attention to chronicling the use and impact of CSF prophylaxis in US patients receiving myelosuppressive chemotherapy for non-myeloid malignancies. Although sargramostim has been used for prophylaxis in a small proportion of cases, filgrastim and pegfilgrastim (a long-acting G-CSF that was approved in 2002) have dominated this field subsequently and are the principal focus of this reviewCitation7 (). We emphasize the interplay of expert opinion, clinical evidence, and economic factors in shaping the use of CSFs in clinical practice over time, and with the recent introduction of new CSF agents and options, we aim to provide useful clinical and economic information for healthcare decision-makers.
Table 1. Currently available CSF agents in US clinical practice.
The 1990s
American Society of Clinical Oncology guidelines
Believing that FDA-approved labeling provided inadequate guidance regarding the appropriate use of CSF, a “considerable number” of practicing physicians requested guidance from the American Society of Clinical Oncology (ASCO), resulting in the publication of the first ASCO clinical practice guidelines in 1994Citation8. For primary prophylaxis, it was recommended that CSF be used only when the estimated FN risk was ≥40%, based on the success of G-CSF in reducing FN risk in the three published RCTs cited aboveCitation4–6. The ASCO guidelines also cited an economic analysis based on data from the pivotal RCT and contemporary costing sources for FN hospitalization that suggested G-CSF primary prophylaxis would only be cost-saving if the risk of FN was ≥40%Citation9. The guidelines acknowledged that very few chemotherapy regimens were associated with the risk of FN ≥40%. A subsequent economic analysis, based on updated and more precise estimates of unit costs, reported the FN risk threshold to be lower (20–25%)Citation10.
For secondary prophylaxis, the recommendation was: “CSFs are recommended in some situations, e.g. after documented FN in a prior chemotherapy cycle to avoid infectious complications and maintain dose-intensity in subsequent treatment cycles when chemotherapy dose-reduction is not appropriate.” A 1996 update to the earlier recommendations did not alter the guidelines for primary and secondary prophylaxisCitation11.
Practice patterns
ASCO conducted surveys of its membership concerning the use of CSFs in 1994 (prior to the release of guidelines) and again in 1997Citation12,Citation13. The survey consisted of two parts: (1) hypothetical scenarios for primary and secondary prophylaxis; and (2) a small number of questions addressing respondents' use of CSFs in clinical practice. In hypothetical scenarios, respondents were reluctant to endorse primary prophylaxis, but much more willing to consider secondary prophylaxis, usually without chemotherapy dose reduction (57–85% of respondents favored secondary prophylaxis depending on the scenario). In response to questions regarding CSF use, many clinicians appeared to use shorter than recommended courses of prophylaxis. Changes in responses were minimal between the two surveys.
A study of 5034 outpatients who received chemotherapy for non-myeloid malignancies at 10 community-based oncology practices from 1996 to 1998 revealed that CSFs were administered in 14% of regimens, and that administration commenced in the first cycle in 49% of casesCitation14. Most CSF administration commenced late in the cycle, however, suggesting that it was for the treatment of FN or a FN.
Another study identified 5843 women with breast cancer diagnosed at age ≥65 years in a Surveillance, Epidemiology, and End Results (SEER)-Medicare-linked database from 1992 to 1999Citation15. Any receipt of filgrastim during chemotherapy was counted, with an attempt to distinguish primary prophylaxis from other uses (sargramostim was received by only 2% of patients and was not considered). Use of filgrastim increased from 4% in 1993 to 27% in 1999. Over the entire study period, there was considerable geographic variation in the percentage of patients receiving filgrastim – from 11% in Seattle to 23% in Atlanta.
The 2000s
American Society of Clinical Oncology guidelines
In 2000, ASCO updated its recommendations for the use of CSFsCitation16. While the recommendation for primary prophylaxis was unchanged from 1994 (i.e. use only when estimated FN risk was ≥40%), dose reduction was strongly endorsed over secondary prophylaxis when FN had occurred in a prior cycle. However, available data suggest that these guidelines had little impact on clinical practice.
Approval and rapid uptake of pegfilgrastim
In 2002, the FDA approved pegfilgrastim citing two RCTs in which a total of 467 breast cancer patients receiving up to four cycles of doxorubicin and docetaxel chemotherapy were randomly assigned to a single injection of pegfilgrastim per cycle or daily doses of filgrastim until their ANC count was ≥10 × 109/L or for 14 d, whichever occurred firstCitation17,Citation18. A combined analysis of the two trials reported a significantly lower risk of FN with pegfilgrastim – relative risk (RR), 0.56 (95% CI 0.35–0.89) Citation19. More recently, three meta-analyses have evaluated the comparative efficacy of pegfilgrastim versus filgrastimCitation20–22. These analyses have included – in addition to the Holmes and Green studies described above – three, or in one case four, smaller studies, and all three meta-analyses have reported a significantly lower risk of FN with pegfilgrastim.
With the price of pegfilgrastim similar to that of 10 doses of filgrastim, its advantages in convenience and compliance (i.e. of once per cycle dosing), as well as the above-cited evidence that it might well be superior in efficacy to filgrastim, pegfilgrastim rapidly replaced filgrastim as the CSF of choice for FN prophylaxis. In a retrospective study of 3535 patients receiving CSF prophylaxis during 12,056 chemotherapy cycles from 2005 to 2009, pegfilgrastim was used in 97% of these cyclesCitation23. Additional justification for the use of pegfilgrastim over filgrastim for prophylaxis may have been accumulating evidence that shorter-than-recommended dosing schedules for filgrastim, commonly used in clinical practice, were associated with higher rates of FNCitation24–27. Not only did pegfilgrastim largely replace filgrastim in US clinical practice, but there also was a dramatic increase in overall CSF use throughout this period as the utilization of pegfilgrastim – at a price that remained almost constant – more than doubled from 2003 to 2010Citation28.
Use of CSFs in general, and pegfilgrastim in particular, almost certainly increased with the widespread adoption of effective, but highly myelosuppressive chemotherapy regimens, which in turn was facilitated by the availability of CSFs for primary prophylaxis. This trend toward high-risk chemotherapy regimens is illustrated by data from two studies of patients receiving chemotherapy for early-stage breast cancer (ESBC) in US clinical practice – one covering the period 1997–2000 and the other, 2004–2010Citation29,Citation30. In the earlier study, 96% of patients received one of five regimens, of which the most popular were cyclophosphamide + methotrexate + fluorouracil (CMF) and doxorubicin + cyclophosphamide (AC). Only 3% of patients received primary prophylaxis and the percentage receiving prophylaxis in any cycle was never greater than 8%. In the latter study, only one of the five common regimens from 1997 to 2000 (AC) was among the seven most common regimens, all of which were dose-dense regimens or included a taxane (i.e. docetaxel or paclitaxel). Overall, 69% of patients received primary prophylaxis. The findings of these two studies are supported by a third study, conducted among Medicare patients receiving adjuvant chemotherapy for breast cancer between 1998 and 2005Citation31.
Practice patterns in the early 2000s
Ramsey et al. identified 2728 patients with breast, colorectal, or non-small cell lung cancer (NSCLC) who received chemotherapy from 2002 to 2005 in Washington StateCitation32. Chemotherapy regimens were classified by FN risk according to guidelines from the National Comprehensive Cancer Network (NCCN): high (>20%), intermediate (10–20%), or low (<10%). Use of CSFs was classified as primary prophylaxis (initial receipt of CSF from 1 d before to 7 d after the first chemotherapy administration) or other (presumably either secondary prophylaxis or treatment of FN/severe neutropenia). Only 35% of all CSF use was primary prophylaxis. In conjunction with high risk-chemotherapy, the proportion receiving primary CSF prophylaxis was greatest for breast cancer patients (38%); the corresponding percentage for NSCLC patients was 12% and none of the 37 colorectal cancer patients received primary prophylaxis. Over the four years of the study, the percentages of patients receiving CSFs for any reason increased, especially for breast cancer patients – from 40% in 2002 to 74% in 2005. Whether these patterns of use were specific to Washington or were nationally representative is unknown.
Crawford et al. analyzed prospectively collected data on neutropenic events among 2692 patients during the first three cycles of chemotherapy for five major cancer types between 2002 and 2005Citation33. All patients were treated at one of 115 representative US community practice sites. Overall, prophylactic CSF use increased over the three cycles from <20% in the first to >40% in the third. Among patients experiencing FN in the first cycle, 78% received prophylactic CSFs in the second cycle.
Morrison et al. obtained retrospective data from the medical records of 1412 patients receiving pegfilgrastim prophylaxis during the first three cycles of chemotherapy; all patients were treated at one of 99 US community oncology practicesCitation34. For 40% of patients, prophylactic pegfilgrastim was initiated in cycle 2 or 3. Collectively, these three studies – as well as the study by Hershman et al. – support the utilization data suggesting a dramatic increase over time in the use of CSFs – in general, and pegfilgrastim in particular – for prophylaxis, and also suggest that, at least during this period of time, secondary prophylaxis was almost as common, or perhaps even more common, than primary prophylaxis.
Finally, findings from a study by Kozma et al. suggest that the number of cancer-related hospitalizations in the US was steady from 1989 to 2007, while hospitalizations for chemotherapy-related neutropenic complications stabilized or decreased during this same periodCitation35. Using data from the US National Inpatient Sample Database and IMS Health Drug Distribution Database, study authors found that discharges for selected cancers remained relatively steady (or increased) over the 19-year observation period; rates of neutropenic complications, however, increased two-fold until the late 1990s, before stabilizing and/or declining. Study authors also found that the mean hospital stay decreased from 10.4 to 7.1 d, and that neutropenic-related mortality decreased from 10% to 5.4%. The use of growth factors and myelosuppressive chemotherapy increased over this same period.
New guidelines for colony-stimulating factors use
In 2005, the NCCN issued its first CSF guidelines, followed by a substantially revised set of guidelines from ASCO in 2006Citation36,Citation37. Both guidelines contained similar recommendations. Primary prophylaxis was recommended when the risk of FN was at least 20% (as opposed to 40% in earlier ASCO guidelines) and this risk was to be based on the average risk associated with the chemotherapeutic regimen and individual risk factors for FN associated with the patient, such as advanced age and pre-existing neutropenia. While there was some ambiguity in prior ASCO guidelines concerning the basis for the 40% risk threshold, the 2006 guidelines explicitly stated that “CSFs should be used when indicated for clinical reasons, not economic ones.” Moreover, for patients with an FN risk <20%, the guidelines stated that primary prophylaxis is often appropriate for those with certain clinical (i.e. risk) factors that predispose to FN (e.g. patient age >65 years, poor performance status, cytopenias, or poor nutritional status). For secondary prophylaxis, CSFs were “recommended for patients who experienced a neutropenic complication from a prior cycle of chemotherapy (for which primary prophylaxis was not received), in which a reduced dose may compromise disease-free or overall survival or treatment outcomes.”
Several key studies published after 2000 were cited by ASCO. A registrational RCT of pegfilgrastim versus placebo among 928 breast cancer patients receiving docetaxel chemotherapy reported a much lower incidence of FN among the pegfilgrastim group (1 versus 17%, p < .001)Citation38. These results were used to support the use of CSFs for primary prophylaxis when the baseline risk of FN was in the neighborhood of 20%. Also cited was a review article that summarized studies of patient-associated risk factors for FNCitation39 and preliminary results from a meta-analysis of CSF prophylaxis studies, which demonstrated significant reductions in the risk of FN and infection-related mortality with CSF prophylaxisCitation40.
ASCO and NCCN guidelines for the prophylactic use of CSFs have remained virtually the same in subsequent updates ()Citation41,Citation42. The broadening of the criteria for the prophylactic use of CSFs may have contributed to the increased use documented above.
Table 2. Current recommendations for use of CSF prophylaxis in US clinical practice.
Meta-analyses
In 2007, two meta-analyses of studies of prophylactic CSF administration were reportedCitation40,Citation43. While both found a substantial benefit of prophylaxis in terms of a reduced risk of FN, they differed as to the mortality benefit of prophylaxis. In the published version of the ASCO-cited meta-analysis (see above), the RR of infection-related mortality was 0.55 (95% CI 0.33–0.90) based on the 12 trials (n = 2917 patients) that included such dataCitation40. This meta-analysis was limited to studies among adults with solid tumors or lymphoma who received CSF within the first 3 d after completion of chemotherapy. The second meta-analysis was much broader in scope and considered 148 trials. In a sub-analysis of 26 trials among adults with solid tumors or lymphoma, the risk reduction in infection-related mortality was not statistically significant – RR 0.70 (95% CI 0.47–1.05)Citation43. Differences in the studies included in these two meta-analyses are probably the principal reason for the disparate findings. In particular, two dose-dense/dose-escalation studies included in the Sung secondary analysis reported a total of nine infection-related deaths in the CSF arms (out of 44 total deaths in the CSF arms) as opposed to only one infection-related death in their placebo arms (out of 59 total deaths)Citation44,Citation45.
A later meta-analysis reported a small benefit in overall mortality attributable to CSF prophylaxis. Lyman et al. performed a meta-analysis of chemotherapy RCTs supported by prophylactic CSFs; all 25 studies had a follow-up period of at least two yearsCitation46. CSF prophylaxis conferred a significantly reduced risk of death – RR = 0.90 (95% CI 0.86–0.94).
Economics of CSF prophylaxis
In this decade, the high economic burden of chemotherapy-induced neutropenia requiring inpatient care was highlighted by two studies. In a study utilizing hospital discharge data from seven states that covered 34% of the US population, Caggiano et al. reported the mean cost of chemotherapy-induced neutropenia to be $11,600 among patients with non-Hodgkin’s lymphoma (NHL) and $8100 among patients with common solid tumorsCitation47. In the second study using data from a large consortium of teaching hospitals (1995–2000), Kuderer et al. reported the mean cost of chemotherapy-induced neutropenia to be $18,400 among lymphoma patients and $13,400 among patients with solid tumorsCitation48. These estimates, however, do not include the considerable cost of neutropenia-related follow-on care.
The economic burden of the initial neutropenic event as well as the follow-on care was highlighted in a subsequent study conducted by Weycker et al.Citation49 Using data from a large US healthcare claims repository (2001–2003) on cancer chemotherapy patients (95% of whom had NHL or solid tumors), the authors reported that the costs of neutropenia-related care totaled $12,400 including $7800 for the initial hospitalization and $4600 for follow-on care ($2300 post-discharge during the cycle in which the hospitalization occurred and $2300 during subsequent chemotherapy cycles).
The high cost of chemotherapy-related neutropenic complications motivated the first cost-effectiveness study of CSF prophylaxis, which – focusing on the first cycle of chemotherapy in which FN risk is greatest – reported that prophylaxis would be cost-saving if FN risk was in the range of 20–25%Citation10. Since the probability of FN in the second and subsequent cycles is substantially lower than in the first, it seems unlikely that CSF prophylaxis would be cost-saving over an entire course of chemotherapy. Primary prophylaxis may be cost-effective, however, in the traditional sense of producing gains in patients’ life-years or quality-adjusted life-years (QALYs) at a cost considered reasonable, if it results in a lower mortality rate, either because of fewer FN deaths or because of improved survival (e.g. by permitting dose-maintenance without dose delays).
Several cost-effectiveness studies were reported during this decade. Timmer-Bonte examined the cost-effectiveness of adding CSF to prophylactic antibiotics during cycle 1 among patients receiving myelosuppressive chemotherapy for small-cell lung cancerCitation50. The authors reported a 14% reduction in the incidence of FN during cycle 1 with the addition of CSF prophylaxis in that cycle at a cost of €681 per patient. The applicability of their results to US practice is uncertain, however, since they are based on Dutch costs and the results of a small (n = 175) Dutch randomized clinical trial. Numnum et al. conducted a cost-effectiveness analysis of pegfilgrastim for the prevention of FN in patients with ovarian cancer receiving chemotherapy conferring a risk of FN of either 5% (“average risk”) or 16% (“high risk”)Citation51. Three strategies were compared: (1) dose delays or modification only after FN; (2) primary prevention; and (3) secondary prevention. Primary prevention was found to dominate (i.e. to be more effective and less costly) the other two strategies at the higher risk, and to be effective but costly at the average risk. Primary prophylaxis with pegfilgrastim also was found to be dominant in an analysis conducted by Eldar-Lissai et al.Citation52 In this analysis, a cost-utility model was employed to evaluate the economic impact of three alternative strategies among patients between the ages of 18 and 65 years receiving chemotherapy for solid tumors: (1) primary prophylactic use of pegfilgrastim; (2) primary prophylactic use of filgrastim; and (3) no prophylactic G-CSF. In the base-case employing the societal perspective and a time horizon defined as the first cycle of chemotherapy, pegfilgrastim was found to yield more quality-adjusted life-days and lower costs than filgrastim and no G-CSF, respectively.
Ramsey et al. examined the cost-effectiveness of primary versus secondary pegfilgrastim prophylaxis in women with ESBC receiving chemotherapy with ≥20% risk of FNCitation53. Assuming a survival benefit from avoiding FN mortality only, they estimated an incremental cost-effectiveness ratio (ICER) for primary pegfilgrastim prophylaxis (versus secondary) of $116,000 per QALY gained. While this ICER exceeds the frequently cited threshold of $50,000 a strong argument has been made that this threshold is unrealistic in the current era and a threshold of $100,000 or $150,000 is more appropriateCitation54.
Comparing pegfilgrastim and filgrastim primary prophylaxis, Lyman et al. completed two cost-effectiveness analyses: the first in women with ESBC, and the second in patients with NHLCitation55,Citation56. In the breast cancer analysis, assuming no survival benefits from reduced FN-related mortality or chemotherapy dose-maintenance without dose delays, pegfilgrastim was reported to be cost-effective versus 6-d filgrastim ($12,904 per FN episode avoided). Adding survival benefits from reduced FN-related mortality yielded an ICER of $31,511 per QALY, and further adding survival benefits from chemotherapy dose-maintenance without dose delays, the ICER was $14,415 per QALY. For patients with NHL, assuming no survival benefits, pegfilgrastim was cost-effective versus 6-d filgrastim ($2167 per FN episode avoided). Adding survival benefits, ICERs were $6190 per QALY gained (FN-related survival benefits) and $1677 per QALY gained (FN- and chemo-related survival benefits), well below the $50,000 per QALY gained threshold. Importantly, however, both studies highlight the limited data available on the relationship between chemotherapy dose intensity and cancer-specific patient survival.
In the same time frame, an analysis from Italy comparing pegfilgrastim and 6-d filgrastim (notably including survival benefits) in ESBC reported an ICER of €429 per QALY gainedCitation57. A similar analysis to that completed by LymanCitation55 in breast cancer was also completed from a UK National Health Service (NHS) perspectiveCitation58. Pegfilgrastim was reported to be cost-effective versus 6-d filgrastim (£4200 per FN event avoided) with no survival benefits. Including survival benefits resulted in ICERs of £8526 per QALY (FN-related survival) and £4161 per QALY gained with all survival benefits (FN- and chemotherapy-related).
The 2010s
Pegfilgrastim: consequences of deviations from guideline-based therapy
The proliferation of myeloid progenitor cells in response to pegfilgrastim is sufficiently rapid that administration on the same day as chemotherapy could adversely affect its efficacy. Therefore, the package insert and guidelines recommend that it be administered 24–72 h subsequent to the last administration of chemotherapy. Nevertheless, probably for reasons of physician and patient convenience (i.e. avoiding an additional healthcare visit), a minority of patients (estimated at 12% in one observational study and 24% in anotherCitation59,Citation60, receive their pegfilgrastim on the same day as chemotherapy. In two small RCTS comparing same-day with next-day pegfilgrastim, the duration of grade 4 neutropenia was significantly longer in the same day groups, although the absolute differences were small (1.2 and 0.9 d, respectively)Citation61.
Among observational studies with data on at least 500 cycles, the results have been mixed, with two studies reporting little or no difference in rates of grade 4 neutropenia and FNCitation60,Citation62 and two reporting significant differences in FN risk favoring delayed pegfilgrastimCitation59,Citation63. In the largest of these four studies (179,000 cycles), only patients receiving intermediate- or high-risk regimens were included and adjustment was made for differences in FN risk factors between the two groupsCitation59. The risk of FN was higher in all cycles for patients receiving same-day pegfilgrastim, odds ratio (OR), 1.5 (95% CI 1.3–1.6). Similar findings were reported in follow-up studies using private healthcare claims data spanning 2010–2015 (OR = 1.3, 95% CI 1.2–1.4) and using Medicare claims data (OR = 1.3, 95% CI 1.2–1.4)Citation64,Citation65. A recent systematic review of the literature on this topic concluded that the literature supports “administration of pegfilgrastim to patients at least 1 d after the completion of a chemotherapy cycle”Citation66.
Given the high cost of pegfilgrastim and the fact that the risk of FN is greatest in the first cycle and diminishes substantially thereafter, some clinicians have limited CSF prophylaxis to the first cycle or the first two cycles. Aarts et al. conducted an RCT in which patients with breast cancer at high-risk for FN were randomly assigned to pegfilgrastim prophylaxis for the first two cycles only or for all cyclesCitation67. The study was prematurely closed due to the high incidence of FN among patients assigned to pegfilgrastim in the first two cycles only – 36 versus 10% among patients receiving pegfilgrastim in every cycle.
In a study based on healthcare claims data, two matched groups of patients receiving intermediate- or high-risk chemotherapy regimens for solid cancers or NHL were comparedCitation68. The first group received pegfilgrastim during the first two cycles; the second received it only during the first. Similar to the Aarts’s study, the odds for FN were significantly higher among those receiving pegfilgrastim in cycle 1 only versus both cycles (FN broad definition: OR = 1.7, 95% CI 1.2–2.5; FN narrow definition: OR = 3.5, 95% CI 2.0–6.0). Comparable findings were reported in follow-up studies using private healthcare claims data spanning 2010–2015 (FN broad definition: OR = 1.7, 95% CI 1.2–2.3; FN narrow definition: OR = 4.3, 95% CI 2.5–7.6) and using Medicare claims data (FN broad definition: OR = 1.9, 95% CI 1.6–2.3; FN narrow definition: OR = 2.1, 95% CI 1.6–2.9)Citation69,Citation70. In both studies, FN ORs with prophylaxis discontinuation in subsequent cycles (i.e. after the first cycle) were largely the same.
Use of pegfilgrastim in US clinical practice
The volume of use of pegfilgrastim prophylaxis appears to have stabilized over the past 6 years. The best evidence comes from publically available utilization data, indicating similar numbers of units sold annually in the US from 2011 to 2015Citation71. In part, this stabilization may have resulted from price increases; the Centers for Medicare and Medicaid Services (CMS) payment limit increased steadily from $2360 (per 6 mg dose) in 2010 to $3828 in 2016Citation28. Another factor limiting any increase in use of pegfilgrastim for prophylaxis may have been controversy about the appropriateness of such use – the subject of several articles published in major journals during this decadeCitation72–75.
Perhaps the best summary statement concerning potential inappropriate use is as follows: “Although there is little debate that both underuse and overuse of the CSFs occur in clinical practice, accurate estimates of the true magnitude and impact of such considerations remain elusive” Citation72. Those that believe the magnitude of inappropriate use is great emphasize: (1) CSF support in conjunction with dose-dense regimens, as well as other high-risk regimens, has not proven to be superior to conventional regimens with a lower risk of FN (e.g. dose-dense regimens for estrogen-receptor [ER]-positive breast cancer); (2) CSF use to permit dose maintenance when it has not proven superior to dose reduction in treating advanced cancer; and (3) the adverse effects of CSF treatmentCitation73–75. Those that believe the magnitude of inappropriate use is not so great emphasize: (1) the substantial clinical and economic consequences of FN; and (2) the high proportion of persons undergoing chemotherapy who have a significant risk of FN when patients’ personal risk-enhancing characteristics are accounted for in addition to the regimen-associated riskCitation72.
To the best of our knowledge, there are only two published studies of actual patterns of CSF prophylaxis subsequent to those described above for the early 2000s. Waters et al. reported the use of pegfilgrastim for primary prophylaxis among ambulatory patients receiving chemotherapy regimens with an average FN risk ≤20% at a single academic institutionCitation76. Of 399 doses administered to 88 patients, 46% were considered “avoidable” because there were no recorded risk factors for FN. However, the authors note that because they relied on written documentation, the prevalence of patient-specific risk factors was potentially underestimated. In addition, the authors stated: “Because this study relied on written documentation to identify additional patient-specific risk factors, patient FN risk is potentially underestimated.” In a retrospective study of patients with non-metastatic cancer of the breast, colon/rectum, lung, or ovaries or NHL receiving chemotherapy regimens with an intermediate/high-risk for FN, use of CSF primary prophylaxis (mostly pegfilgrastim, >96%) increased only slightly from 52% in 1Q2010 to 58% in 4Q2016, suggesting that although primary prophylaxis is commonplace in current US clinical practice, underutilization in patients for whom prophylaxis is recommended may still be an issueCitation77.
Notwithstanding the recent focus on the inappropriate use of CSF agents in US clinical practice, the findings from a 2016 evaluation highlighted (again) the clinical benefits of pegfilgrastim prophylaxis, especially with regimens associated with a higher risk of FNCitation78. In this study, women with breast cancer receiving selected intermediate-risk regimens (TC: docetaxel + cyclophosphamide; TCH: docetaxel + carboplatin + trastuzumab) and primary prophylaxis (∼60% of women receiving TC or TCH) were reported to have 70–80% lower risk of FN than those not receiving primary prophylaxis. Among women with breast cancer receiving a low-risk regimen (AC: doxorubicin + cyclophosphamide [conventional-dose]), however, no such benefits were reported. One other recent study, which comprised separate analyses of commercially insured patients and Medicare patients, respectively, documented the effectiveness of pegfilgrastim prophylaxis among patients with non-metastatic breast cancer or NHL receiving chemotherapy with an intermediate/high-risk for FNCitation79. In the first analysis, which utilized data from two large private healthcare claims databases (2010–2016), FN odds in cycle 1 were significantly higher among patients not receiving prophylaxis versus those receiving pegfilgrastim prophylaxis (FN broad definition: OR = 2.6, 95% CI 2.3–2.8; FN narrow definition: OR = 4.2, 95% CI 3.8–4.7). In the second analysis, which utilized the same design and Medicare claims data, FN odds were 1.6 (95% CI 1.5–1.7) to 2.0 (1.9–2.2) times higher (broad and narrow definitions, respectively) in cycle 1. In both analyses, findings for subsequent cycles were similar.
Meta-analyses
More recently, Lyman et al. provide additional evidence supporting the favorable risk-benefit profile of CSFs with an update to the 2013 analysisCitation80. Based on random-effects meta-analysis, CSF support was reported to significantly improve survival compared to no CSF support (mortality RR = 0.92; 95% CI: 0.90–0.95; p < .001), even more so for patients receiving dose-dense chemotherapy regimens (mortality RR = 0.86; 95% CI: 0.80–0.92; p<.001). While patients who received CSF support experienced a significantly higher risk of secondary malignancies compared with controls (RR = 1.85; 95% CI: 1.19–2.88; p < .01), the number needed to treat (NNT) to observe one secondary malignancy was 213 (p<.001) (i.e. for every secondary malignancy observed, 7.4 deaths were avoided). Importantly, the authors note that it was not possible to distinguish whether the risk of secondary malignancies is associated with supportive CSF use or the leukemogenic effects of intensified chemotherapeutic agents. The issue of competing risk (where an event, in this case improved survival, modifies the chance that the event of interest, secondary malignances, occurs) was also not accounted for in the analysis.
Economics of CSF prophylaxis
Six major cost-effectiveness studies conducted in this decade attributed to prophylaxis both a modest mortality reduction from FN prevention and a slight survival benefit from maintenance of chemotherapy relative dose intensity (RDI). Whyte et al. reported the cost-effectiveness of primary and secondary pegfilgrastim prophylaxis in the UK for women with stage II breast cancer and a 24% risk of FNCitation81. For secondary prophylaxis versus no-CSF, the ICER was £6500 per life-year gained, and for primary versus secondary prophylaxis, £38,482 per life-year gained. When FN risk was >38%, primary pegfilgrastim was the most cost-effective strategy. In a US analysis based on NHL patients, Hill and colleagues reported an ICER of $29,500 per QALY gained for primary pegfilgrastim versus secondary pegfilgrastimCitation82.
Fust et al. evaluated primary prophylaxis or secondary prophylaxis with pegfilgrastim as well as filgrastim (versus no prophylaxis) in patients with recurrent ovarian cancer receiving docetaxel or topotecanCitation83. For patients receiving docetaxel, the ICER for primary versus secondary prophylaxis with pegfilgrastim was $7900 per QALY gained; pegfilgrastim primary prophylaxis dominated all comparators. For patients receiving topotecan, pegfilgrastim primary prophylaxis was dominant versus all comparators.
From a Canadian perspective, and assuming a mortality reduction from FN prevention, Lee et al. examined the cost-effectiveness of secondary prophylaxis, primary prophylaxis starting with the first cycle of D, and primary prophylaxis starting with the first cycle of FEC in patients with ESBCCitation84. Starting filgrastim prophylaxis with the first cycle of D and FEC, compared to using secondary prophylaxis, resulted in ICER values of $57,886 per QALY gained and $116,186 per QALY gained, respectively. With pegfilgrastim, the ICERs for the same strategies were $90,735 per QALY gained and $149,483 per QALY gained.
Also from a Canadian perspective and assuming a survival benefit from FN prevention, Skedgel et al. assessed the cost-effectiveness of primary prophylaxis, secondary prophylaxis, and no G-CSF among women with breast cancer receiving adjuvant taxotere plus cyclophosphamide (TC)Citation85. Secondary prophylaxis dominated no prophylaxis, while the cost of primary prophylaxis (versus secondary prophylaxis) was $94,327 per QALY gained. Finally, from a Belgium perspective, Fust et al. analyzed cost-effectiveness among patients with ESBC and NHL. The authors reported that secondary prevention dominated the no prophylaxis strategy, and primary prevention had highly favorable ICERs versus secondary prevention: €15,500 per QALY gained for breast cancer and €7800 for NHLCitation86.
On the other hand, if FN-related mortality and chemotherapy RDI-related survival benefits are not assumed (and the only QALY advantage of prophylaxis is the avoidance of the very minimal quality-of-life decrement associated with FN hospitalization), ICERs are highly unfavorable. In such a study, Lathia et al. examined the cost-effectiveness of primary pegfilgrastim prophylaxis and primary filgrastim prophylaxis among Canadian patients with NHL and a 64% risk of FN during the chemotherapy course; they reported an ICER of $5.8 million per QALY gained for filgrastim (versus no prophylaxis) and an ICER of $2.6 million per QALY gained for pegfilgrastim (versus filgrastim primary prophylaxis)Citation87. Similarly, Chan and colleagues completed a Canadian analysis comparing primary and secondary prophylaxis with filgrastim among elderly patients with diffuse aggressive lymphomaCitation88. Without FN-related mortality and chemotherapy RDI-related survival benefits, the ICER was $700,500 per QALY gained.
The forseeable future
New products may alter the practice of FN prophylaxis. In 2012, a new once-a-day recombinant G-CSF (tbo-filgrastim) received FDA approval, and in 2015, the first filgrastim biosimilar (filgrastim-sndz) was approved, followed by a second biosimilar (filgrastim-aafi) in 2018. However, these daily dosed products are not expected to become a substitute for the most widely used option for prophylaxis–pegfilgrastim.
Other developments may, however, increase the use of CSFs. Amgen’s 2015 launch of a pegfilgrastim on-body injector kit (Onpro), which eliminates the need for patients to return the day after chemotherapy for CSF prophylaxis, may provide a mechanism for physicians to provide appropriate prophylaxis where it may have been limited to the first one or two cycles, or not provided at all, previously. In 2018, the FDA approved the first pegfilgrastim biosimilars, pegfilgrastim-jmdb and pegfilgrastim-cbqv, followed by pegfilgrastim-bmez in 2019. Approval of the pegfilgrastim biosimilars is expected to produce downward pressure on prices, which may lead to increased use of CSF prophylaxis in clinical practice. While patient and caregiver travel burden has been identified as an important factor for physicians in CSF decision-making, cost/insurance coverage has also been identified as a common reason why patients do not receive CSF prophylaxisCitation89,Citation90. In addition, we note that cost-effectiveness ratios for CSF prophylaxis discussed above have been found to be sensitive to corresponding drug acquisition costs. Reductions in such costs of 10% or more have been shown to yield meaningful improvements in the cost-effectiveness of CSF prophylaxis (81.82). Thus, to the extent that the introduction of pegfilgrastim biosimilars results in lower drug acquisition costs, this development will undoubtedly improve the cost-effectiveness profile of CSF prophylaxis.
FN continues to be an important issue for patients receiving myelosuppressive chemotherapy. Based on data from the 2012 National Inpatient Sample (NIS), there were 91,560 cancer-related neutropenia hospitalizations among US adults, at a mean cost of $24,770 per stay ($14,250 for metastatic cancer ([n = 16,000 hospitalizations]); $25,676 for NHL [n = 14,000]; $12,552 for lung [n = 8000])Citation91. Thus, the need for CSF prophylaxis persists. More appropriate use of prophylaxis in the future may lead to a decrease in the burden of FN. In a recent retrospective study based on healthcare claims data, one-half of all FN hospitalizations among patients who were appropriate candidates for CSF prophylaxis occurred among those who did not receive it or received in a manner inconsistent with guidelinesCitation68. Widespread adoption of an accurate clinical tool, possibly including genetic factors and/or biomarkers to predict FN risk in individual patients – in lieu of relying on physician judgment – could lead to improved appropriateness of use of CSF prophylaxisCitation41,Citation46,Citation92.
Summary
Despite its high cost, filgrastim prophylaxis, both primary and secondary was rapidly incorporated into clinical practice in the 1990s. When pegfilgrastim became available in 2002, it quickly replaced filgrastim as the CSF of choice for prophylaxis, because of its greater convenience, and possibly greater efficacy. Use of prophylaxis increased markedly in the first decade of this century, probably due to the widespread use of dose-dense chemotherapy for breast cancer and other highly myelosuppressive chemotherapy regimens. In addition, the increase in use may have been facilitated by ASCO and NCCN guidelines in 2005–2006 that expanded the indication for primary prophylaxis and endorsed secondary prophylaxis in situations where adopting the alternative strategy – dose reduction – might compromise survival or other treatment outcomes. During the present decade, use of prophylaxis appears to have stabilized.
Data concerning real-world CSF prophylactic practice patterns are limited, but suggest that both primary and secondary prophylaxis are common and that use is frequently inappropriate according to guidelines. The extent of inappropriate use is controversial, as are issues concerning the cost-effectiveness of prophylaxis versus no prophylaxis, and the cost-effectiveness of primary prophylaxis versus secondary prophylaxis. Nevertheless, CSF prophylaxis is firmly established as a valuable adjunct to chemotherapy and will almost certainly continue to be widely used for the foreseeable future.
Transparency
Declaration of funding
Funding for this research was provided by Amgen Inc. to Policy Analysis Inc. (PAI) and the Fred Hutchinson Cancer Research Center.
Declaration of financial/other relationships
DW and JE are employed by PAI, which received funding for this research from Amgen. GHL is a principal investigator on a research grant provided to Fred Hutchinson Cancer Research Center by Amgen. MB and CB are employed by, and own stock in, Amgen. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Acknowledgements
None reported.
References
- Pizzo PA. Management of fever in patients with cancer and treatment-induced neutropenia. N Engl J Med. 1993;328:1323–1332.
- Bodey GP, Buckley M, Sathe YS, et al. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64(2):328–340.
- Groopman JE, Molina JM, Scadden DT. Hematopoietic growth factors. N Engl J Med. 1989;321(21):1449–1459.
- Crawford J, Ozer H, Stoller R, et al. Reduction by granulocyte colony-stimulating factor of fever and neutropenia induced by chemotherapy in patients with small-cell lung cancer. N Engl J Med. 1991;325(3):164–170.
- Pettengell R, Gurney H, Radford JA, et al. Granulocyte colony-stimulating factor to prevent dose-limiting neutropenia in non-Hodgkin's lymphoma: a randomized controlled trial. Blood. 1992;80(6):1430–1436.
- Trillet-Lenoir V, Green J, Manegold C, et al. Recombinant granulocyte colony stimulating factor reduces the infectious complications of cytotoxic chemotherapy. Eur J Cancer. 1993;29A:319–324.
- Weycker D, Malin J, Barron R, et al. Comparative effectiveness of filgrastim, pegfilgrastim, and sargramostim as prophylaxis against hospitalization for neutropenic complications in cancer chemotherapy patients. Am J Clin Oncol. 2012;35(3):267–274.
- American Society of Clinical Oncology. Recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. J Clin Oncol. 1994;12(11):2471–2508.
- Lyman GH, Lyman CG, Sanderson RA, et al. Decision analysis of hematopoietic growth factor use in patients receiving cancer chemotherapy. J Natl Cancer Inst. 1993;85(6):488–493.
- Lyman GH, Kuderer N, Greene J, et al. The economics of febrile neutropenia: implications for the use of colony-stimulating factors. Eur J Cancer. 1998;34(12):1857–1864.
- American Society of Clinical Oncology. Update recommendations for the use of hematopoietic colony stimulating factors: evidence-based clinical practice guidelines. J Clin Oncol. 1996;14:1957–1960.
- Bennett CL, Smith TJ, Weeks JC, et al. Use of hematopoietic colony-stimulating factors: the American society of clinical oncology survey. The health services research committee of the American society of clinical oncology. J Clin Oncol. 1996;14(9):2511–2520.
- Bennett CL, Weeks JC, Somerfield MR, et al. Use of hematopoietic colony-stimulating factors: comparison of the 1994–1997 American society of clinical oncology surveys regarding ASCO clinical practice guidelines. J Clin Oncol. 1999;17(11):3676–3681.
- Swanson G, Bergstrom K, Stump E, et al. Growth factor usage patterns and outcomes in the community setting: collection through a practice-based computerized clinical information system. J Clin Oncol. 2000;18(8):1764–1770.
- Du XL, Lairson DR, Begley CE, et al. Temporal and geographic variation in the use of hematopoietic growth factors in older women receiving breast cancer chemotherapy: findings from a large population-based cohort. J Clin Oncol. 2005;23(34):8620–8628.
- Ozer H, Armitage JO, Bennett CL, et al. 2000 Update of recommendations for the use of hematopoietic colony-stimulating factors: evidence-based, clinical practice guidelines. J Clin Oncol. 2000;18(20):3558–3585.
- Holmes FA, Jones SE, O’Shaughnessy J, et al. Comparable efficacy and safety profiles of once-per-cycle pegfilgrastim and daily injection filgrastim in chemotherapy-induced neutropenia: a multicenter dose-finding study in women with breast cancer. Ann Oncol. 2002;13(6):903–909.
- Green MD, Koelbl H, Baselga J, et al. A randomized double-blind multicenter phase III study of fixed-dose single-administration pegfilgrastim versus daily filgrastim in patients receiving myelosuppressive chemotherapy. Ann Oncol. 2003;14(1):29–35.
- Siena S, Piccart MJ, Holmes FA, et al. A combined analysis of two pivotal randomized trials of a single dose of pegfilgrastim per chemotherapy cycle and daily Filgrastim in patients with stage II-IV breast cancer. Oncol Rep. 2003;10(3):715–724.
- Pinto L, Liu Z, Doan Q, et al. Comparison of pegfilgrastim with filgrastim on febrile neutropenia, grade IV neutropenia and bone pain: a meta-analysis of randomized controlled trials. Curr Med Res Opin. 2007;23(9):2283–2295.
- Cooper KL, Madan J, Whyte S, et al. Granulocyte colony-stimulating factors for febrile neutropenia prophylaxis following chemotherapy: systematic review and meta-analysis. BMC Cancer. 2011;11(1):404.
- Wang L, Baser O, Kutikova L, et al. The impact of primary prophylaxis with granulocyte colony-stimulating factors on febrile neutropenia during chemotherapy: a systematic review and meta-analysis of randomized controlled trials. Support Care Cancer. 2015;23(11):3131–3140.
- Naeim A, Henk HJ, Becker L, et al. Pegfilgrastim prophylaxis is associated with a lower risk of hospitalization of cancer patients than filgrastim prophylaxis: a retrospective United States claims analysis of granulocyte colony-stimulating factors (G-CSF). BMC Cancer. 2013;13(1):11.
- Scott SD, Chrischilles EA, Link BK, et al. Days of prophylactic filgrastim use to reduce febrile neutropenia in patients with non-Hodgkin’s lymphoma treated with chemotherapy. J Manag Care Pharm. 2003;9(2):15–S21.
- Chrischilles EA, Rubenstein LM, Voelker MD, et al. Granulocyte colony-stimulating factor use during first course chemotherapy for non-Hodgkin’s lymphoma: national SEER-Medicare study. Blood. 2003;102(11):499A.
- Weycker D, Hackett J, Edelsberg JS, et al. Are shorter courses of filgrastim prophylaxis associated with increased risk of hospitalization? Ann Pharmacother. 2006;40(3):402–407.
- Weycker D, Malin J, Kim J, et al. Risk of hospitalization for neutropenic complications of chemotherapy in patients with primary solid tumors receiving pegfilgrastim versus filgrastim prophylaxis: a retrospective cohort study. Clin Ther. 2009;31(5):1069–1081.
- SEC Filing. Amgen’s website. [cited 2016 Oct 20]. Available from: http://investors.amgen.com/financial-information/annual reports.
- Lyman GH, Dale DC, Crawford J. Incidence and predictors of low dose-intensity in adjuvant breast cancer chemotherapy: a nationwide study of community practices. J Clin Oncol. 2003;21(24):4524–4531.
- Weycker D, Barron R, Edelsberg J, et al. Incidence of reduced chemotherapy relative dose intensity among women with early stage breast cancer in US clinical practice. Breast Cancer Res Treat. 2012;133(1):301–310.
- Hershman D, Wilde ET, Wright JD, et al. Uptake and economic impact of first-cycle colony-stimulating factor and use during adjuvant treatment of breast cancer. J Clin Oncol. 2012;30(8):806–812.
- Ramsey SD, McCune JS, Blough DK, et al. Colony-stimulating factor prescribing patterns in patients receiving chemotherapy for cancer. Pharmacotherapy. 2010;16(9):678–686.
- Crawford J, Dale DC, Kuderer NM, et al. Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J Natl Compr Canc Netw. 2008;6(2):109–118.
- Morrison VA, Wong M, Hershman D, et al. Observational study of the prevalence of febrile neutropenia in patients who received filgrastim or pegfilgrastim associated with 3-4 week chemotherapy regimens in community oncology practices. J Manag Care Pharm. 2007;13(4):337–348.
- Kozma CM, Dickson M, Chia V, et al. Trends in neutropenia-related inpatient events. J Oncol Pract. 2012;8(3):149–150.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: myeloid growth factors in cancer treatment. Version 2. 2005. Available from: www.nccn.org
- Smith TJ, Khatcheressian J, Lyman GH, et al. 2006 Update of recommendations for the use of white blood cell growth factors: an evidence-based clinical practice guideline. J Clin Oncol. 2006;24(19):3187–3205.
- Vogel CL, Wojtukiewicz MZ, Carroll RR, et al. First and subsequent cycle of use of pegfilgrastim prevents febrile neutropenia in patients with breast cancer: a multicenter, double-blind, placebo-controlled phase III study. J Clin Oncol. 2005;23(6):1178–1184.
- Lyman GH, Kuderer NM. Epidemiology of febrile neutropenia. Support Cancer Ther. 2003;1(1):23–35.
- Kuderer NM, Dale DC, Crawford J, et al. Impact of primary prophylaxis with granulocyte colony-stimulating factor on febrile neutropenia and mortality in adult cancer patients receiving chemotherapy: a systematic review. J Clin Oncol. 2007;25(21):3158–3167.
- Smith TJ, Bohlke K, Lyman GH, et al. Recommendations for the use of WBC growth factors: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2015;33(28):3199–3212.
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology (NCCN guidelines): myeloid growth factors, version 2.2019, 03/27/19. Fort Washington (PA): National Comprehensive Cancer Network, Inc; 2019.
- Sung L, Nathan P, Alibhai S, et al. Meta-analysis: effect of prophylactic hematopoietic colony-stimulating factors on mortality and outcomes of infection. Ann Intern Med. 2007;147(6):400–411.
- Bunn PA, Jr., Crowley J, Kelly K, et al. Chemoradiotherapy with or without granulocyte-macrohage colony-stimulation factor in the treatment of limited-stage small-cell lung cancer: a prospective phase III randomized study of Southwest Oncology Group. J Clin Oncol. 1995;13(7):1632–1641.
- Fridrik MA, Greil R, Hausmaninger H, et al. Randomized open label phase III trial of CEOP/IMVP-Dexa alternating chemotherapy and filgrastim versus CEOP/IMVP-Dexa alternating chemotherapy for aggressive non-Hodgkin’s lymphoma (NHL). A multicenter trial by the Austrian Working Group for medical tumor therapy. Ann Hematol. 1997;75:135–140.
- Lyman GH, Dale DC, Culakova E, et al. The impact of the granulocyte colony-stimulating factor on chemotherapy dose intensity and cancer survival: a systematic review and meta-analysis of randomized controlled trials. Ann Oncol. 2013;24(10):2475–2484.
- Caggiano V, Weiss RV, Rickert TS, et al. Incidence, cost and mortality of neutropenia hospitalization associated with chemotherapy. Cancer. 2005;103(9):1916–1924.
- Kuderer NM, Dale DC, Crawford J, et al. Mortality, morbidity, and cost associated with febrile neutropenia in adult cancer patients. Cancer. 2006;106(10):2258–2266.
- Weycker D, Malin J, Edelsberg J, et al. Cost of neutropenic complications of chemotherapy. Ann Oncol. 2008;19(3):454–460.
- Timmer-Bonte JN, Adang EM, Smit HJ, et al. Cost-effectiveness of adding granulocyte colony-stimulating factor to primary prophylaxis with antibiotics in small-cell lung cancer. J Clin Oncol. 2006;24(19):2991–2997.
- Numnum TM, Kimball KJ, Rocconi RP, et al. Pegfilgrastim for the prevention of febrile neutropenia in patients with epithelial ovarian carcinoma—a cost-effectiveness analysis. Int J Gynecol Cancer. 2007;17(5):1019–1024.
- Eldar-Lissai A, Cosler LE, Culakova E, et al. Economic analysis of prophylactic pegfilgrastim in adult cancer patients receiving chemotherapy. Value Health. 2008;11(2):172–179.
- Ramsey SD, Liu Z, Boer R, et al. Cost-effectiveness of primary versus secondary prophylaxis with pegfilgrastim in women with early-stage breast cancer receiving chemotherapy. Value Health. 2009;12(2):217–225.
- Neumann PJ, Cohen JT, Weinstein MC. Updating cost-effectiveness—the curious resilience of the $50,000-per-QALY threshold. N Eng J Med. 2014;371(9):796–797.
- Lyman G, Lalla A, Barron R, et al. Cost-effectiveness of pegfilgrastim versus 6-day filgrastim primary prophylaxis in patients with non-Hodgkin's lymphoma receiving CHOP-21 in United States. Curr Med Res Opin. 2009;25(2):401–411.
- Lyman GH, Lalla A, Barron RL, et al. Cost-effectiveness of pegfilgrastim versus filgrastim primary prophylaxis in women with early-stage breast cancer receiving chemotherapy in the United States. Clin Ther. 2009;31(5):1092–1104.
- Danova M, Chiroli S, Rosti G, et al. Cost-effectiveness of pegfilgrastim versus six days of filgrastim for preventing febrile neutropenia in breast cancer patients. Tumori. 2009;95(2):219–226.
- Liu Z, Doan QV, Malin J, et al. The economic value of primary prophylaxis using pegfilgrastim compared with filgrastim in patients with breast cancer in the UK. Appl Health Econ Health Policy. 2009;7(3):193–205.
- Weycker D, Li X, Figueredo J, et al. Risk of chemotherapy-induced febrile neutropenia in cancer patients receiving pegfilgrastim prophylaxis: does timing of administration matter? Support Care Cancer. 2015;24:2309–2316.
- Billingsley CC, Jacobson SN, Crafton SM, et al. Evaluation of the hematologic safety of same day versus standard administration (24- to 72- hour delay) of pegfilgrastim in gynecology oncology patients undergoing cytotoxic chemotherapy. Int J Gynecol Cancer. 2015;25(7):1331–1336.
- Burris HA, Belani CP, Kaufman PA, et al. Pegfilgrastim on the same day versus next day of chemotherapy in patients with breast cancer, non-small-cell lung cancer, ovarian cancer, and non-Hodgkin’s lymphoma: results of four multicenter, double-blind, randomized phase II studies. J Oncol Pract. 2010;6(3):133–140.
- Whitworth JM, Matthews KS, Shipman KA, et al. The safety and efficacy of day 1 versus day 2 administration of pegfilgrastim in patients receiving myelosuppressive chemotherapy for gynecologic malignancies. Gynecol Oncol. 2009;112(3):601–604.
- Cheng C, Gallagher EM, Yeh J-Y, et al. Rates of febrile neutropenia with pegfilgrastim on same versus next day of CHOP with or without rituximab. Anticancer Drugs. 2014;25(8):964–969.
- Weycker D, Bensink M, Lonshteyn A, et al. Risk of chemotherapy-induced febrile neutropenia by day of pegfilgrastim prophylaxis in US clinical practice from 2010 to 2015. Curr Med Res Opin. 2017;33:2107–2113.
- Weycker D, Hanau A, Lonshteyn A, et al. Risk of chemotherapy-induced febrile neutropenia with same-day versus next-day pegfilgrastim prophylaxis among patients aged ≥65 years: a retrospective evaluation using Medicare claims. Curr Med Res Opin. 2018;34(9):1705–1711.
- Lyman GH, Allcott K, Garcia J, et al. The effectiveness and safety of same-day versus next-day administration of long-acting granulocyte colony-stimulating factors for the prophylaxis of chemotherapy induced neutropenia: a systematic review. Support Care Cancer. 2017;25(8):2619–2629.
- Aarts MJ, Peters FP, Mandigers CM, et al. Primary granulocyte colony-stimulating factor prophylaxis during the first two cycles only or throughout all chemotherapy cycles in patients with breast cancer at risk for febrile neutropenia. J Clin Oncol. 2013;31(34):4290–4296.
- Weycker D, Li X, Barron R, et al. Risk of chemotherapy-induced febrile neutropenia with early discontinuation of pegfilgrastim prophylaxis in US clinical practice. Support Care Cancer. 2016;24(6):2481–2490.
- Weycker D, Bensink M, Wu H, et al. Risk of chemotherapy-induced febrile neutropenia with early discontinuation of pegfilgrastim prophylaxis based on real-world data from 2010 to 2015. Curr Med Res Opin. 2017;33:2115–2120.
- Weycker D, Hanau A, Lonshteyn A, et al. Risk of chemotherapy-induced febrile neutropenia with early discontinuation of pegfilgrastim prophylaxis: a retrospective evaluation using medicare claims. Curr Med Res Opin. 2019;35(4):725–730.
- Drugs.com. Neulasta sales data. [cited 2016 Dec 25]. Available from: https://www.drugs.com/stats/neulasta.
- Kuderer NM, Lyman GH. Personalized medicine and cancer supportive care: appropriate use of colony-stimulating factor support of chemotherapy. J Natl Cancer Inst. 2011;103(12):910–913.
- Smith TJ, Hillner BE. Bending the cost curve in cancer care. N Engl J Med. 2011;364(21):2060–2065.
- Schnipper LE, Smith TJ, Raghavan D, et al. American society of clinical oncology identifies five key opportunities to improve care and reduce costs: the top five list for oncology. J Clin Oncol. 2012;30(14):1715–1724.
- Smith TJ, Hillner BE. A way forward on the medically appropriate use of white cell growth factors. J Clin Oncol. 2012;30(14):1584–1587.
- Waters GE, Corrigan P, Gatesman M, et al. Comparison of pegfilgrastim prescribing practice to national guidelines at a university hospital outpatient oncology clinic. J Oncol Pract. 2013;9(4):203–206.
- Weycker D, Bensink M, Lonshteyn A, et al. Use of colony-stimulating factor primary prophylaxis and incidence of febrile neutropenia from 2010–2016: a longitudinal assessment. Curr Med Res Opin. 2019;35(6):1073–1080.
- Agiro A, Ma Q, Acheson AK, et al. Risk of neutropenia-related hospitalization in patients who received colony-stimulating factors with chemotherapy for breast cancer. J Clin Oncol. 2016;34(32):3872–3879.
- Weycker D, Lonshteyn A, Doroff R, et al. Use and effectiveness of pegfilgrastim prophylaxis in US clinical practice: a retrospective observational study. BMC Cancer. 2019;19(1):792.
- Lyman GH, Yau L, Nakov R, et al. Overall survival and risk of second malignancies with cancer chemotherapy and G-CSF support. Ann Oncol. 2018;29(9):1903–1910.
- Whyte S, Cooper KL, Stevenson MD, et al. Cost-effectiveness of granulocyte colony-stimulating factor prophylaxis for febrile neutropenia in breast cancer in the United Kingdom. Value Health. 2011;14(4):465–474.
- Hill G, Barron R, Fust K, et al. Primary vs secondary prophylaxis with pegfilgrastim for the reduction of febrile neutropenia risk in patients receiving chemotherapy for non-Hodgkin's lymphoma: cost-effectiveness analyses. J Med Econ. 2014;17(1):32–42.
- Fust K, Li X, Maschio M, et al. Cost-effectiveness of prophylaxis treatment strategies for febrile neutropenia in patients with recurrent ovarian cancer. Gynecol Oncol. 2014;133(3):446–453.
- Lee EK, Wong WWL, Trudeau ME, et al. Cost-effectiveness of prophylactic granulocyte colony-stimulating factor for febrile neutropenia in breast cancer patients receiving FEC-D. Breast Cancer Res Treat. 2015;150(1):169–180.
- Skedgel C, Rayson D, Younis T. Is febrile neutropenia prophylaxis with granulocyte-colony stimulating factors economically justified for adjuvant TC chemotherapy in BC? Support Care Cancer. 2016;24(1):387–394.
- Fust K, Li X, Maschio M, et al. Cost-effectiveness analysis of prophylaxis treatment strategic to reduce the incidence of febrile neutropenia in patients with early-stage breast cancer or non-Hodgkin lymphoma. Pharmacoeconomics. 2017;35(4):425–438.
- Lathia N, Isogai PK, Angelis CD, et al. Cost-effectiveness of filgrastim and pegfilgrastim as primary prophylaxis against neutropenia in lymphoma patients. J Natl Cancer Inst. 2013;105(15):1078–1085.
- Chan KK, Siu E, Krahn MD, et al. Cost-utility analysis of primary prophylaxis versus secondary prophylaxis with granulocyte colony-stimulating factor in elderly patients with diffuse aggressive lymphoma receiving curative-intent chemotherapy. J Clin Oncol. 2012;30(10):1064–1071.
- Marion S, Tzivelekis S, Darden C, et al. Same-day” administration of pegfilgrastim following myelosuppressive chemotherapy: clinical practice and provider rationale. Support Care Cancer. 2016;24(9):3889–3896.
- National Comprehensive Cancer Network. NCCN trends. Results: August 2013, granulocyte-colony stimulating factor. National Comprehensive Cancer Network, Inc.; 2013.
- Tai E, Guy GP, Dunbar A, et al. Cost of cancer-related neutropenia or fever hospitalizations, United States, 2012. J Oncol Pract. 2017;13(6):e552–e561.
- Lyman GH, Dale DC, Legg JC, et al. Assessing patients’ risk of febrile neutropenia: is there a correlation between physician-assessed risk and model-predicted risk? Cancer Med. 2015;4(8):1153–1160.