Abstract
Objective: Comorbidities and comedications are important factors influencing optimal therapy because people are living longer with HIV infection. This study describes the long-term comorbidity profile and treatment burden among people with HIV-1 infection.
Methods: This retrospective study included Medicaid claims data from patients with ≥1 antiretroviral (ARV) claim between 2016 and 2017 (most recent claim defined the index date), ≥1 HIV diagnosis within 1 year before index, age ≥18 years at first HIV diagnosis and <65 years at index, ≥12 months of continuous eligibility before index, and no history of HIV-2 infection. Comorbidities, concomitant medication use, and pill burden were assessed in the 4 years before index. Analyses were stratified by patient age and treatment experience.
Results: Among 3456 patients, the mean (standard deviation [SD]) age was 47.1 (10.4) years; the majority were black (55%) and men (63%). In general, the prevalence of comorbidities increased from the fourth year to the first year before index and included cardiovascular disease (28–40%), hypertension (24–37%), hyperlipidemia (12–17%), and asthma/chronic obstructive pulmonary disease (13–19%). Concomitant medication use corresponding to these comorbidities slightly increased over time. In the year before index, mean (SD) daily pill burden was 2.1 (1.4) for ARVs and 5.9 (5.9) for non-ARVs. Older age and prior treatment experience were associated with higher rates of comorbidities and greater pill burden.
Conclusions: In people with HIV infection, comorbidities and concomitant medication use increased with age, supporting considerations for streamlined ARV regimens highlighted in treatment guidelines.
Introduction
HIV-1 is a retrovirus spread by sexual, percutaneous, intravenous, and perinatal routes and, if left untreated, ultimately causes AIDSCitation1,Citation2, a stage of infection when the immune system is too damaged to fight infectionCitation3. From 2012 to 2017, approximately 39,000 people became infected with HIV each year in the United StatesCitation4. At the end of 2015, an estimated 1.1 million people were living with HIV infection and approximately 16,000 people with HIV/AIDS died in 2016Citation4.
Since the advent of highly active antiretroviral therapy (HAART), the infection rate and number of deaths attributed to HIV/AIDS have dramatically declinedCitation5, thus transforming HIV infection from an acute infection with high mortality to a chronic, manageable disease allowing people with HIV infection to reach their full life expectancy. In 2015, approximately 47% of people living with HIV infection in the United States were aged >50 yearsCitation6. Guidelines recommend that HAART is generally comprised of three antiretrovirals (ARVs) from ≥2 distinct fully active classes that disrupt different components of HIV replicationCitation7. With potentially complex regimens involving multiple drugs per day, a substantial burden is placed on people living with HIV infection who are attempting to adhere to the correct administration of the regimen. People living with HIV infection experience additional, non-HIV-related comorbidities as they become older and require multiple treatments and management strategiesCitation7. Antiretrovirals may also interact with other concomitant medications or may be contraindicated in patients with comorbidities, making treatment with a multiple-drug regimen even more cumbersome. Long-term drug toxicities and effects of HIV infection – such as cardiovascular disease (CVD), kidney and liver dysfunctions, and reduced bone mineral density – have also become important aspects of disease managementCitation8,Citation9. All of these complexities can present challenges for people living with HIV infection to maintain virologic suppression and avoid treatment-emergent resistanceCitation10.
Given this context, it is clear that the profile of the patient population with HIV infection is evolving with respect to complexities in comorbidities, increasing comedication burden, and improved life expectancy. As a result, it is important to better understand this evolution over time of comorbidities and concomitant drug use among people living with HIV infection, because doing so can allow for better targeting of treatments to patients and inform policies or medical practice to effectively address the burden of illness in this population (e.g. streamlining HAART regimens)Citation11. This retrospective study was conducted to assess the comorbidity and treatment burden of patients with HIV infection treated with HAART to build upon the literature on the long-term clinical and demographic profiles of this patient population.
Methods
Study design and patient selection
A retrospective, longitudinal cohort study was conducted utilizing deidentified data from a Medicaid administrative medical and pharmacy claims database, which includes information from Iowa, Kansas, Missouri, and Mississippi. Thus, no approval or consent from an ethics or institutional review board was required.
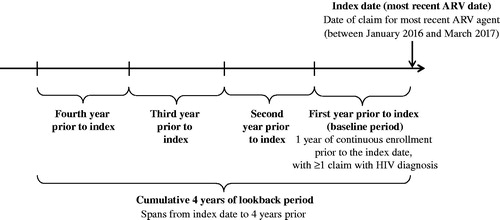
The study period spanned from January 2012 to March 2017. The index date was defined as the date of the most recent pharmacy claim with an ARV (i.e. nucleoside reverse transcriptase inhibitor (NRTI), integrase strand transfer inhibitor (INSTI), non-NRTI (NNRTI), protease inhibitor (PI)) on or after 1 January 2016, and the baseline period was defined as the 1 year prior. The pre-index period was divided into 1-year increments (up to the fourth year before index) and a cumulative 4-year period, during which patients with continuous eligibility during the respective year before index were assessed ().
The study population included patients with: (a) ≥1 medical claim with a diagnosis of HIV infection (International Classification of Diseases, ninth revision, clinical modification (ICD-9-CM): V08, 042xx; International Classification of Diseases, 10th revision, clinical modification (ICD-10-CM): Z21, B20) during the first year before index, (b) age ≥18 years on the date of the first claim with an HIV diagnosis, (c) ≥1 pharmacy claim on or after 1 January 2016, for an ARV after initial HIV diagnosis, (d) ≥1 year of continuous eligibility before the index date, and (e) age ≤65 years on the index date. Patients with a medical claim and a diagnosis of HIV-2 infection at any time (ICD-9-CM: 079.53; ICD-10-CM: B97.35) in the database were excluded from the analysis.
Statistical analyses
Patient demographic characteristics – including core ARV agent, age, year of index date, race, sex, location, number of family members in a household unit, household income level, and Medicare dual eligibility (as available in the data) – were assessed in the year before the index date. Core ARV agent was defined as the core agent class (i.e. INSTI, NNRTI, PI; Supplemental Table 1) that the patient was treated with in the 90 days preceding and including the index date.
Patient clinical characteristics were assessed for ≤4 years before index for each year that the patient met the eligibility criteria. Clinical characteristics included select comorbidities, concomitant and contraindicated medication use, and pill burden. Comorbidities were grouped into the following categories: AIDS-defining illnesses as identified by the Centers for Disease Control and Prevention (e.g. Pneumocystis jirovecii pneumonia, candidiasis, encephalopathy), select adverse events associated with ARVs (e.g. impaired renal function, fractures) and additional comorbidities (e.g. CVD, hypertension; Supplemental Table 2). Concomitant medication use of the following drug classes was assessed: antihypertensive agents, antihyperlipidemic agents, angiotensin-converting enzyme (ACE) inhibitors, calcium channel blockers, beta blockers, antidiabetic agents, antineoplastic agents, contraceptives, and bisphosphonates (Supplemental Table 3). In addition, medications with potential drug–drug interactions with common ARVs were identified, including steroids, therapies for gastroesophageal reflux disease (GERD) or benign prostatic hyperplasia (BPH), lipid-lowering agents, antimycobacterial agents, neurologic agents, cardiac agents, and hepatitis C virus agents. Per-patient mean daily pill burden was calculated as the mean number of pills per day for days covered with the medication of interest during each year prior to index. Days on which no pills were received were excluded from the denominator of the calculation. Results were presented for total pill burden, ARV pill burden, and non-ARV pill burden. As patients were required to have ≥1 year of continuous eligibility prior to index, baseline clinical characteristics and treatment patterns were assessed among all patients during this baseline period.
Descriptive statistics were reported as means and standard deviations (SDs) and medians and interquartile ranges (IQRs) for continuous variables and frequencies and proportions for categorical variables.
Stratifications
Analyses were stratified by age (≥50 vs. <50 years) and treatment experience (treatment-naive vs. treatment-experienced). Patients were defined as being treatment-naive if they did not have any claims for ARVs prior to the 90 days preceding the index date and as treatment-experienced if otherwise (i.e. if they had ARV claims prior to the 90-day period preceding index date). Comparisons between strata were made using the Wilcoxon rank sum test for continuous variables and chi-square test for categorical variables. p Values <.05 were identified as statistically significant. All analyses were carried out using SAS Enterprise Guide 7.1 (Cary, NC).
Results
A total of 3456 patients met the study inclusion criteria, of whom 3059 had 2 years of continuous eligibility prior to index, 2632 had 3 years, and 2281 had 4 years. In stratified analyses, 1559 patients were aged ≥50 years, whereas 1842 were aged <50 years; 3336 were treatment-experienced and 65 were treatment-naive.
Baseline demographics
In the 90 days preceding and including the index date, 40.1% (N = 1385) of patients were most recently treated with an INSTI as their core ARV agent, 26.0% (N = 898) with an NNRTI, 15.9% (N = 551) with a boosted PI, and 16.4% (N = 567) with multiple, overlapping, core-agent ARV classes (). There were 1.6% (N = 55) of patients with claims for NRTI alone and no other classes of ARVs, making NRTI their index core agent. These patients were excluded from the analysis of clinical characteristics due to the likelihood that claims for the core-agent ARV that the patients received were not available (or patients obtained it in another way) or that patients received NRTI alone as pre-exposure prophylaxis as opposed to a complete, multiclass ARV regimen for treatment.
Table 1. Demographic characteristics.
The mean (SD) age of patients was 47.1 (10.4) years. Patients were mostly men (62.8%) and black (55.1%). The mean (SD) number of family members per patient household was 3.1 (2.7), and 60.9% of patients were from a household with an income level between $25,000 and $50,000. Nearly one-third of patients were dually eligible for Medicare (30.1%). These trends were generally consistent for all subgroups defined by age and treatment experience, although patients in the treatment-naive cohort were, on average, younger by approximately 10 years (38.8 ± 11.5 years).
Comorbidities
Common comorbidities included CVD (40.3%), hypertension (36.8%), hyperlipidemia (16.8%), asthma/chronic obstructive pulmonary disease (COPD; 18.4%), depression (20.7%), and anxiety (14.5%) in the first year prior to the index (). The comorbidity prevalence was greater among older patients compared with younger patients (CVD: 51.6% vs. 30.7% (p < .0001), hypertension: 46.6% vs. 28.5% (p < .0001), hyperlipidemia: 22.5% vs. 12.1% (p < .0001), asthma/COPD: 22.8% vs. 14.7% (p < .0001); Supplemental Table 4). Although they were not significantly different, the prevalence of comorbidities tended to be higher among treatment-experienced compared with treatment-naive patients (CVD: 40.5% vs. 29.2% (p = .0666); hypertension: 37.0% vs. 26.2% (p = .0720); hyperlipidemia: 17.0% vs. 9.2% (p = .0987); asthma/COPD: 18.5% vs. 12.3% (p < .2021)). Among all patients, the prevalence of comorbidities generally increased over time, which may explain the subgroup trends observed: 27.9% of patients had CVD in the fourth year prior to index, increasing to 40.3% in the first year prior to index. Similar increases were seen for hypertension (24.3–36.8%), hyperlipidemia (11.5–16.8%), and asthma/COPD (12.7–18.4%).
Table 2. Comorbidities by years prior to index.
Concomitant medications
The most common concomitant drug classes used included antihypertensive agents (27.4%), antihyperlipidemic agents (23.5%), ACE inhibitors (19.8%), beta blockers (16.1%), and calcium channel blockers (14.6%) in the first year prior to index ().
Table 3. Concomitant medications by years prior to index.
Rates of concomitant medication use were higher among older patients than younger patients. For instance, 36.4% of older patients compared with 19.8% of younger patients used an antihypertensive in the first year prior to index (p < .0001). Similar trends were observed for antihyperlipidemic agents (35.0% vs. 13.7%; p < .0001), ACE inhibitors (27.2% vs. 13.6%; p < .0001), calcium channel blockers (19.4% vs. 10.4%; p < .0001), and beta blockers (20.9% vs. 12.1%; p < .0001).
Similarly, treatment-experienced patients had greater rates of concomitant medication use than treatment-naive patients (Supplemental Table 5). Although 27.6% of treatment-experienced patients used an antihypertensive in the first year prior to index, 15.4% of treatment-naive patients used an antihypertensive agent during the same period (p = .0283). Antihyperlipidemic agents (23.7% vs. 12.3%; p = .0321), ACE inhibitors (20.0% vs. 9.2%; p = .0306), calcium channel blockers (14.7% vs. 7.7%; p = .1132), and beta blockers (16.2% vs. 10.8%; p = .2367) showed the same trends.
As observed with comorbidities, in general, concomitant medication use moderately increased over time, thus likely contributing to the differences observed in older versus younger and treatment-experienced versus treatment-naive patients (): 26.6% of patients used an antihypertensive agent in the fourth year prior to index, increasing to 27.4% in the first year prior to index. Similar increases were found for antihyperlipidemic agents (19.6–23.5%), calcium channel blockers (12.5–14.6%), and beta blockers (13.2–16.1%). However, use of ACE inhibitors appeared to decline among this group of patients over time (21.3–19.8%).
Many patients used medications commonly with potential drug–drug interactions with ARVs, such as steroids and therapies for GERD and BPH. As observed for concomitant medications, contraindicated medication use increased over time. Approximately 16.2% of patients used steroids in the fourth year prior to index, a percentage that increased to 18.2% by the first year prior to index. Increases were also observed for therapies used for GERD (14.4–18.1%) and BPH (4.0–6.6%).
Pill burden
In the first year prior to index, mean (SD) of average daily pill burden was 2.1 (1.4) for ARVs and 5.9 (5.9) for non-ARVs for the total study population (). In the first year prior to index, treatment-experienced patients had higher daily pill burdens (2.1 (1.4) for ARVs and 6.0 (5.9) for non-ARVs) than treatment-naive patients (1.8 (1.2) for ARVs (p = .0394) and 4.0 (3.2) for non-ARVs (p < .0001)), and older patients had higher daily pill burdens (2.3 (1.5) for ARVs and 6.9 (6.5) for non-ARVs) than younger patients (2.0 (1.3) for ARVs (p < .0001) and 5.0 (5.2) for non-ARVs (p < .0001); Supplemental Table 6). Patient total daily pill burdens decreased from the fourth year prior to index to the first year prior to the index (mean of 2.4–2.1 for ARVs and 6.5–5.9 for non-ARVs), possibly because of the greater number of treatment options with coformulations (including single-tablet regimens) in recent years.
Table 4. Pill burden by years prior to index.
Discussion
This study found that people living with HIV infection experience more comorbidities (similar to the population not infected with HIV, as previously shownCitation12) and use more medications as they age. Rates of common comorbidities (e.g. CVD, hypertension, hyperlipidemia, asthma/COPD) and medication use (e.g. antihypertensives, antihyperlipidemic agents, ACE inhibitors) were persistently high in the overall population and were higher in subgroups of older and treatment-experienced patients (compared with younger and treatment-naive patients, respectively).
The medical literature indicates that people with HIV infection, especially the elderly, experience a high comorbidity burden. Compared with the prevalence of diseases in the general adult US population, as reported by the Centers for Disease Control and Prevention, patients in the current study had a higher prevalence of CVD (40.3% vs. 10.7%), hypertension (36.8% vs. 24.9%), and diabetes (12.3% vs. 11.9%)Citation13,Citation14. Other retrospective studies have shown that these diseases, as well as decreased renal function and bone fractures, are also common in people with HIV infectionCitation15,Citation16. Thus, the current study supports those previous findings and adds to the literature on the long-term profile of this patient population with specific measures of notable comorbidity burden, demonstrating need for safe treatments with favorable toxicity profiles that will not exacerbate their current comorbidities or increase this patient population’s need for additional medications.
In addition, this study demonstrated that older patients and treatment-experienced patients were more likely than younger patients and treatment-naive patients, respectively, to experience comorbidities. A retrospective case-control study of patients with HIV infection who were treated in Italy also found that the comorbidity burden was higher among older patients with HIV infection compared with younger patientsCitation16. In this study, rates of comorbidities assessed by year before the index date generally increased over time. In addition to the trends of greater comorbidity burden and concomitant medication use as patients age, some studies have shown an association between the development of certain comorbidities and the long-term use of specific ARVs. For example, researchers have described associations between renal impairment and the prolonged use of tenofovir disoproxil fumarate and certain PIsCitation17. Aging and prolonged exposure to treatment for HIV infection exacerbates the clinical and pharmacologic complexity of these patients, suggesting the need for tolerable treatments with minimal side effects. Furthermore, although younger and treatment-naive patients typically have a lower burden of comorbidities and less concomitant medication use than their older counterparts, this burden is still substantial, as evidenced by the fact that 29% and 31% younger patients had CVD and hypertension (Supplemental Table 4), respectively, and a mean non-ARV daily pill burden of 5.0 (Supplemental Table 6). Hence, younger patients may also benefit from treatment with less-toxic regimens that have fewer drug–drug interactions early on.
In this study, large proportions of patients used concomitant medications and faced substantial daily pill burdens (two pills per day for ARV regimens and six pills per day for non-ARV regimens), consistent with findings from other studies. Zhou et al.Citation18 estimated that each person living with HIV infection received a median number of eight medications and reported that antihypertensive agents were among the most common classes of medications prescribed to this group. Another descriptive, retrospective study of patients aged 50–64 years found that long-term polypharmacy (taking ≥5 non-ARV medications) was more common among men with HIV infection compared with men in the general populationCitation19. The current study adds contemporary, supportive evidence to the literature triangulating these previous findings. Therefore, patients with HIV infection are burdened with managing more extensive medication regimens, which can impact treatment adherence and patient outcomes.
An increase in concomitant medication use in addition to ARV use not only creates a more complicated treatment regimen but also increases the risks of drug–drug interactions. The present study showed that the use of medications frequently contraindicated with certain ARVs was high in the overall study sample. Other researchers have also found high proportions of contraindicated medication use, including a study that reported that 54% of people with HIV infection and taking contraindicated medications experienced an interaction involving their ARV therapyCitation20.
Ultimately, the unmet need for ARVs with minimal toxicities and for simplified ART regimens has driven researchers to consider alternatives such as two-drug regimens. Benefits of two-drug regimens include exposing people to one or two fewer ARV medications, minimizing toxicities, and decreasing risk for drug–drug interactionsCitation21. Furthermore, two-drug regimens are being considered in people with stable disease, including in the approved combination of dolutegravir plus rilpivirineCitation22 and in treatment-naive people with HIV infectionCitation21. These dual therapies could potentially minimize toxicities associated with prolonged drug exposure, reduce the risk of drug–drug interaction for patients who take contraindicated or concomitant medications for diseases associated with aging (e.g. hypertension, hyperlipidemia, diabetes), and reduce pill burden, possibly resulting in improved rates of adherence. Indeed, findings from this study support the need for these streamlined regimens, even in the current HIV landscape of patients who live with HIV infection as a chronic condition.
The current study had several limitations. First, because it considered only a continuous timeframe of eligibility when assessing lookback periods, the sample size decreased with each incremental period before the index date. Although the study was designed to comprehensively capture patient medical history over 4 years, there was only information from all 4 years for approximately two-thirds of the study sample. However, the study was novel because it analyzed a longitudinal cohort to examine trends over time on a year-to-year basis to show how the profile of patients living with HIV infection may change, rather than as a cross-sectional analysis focused on a single point in time. Thus, the study demonstrated that the comorbidity burden of patients living with HIV infection becomes increasingly complex over time. Second, patients on Medicaid are not representative of the general population because they tend to have lower levels of education, lower income, and higher rates of disability. However, Medicaid is the largest single source of insurance coverage for American patients with HIV infection, making it a more appropriate data source for this study to ensure better generalizability and contextualization of results to the population of US patients living with HIV infection than other commercial payer databasesCitation23. Longitudinal data for patients on Medicaid were available for longer periods, allowing for the assessment of patient comorbidities and medication use over time. Although people aged ≥65 years were excluded from the analysis, approximately 30% were still dually eligible for Medicare and Medicaid; therefore, it is possible that some data may be missing if patients used Medicare coverage rather than Medicaid to file insurance claims. However, this proportion of dually eligible patients is consistent with Kaiser Family Foundation statistics on dual eligible patients both with HIV infection and those in the general populationCitation24. Third, comorbidities were identified using ICD-9-CM/ICD-10-CM diagnosis codes, and it is possible that not all diagnoses captured in the Medicaid claims data were analyzed. Similarly, pharmacy claims data reflected drugs being dispensed, not drugs actually administered. For instance, although patients may have had claims for concomitant medications and ARVs, they may not have taken them as prescribed (or at all). Lastly, the analyses were generally exploratory and descriptive in nature rather than focused on drawing comparative conclusions. Future research may consider testing hypotheses with statistical comparisons and adjusting for potential confounders (e.g. age) that could impact patient outcomes.
Conclusions
This study showed that people currently living with HIV infection may experience substantial comorbidity burden and have a greater need for medications that in turn increases with age. Frequencies of comorbidities and concomitant medication use generally increased over the study period for all individuals, with older and treatment-experienced people facing the greatest burden. Consequently, as people with HIV infection continue to live longer, there may be increasing interest in simplified and streamlined ARV regimens with minimal side effects to avoid increasing patients’ comorbidities and medication use, particularly contraindicated medications. Treatment guidelines suggest that streamlined ARV regimens may be considered as patient complexity evolves over time.
Transparency
Declaration of funding
Support for research and writing was funded by ViiV Healthcare. Analysis Group, Inc., received research funding from ViiV Healthcare for the conduct of the study.
Declaration of financial/other relationships
MDS, RHB, EG, MM, CS, and MSD are employees of Analysis Group, Inc. AO and JP are employees of ViiV Healthcare and own stock in GlaxoSmithKline. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (34.7 KB)Acknowledgements
Editorial assistance was provided under the direction of the authors by Jeffrey Stumpf and Sherri Damlo of MedThink SciCom and funded by ViiV Healthcare.
Data availability statement
Study documents can be requested for further research from www.clinicalstudydatarequest.com.
References
- Cohen MS, Shaw GM, McMichael AJ, et al. Acute HIV-1 infection. N Engl J Med. 2011;364(20):1943–1954.
- Hladik F, McElrath MJ. Setting the stage: host invasion by HIV. Nat Rev Immunol. 2008;8(6):447–457.
- Lackner AA, Lederman MM, Rodriguez B. HIV pathogenesis: the host. Cold Spring Harb Perspect Med. 2012;2(9):a007005.
- HIV/AIDS basic statistics [Internet]. Atlanta (GA): Centers for Disease Control and Prevention; 2018; [updated 2018 Nov 19; cited 2018 Dec 11]. Available from: https://www.cdc.gov/hiv/basics/statistics.html
- Palella FJ Jr, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr. 2006;43(1):27–34.
- HIV among people aged 50 and older [Internet]. Atlanta (GA): Centers for Disease Control and Prevention; 2018; [updated 2018 Sep 18; cited 2018 Dec 11]. Available from: https://www.cdc.gov/hiv/group/age/olderamericans/index.html
- Clinical guidelines [Internet]. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Rockville (MD): U.S. Department of Health and Human Services; 2018; [updated 2018 Oct 25; cited 2018 Dec 11]:[332 p.]. Available from: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf
- Bedimo R, Maalouf NM, Zhang S, et al. Osteoporotic fracture risk associated with cumulative exposure to tenofovir and other antiretroviral agents. AIDS. 2012;26(7):825–831.
- Margolis AM, Heverling H, Pham PA, et al. A review of the toxicity of HIV medications. J Med Toxicol. 2014;10(1):26–39.
- Al-Dakkak I, Patel S, McCann E, et al. The impact of specific HIV treatment-related adverse events on adherence to antiretroviral therapy: a systematic review and meta-analysis. AIDS Care. 2013;25(4):400–414.
- Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection [Internet]. Clinical guidelines: managing common coinfections and comorbidities. Geneva, Switzerland: World Health Organization; 2016; [cited 2019 Nov 7]:[46 p.]. Available from: https://www.who.int/hiv/pub/arv/chapter5.pdf?ua=1
- Salter ML, Lau B, Go VF, et al. HIV infection, immune suppression, and uncontrolled viremia are associated with increased multimorbidity among aging injection drug users. Clin Infect Dis. 2011;53(12):1256–1264.
- Summary Health Statistics [Internet]. National Health Interview Survey; 2016. Atlanta (GA): Centers for Diseases Control and Prevention; 2018; [cited 2018 Dec 11]:[9 p.]. Available from: https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2016_SHS_Table_A-1.pdf
- Health, United States, 2017. Data finder [Internet]. Selected health conditions and risk factors, by age: United States, selected years 1988–1994 through 2015–2016; 2017. Atlanta (GA): Centers for Disease Control and Prevention; 2018; [cited 2018 Dec 11]:[2 p.]. Available from: https://www.cdc.gov/nchs/data/hus/2017/053.pdf
- Gallant J, Hsue PY, Shreay S, et al. Comorbidities among US patients with prevalent HIV infection-a trend analysis. J Infect Dis. 2017;216(12):1525–1533.
- Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clin Infect Dis. 2011;53(11):1120–1126.
- Ryom L, Mocroft A, Kirk O, et al. Association between antiretroviral exposure and renal impairment among HIV-positive persons with normal baseline renal function: the D:A:D study. J Infect Dis. 2013;207(9):1359–1369.
- Zhou S, Martin K, Corbett A, et al. Total daily pill burden in HIV-infected patients in the southern United States. AIDS Patient Care STDS. 2014;28(6):311–317.
- Gimeno-Gracia M, Crusells-Canales MJ, Armesto-Gomez FJ, et al. Polypharmacy in older adults with human immunodeficiency virus infection compared with the general population. Clin Interv Aging. 2016;11:1149–1157.
- Jakeman B, Nasiri M, Ruth L, et al. Comparing the frequencies of contraindicated drug–drug interactions between differing antiretroviral regimens in HIV-infected patients. Ann Pharmacother. 2017;51(5):365–372.
- Corado KC, Caplan MR, Daar ES. Two-drug regimens for treatment of naive HIV-1 infection and as maintenance therapy. Drug Des Devel Ther. 2018;12:3731–3740.
- Juluca [package insert]. Research Triangle Park (NC): ViiV Healthcare; 2017.
- Medicaid and HIV [Internet]. Medicaid and HIV fact sheet. San Francisco (CA): Kaiser Family Foundation; 2018; [updated 2016 Oct 24; cited 2018 Dec 11]:[6 p.]. Available from: http://files.kff.org/attachment/Fact-Sheet-Medicaid-and-HIV
- State health facts: Medicare [Internet]. San Francisco (CA): Kaiser Family Foundation; 2018. Dual eligibles’ share of Medicaid spending; 2013 FY [cited 2018 Dec 11]; [about 2 screens]. Available from: https://www.kff.org/medicaid/state-indicator/duals-share-of-medicaid-spending/?currentTimeframe=0&sortModel=%7B%22colId%22:%22Location%22,%22sort%22:%22asc%22%7D