Abstract
Objective: Varenicline, a selective partial agonist of the α4β2 nicotinic acetylcholine receptor, is a smoking cessation pharmacotherapy that more than doubles the chance of quitting smoking at 6 months compared with placebo. This article reviews salient knowledge of the discovery, pharmacological characteristics, and the efficacy and safety of varenicline in general and in specific populations of smokers and provides recommendations to support use in clinical practice.
Methods: Literature searches for varenicline were conducted using PubMed, with date limitations of 2000–2018 inclusive, using search terms covering the discovery, mechanism of action, pharmacokinetics, efficacy and safety in different populations of smokers, alternative quit approaches and combination therapy. Selection of safety and efficacy data was limited to clinical trials, meta-analyses and observational studies.
Results: Standard administration of varenicline is efficacious in helping smokers to quit, including smokers with cardiovascular disease and chronic obstructive pulmonary disease. Furthermore, varenicline efficacy may be improved with pre-loading, a gradual quitting approach for smokers unwilling or unable to quit abruptly, and extended treatment in smokers who have recently quit to help maintain abstinence. Initial concerns regarding the association of varenicline with increased risk of neuropsychiatric and cardiovascular adverse events have been disproven after extensive clinical evaluations, and the benefit-risk profile of varenicline is considered favorable.
Conclusions: Varenicline is efficacious and safe for all adult smokers with a range of clinical characteristics. Evidence suggests that approaches offering greater flexibility in timing and duration of treatment may further extend treatment efficacy and clinical reach.
Introduction
Cigarette smoking takes the life of every other smoker prematurely, and causes preventable illness in most organ systemsCitation1. Given the dire consequences of tobacco use, helping smokers to quit belongs at the fore of preventive and curative medicine, yet the development of treatment options for tobacco addiction appears slower compared with other major modifiable risk factors, for example hyperlipidemia and hypertension. For tobacco addiction treatment to achieve clinical priority requires that health care professionals recognize effective therapies to be safe and well tolerated, and reimbursable in some areas of the world.
Early attempts to treat nicotine dependence with pharmacotherapy involved anxiolytics and tranquilizersCitation2, but with growing understanding of the neurobiology of tobacco addiction, a small number of better targeted pharmacotherapeutic aids emerged. Most notable was the development of nicotine replacement therapy (NRT) in the late 1970sCitation3,Citation4. This was followed by the development of bupropion, initially indicated as an anti-depressant, and first suggested as a smoking cessation aid in 1994Citation5. During the following decade and a half, both NRT and bupropion underwent post-approval scrutiny, particularly with regard to cardiovascular (CV) safety. In the meantime, smokers went untreated, despite the proven efficacy and safety of both treatmentsCitation6,Citation7. In the early 1990s, pharmaceutical industry scientists had begun development of novel, non-nicotine drugs designed to alleviate withdrawal symptoms while blocking the rewards of smokingCitation8–10. This ultimately led to the regulatory approval of varenicline, which was demonstrated in pre-approval pivotal trials to have superior efficacy compared with both placebo and bupropion, and an acceptable safety/tolerability profileCitation11,Citation12. Other attempts to develop smoking cessation pharmacotherapies, such as rimonabant (a selective cannabinoid antagonist)Citation13 and nicotine vaccinesCitation14 have been unsuccessful and no new medications for smoking cessation have been approved since varenicline.
The objective of this monograph is to summarize the current state of knowledge about varenicline as a smoking cessation therapy, including its discovery, pharmacological characteristics, efficacy and safety, and to discuss its clinical use through various administration paradigms and alternative treatment options that may increase efficacy and clinical reach.
Methods
For this narrative review, a literature search was conducted using PubMed, with date limitations of 2000–2018 inclusive. The primary search term, “varenicline”, returned 1564 results. Additional sub-searches were conducted using the following search terms: varenicline and discovery, or mechanism, pharmacology, α4β2 nicotinic acetylcholine receptors (nAChR), pharmacokinetics, absorption, metabolism, distribution, excretion, renal impairment, hepatic impairment, interactions; varenicline and efficacy; varenicline and efficacy and COPD, or cardiovascular disease, neuropsychiatric, psychiatric, combination; varenicline and high dose, or flexible quit, smoking reduction, retreatment, predictors, maintenance; varenicline and safety; varenicline and safety and neuropsychiatric, or cardiovascular disease. Articles considered by the Authors to be relevant to the objectives of this monograph i.e. which demonstrate important pharmacological characteristics, or that inform evidence-based recommendations for practitioners when prescribing varenicline, were selected for inclusion. For the latter objective, selection of safety and efficacy data was limited to clinical trials, meta-analyses and observational studies.
Results
Discovery, and mechanism of action
Discovery rationale
The smoking cessation drug discovery program initiated by Pfizer was based on the rationale that α4β2 nAChRs mediate the addictive properties of nicotine, and that because of a dual agonist-antagonist action, an α4β2 nAChR partial agonist might improve abstinence rates compared to NRT, with less abuse liability than the full agonist, nicotineCitation10. The theory was that in the absence of nicotine during a quit attempt, the agonist activity of a partial agonist would to some extent replace nicotine and reduce craving and other withdrawal symptoms. During a relapse, the antagonist activity would blunt nicotine reinforcement by competing with inhaled nicotine for the same α4β2 nAChR binding sites. Varenicline, synthesized in 1997, was derived from a bicyclic benzazepine that was found to be a potent α4β2 nAChR antagonist, after initial searches for drug candidates based on cytisine, a natural product and α4β2 nAChR partial agonist, were unsuccessfulCitation10.
Pharmacology
Varenicline was selected for development, in part because it demonstrated high affinity for α4β2 nAChRs. It was later shown to be a high affinity partial agonist at α6β2-containing (α6β2*) nAChRs, which also play a key role in nicotine dependenceCitation15. Varenicline has a preclinical in vivo profile consistent with that of an α4β2/α6β2* partial agonist. It stimulates basal mesolimbic dopamine release to approximately 50% of the maximal effect of nicotine, inhibits nicotine-induced dopamine release, and reduces nicotine self-administrationCitation10,Citation16.
A dual agonist-antagonist mechanism consists of two separate pharmacological activities (). The agonist activity is the net result of receptor desensitization and activationCitation17. Nicotine from smoking causes extensive nAChR desensitization with minimal activation, and if the activity of another nAChR agonist effectively replaces that of nicotine from smoking, it can reduce craving during a quit attempt. Antagonist activity is due to the competition of a nAChR agonist with inhaled nicotine for the nAChR binding sites, resulting in a decrease in nicotine receptor occupancy of α4β2 and α6β2* nAChRs, which will attenuate reinforcement during a relapse. In vivo activity is dependent on the dual agonist’s binding affinity and functional potency at unbound concentrations in the human brain after clinical doses.
Figure 1. Concentration-dependent functional effects of a partial nAChR agonist. Short exposures to high concentrations (μM) of a partial agonist cause activation of nAChRs (dotted line). A partial agonist like varenicline causes only partial activation (here 20%) versus the full agonist ACh that causes maximal nAChR activation (100%). Prolonged exposures to low partial agonist concentrations (nM) causes receptor desensitization, shown by the decrease in the ACh response in the presence of increasing concentrations of the partial agonist (solid line). In the sustained presence of low agonist concentrations present in human brain (nM range, gray bar), only a small fraction of the nAChRs that are not desensitized can be activated, resulting in extensive nAChR desensitization and low-level activation at steady state. Abbreviations. Ach, acetylcholine; nAChRs, nicotinic acetylcholine receptors.
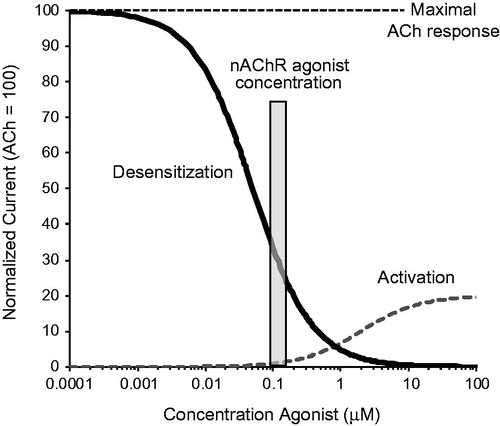
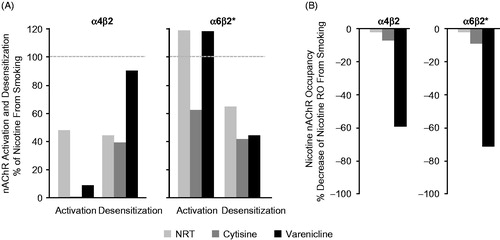
A comparison of agonist and antagonist activities after clinical doses of α4β2 nAChR agonists with those of smoked nicotineCitation17, showed that agonists cause α4β2/α6β2* desensitization and α6β2* activation and can, to some extent, replace the effects of nicotine as shown for NRT, varenicline and cytisine in . However, most α4β2 nAChR agonists lack pharmacologically relevant antagonist activity, with the exception of varenicline (), which because of its high binding affinity and brain exposure, competes with inhaled nicotine for α4β2 and α6β2* binding sites. The predicted agonist-antagonist activities of varenicline are consistent with efficacy data from the pivotal clinical trialsCitation11,Citation12 and later clinical studies showing that varenicline not only reduces craving in a concentration-dependent mannerCitation18–20, but also attenuates the rewarding effects from smokingCitation18,Citation21. The potent dual agonist-antagonist activity at α4β2 and α6β2* nAChRs is considered the key mechanism that underlies the clinical efficacy of varenicline.
Figure 2. Comparison of agonist and antagonist activities of NRT, cytisine and varenicline. (A) The degree of activation and desensitization (agonist activity) of α4β2 and α6β2* nAChRs by clinical doses of each agonist relative to the effects of nicotine from smoking (= 100%). (B) The decrease in nicotine receptor occupancy of α4β2 and α6β2* nAChRs (antagonist activity) by clinical doses of each agonist relative to the decrease in nicotine receptor occupancy when smoking without nAChR agonist treatment (= 0%). Bars represent the magnitude of activities expressed as a percentage of the corresponding effects of nicotine from smoking. Smoking activation and desensitization = 100%, decrease in nicotine receptor occupancy = 0%, indicated by dotted lines. Abbreviations. nAChRs, nicotinic acetylcholine receptors; NRT, nicotine replacement therapy; RO, receptor occupancy.
Pharmacokinetics
Absorption, distribution, metabolism and excretion
Following oral administration, varenicline is almost completely absorbed and has high bioavailability (90%)Citation22. Time to maximum plasma concentration is 3–4 h after single dose oral administrationCitation23 and the plasma elimination half-life is approximately 24 hCitation22,Citation24,Citation25. At the recommended therapeutic dose of 1 mg twice daily (BID), varenicline steady-state plasma concentrations are reached on day 4 of continuous administration, and range from 6.0 to 9.2 ng/mL (30–43 nM) in adult smokersCitation25. The plasma protein binding is ≤20%, and average apparent volume of distribution at steady state is 415 liters, suggesting a large distribution to all tissues, including the brainCitation25. The majority of varenicline (92%) undergoes minimal metabolism in the body and is excreted unchanged in the urineCitation25. In addition, varenicline neither inhibits nor induces the activity of cytochrome P450 enzymes, which reduces the likelihood of clinically meaningful pharmacokinetic drug-drug interactions.
Effects of renal and hepatic impairment
Pharmacokinetic parameters following varenicline administration are unchanged in subjects with mild renal impairment (creatinine clearance [CrCl] 50–80 mL/min)Citation25,Citation26. However, in subjects with moderate (30 ≥CrCl ≤50 mL/min) or severe (CrCl <30 mL/min) renal impairment, systemic exposure increases 1.5- and 2.1-fold, respectively, compared with individuals without renal impairment. Consequently, a starting dose of 0.5 mg once daily (QD) and a maximum dose of 0.5 mg BID, is indicated for smokers with severe renal impairmentCitation27.
Although no studies have been conducted in subjects with hepatic insufficiency, hepatic metabolism of varenicline is minimal and therefore pharmacokinetic parameters should be unaffectedCitation27.
Efficacy
Efficacy in the general population of smokers
Randomized controlled trials
The efficacy of varenicline compared with placebo has been demonstrated in numerous randomized controlled trials (RCTs) that enrolled generally healthy adult smokers who were motivated to quitCitation11,Citation12,Citation28–31 (). The standard treatment paradigm was varenicline 1 mg BID for 12 weeks (starting with a 1-week up-titration), with a target quit date (TQD) set at 1 week after the start of treatment. The trials also included non-treatment follow-up periods of 12 or 40 weeks. Brief, but frequent behavioral counseling was also provided to all participants throughout the studies. Cessation was assessed as carbon monoxide (CO)-confirmed continuous abstinence rates (CARs) for the last 4 weeks of treatment (CAR9–12) and longer-term CARs including for weeks 9–24 (CAR9–24) and/or weeks 9–52 (CAR9–52). The trials ranged in size and some included an active comparator group as described below. Some trials were conducted in specific geographic regions, most notably Asia. In all trials, CAR9–12 were significantly higher for varenicline versus placebo (). Similar findings were observed at later time points, although CARs were generally lower than at end of treatment for both varenicline- and placebo-treated participants (Supplementary Table 1).
Figure 3. Comparison of continuous abstinence rates in randomized trials that were included in the clinical program: 12 weeks’ varenicline treatment for smoking cessation at weeks 9‒12. Pfizer-sponsored studies by (A) General populations, (B) Comorbid populations or (C) Alternative treatment paradigms. *Varenicline 1 mg BID vs. placebo or active treatment comparator: difference significant at week 12; N, intention-to-treat population (varenicline plus placebo or active treatment comparator). ORs (95% CI) are varenicline vs. placebo or active treatment comparator. Abbreviations. BID, twice daily; CI, confidence interval; COPD, chronic obstructive pulmonary disease; CVD, cardiovascular disease; NPS, neuropsychiatric; NRT, nicotine replacement therapy; OR, odds ratio.
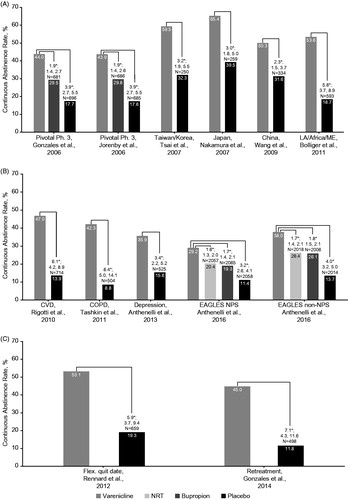
The pivotal studies included bupropion as an active comparator. In a pooled analysis of these two studies (N = 1025Citation11; N = 1027Citation12), varenicline demonstrated superior CAR9–12 compared with bupropion (44.0% vs. 29.7%, odds ratio [OR], 1.86; 95% confidence interval [CI]: 1.49, 2.33) or placebo (44.0% vs. 17.7%, OR, 3.66; 95% CI: 2.86, 4.68). Similar findings were reported for CAR9–24 (varenicline, 29.6% vs. bupropion, 20.4%, OR, 1.54; 95% CI: 1.28, 2.10; varenicline, 29.6% vs. placebo, 11.8%, OR, 3.14; 95% CI: 2.36, 4.16) and CAR9–52 (varenicline, 22.4% vs. bupropion, 15.4%, OR, 1.59; 95% CI: 1.21, 2.10; varenicline, 22.4% vs. placebo, 9.3%, OR, 2.80; 95% CI: 2.05, 3.83)Citation32. These results were confirmed in the large-scale Evaluating Adverse Events in a Global Smoking Cessation Study (EAGLES)Citation33 which directly compared varenicline and bupropion with an active (NRT patch) and placebo control in two cohorts of smokers, one with, and one without, a history of psychiatric disorders. In the non-psychiatric cohort (N = 4028), which is similar to the general population of smokers, varenicline demonstrated superior efficacy to bupropion, NRT patch and placebo (; Supplementary Table 1).
Meta-analyses of randomized controlled trials
The efficacy of varenicline has been assessed in a number of large-scale, retrospective, meta-analysesCitation17,Citation34–36 conducted using end-of-treatment and long-term abstinence data.
Consistent with individual trial data presented in , a recent meta-analysis of placebo-controlled RCTs with varenicline that reported end-of-treatment CARs (34 trials; ∼15,000 participants), demonstrated that varenicline significantly increased the odds of achieving abstinence compared with placebo (OR, 3.52; 95% CI: 3.27, 3.70)Citation17. Further, a 2016 Cochrane meta-analysis of nicotine receptor partial agonists for smoking cessation found that varenicline increased the chances of successful long-term (≥6 months) abstinence compared with placebo (pooled risk ratio [RR] 2.24; 95% CI: 2.06, 2.43), based on 27 trials (including EAGLES) totaling 12,625 participantsCitation36 (Supplementary Figure 1). In addition, more participants quit successfully with varenicline than with bupropion (RR, 1.39; 95% CI: 1.25, 1.54; based on five trials [5877 participants]) or NRT (RR, 1.25; 95% CI: 1.14, 1.37; based on eight trials [6264 participants])Citation36. The number needed to treat (NNT) for varenicline, all types of NRT and bupropion was 11 (95% CI: 9, 13), 23 (95% CI: 20, 25) and 22 (95% CI: 18, 28), respectively. In an earlier network meta-analysis of pharmacological interventions for smoking cessation based on previous Cochrane reviews including direct and indirect comparisonsCitation35, varenicline increased the odds of quitting compared with placebo, and was superior to NRT (overall and to individual forms: patch, gum, inhaler, spray, tablets and lozenges), and to bupropion, but was not more effective than combination NRT treatment. In addition, results of a systematic review and multiple treatment meta-analysis based on 146 studies (not including EAGLES) comparing the abstinence rates of high-dose and combination NRT, with standard-dose NRT patch, varenicline and bupropion, found that only varenicline demonstrated efficacy versus other treatment options and sustained long-term efficacy over standard dose NRTCitation34. This review also included analyses of quit rates at end of treatment (3 months). Varenicline resulted in significantly higher quit rates compared with placebo (9 RCTs, 4072 participants; RR, 2.26; 95% CI: 1.69, 3.02), bupropion (3 RCTs, 1870 participants; RR, 1.41; 95% CI: 1.13, 1.77), and standard dose NRT (1 RCT, 746 participants; RR, 1.29; 95% CI: 1.11, 1.49), but not high dose NRT (1 RCT, 32 participants; RR, 0.92; 95% CI: 0.60, 1.38).
Efficacy in smokers with comorbidities
Smokers with chronic obstructive pulmonary disease (COPD)
The efficacy of varenicline was evaluated in subjects with mild to moderate COPD in a double-blind, placebo-controlled RCT of 504 smokers. CAR9–12 were significantly higher with varenicline (42.3%) versus placebo (8.8%) (OR, 8.40; 95% CI: 4.99, 14.14), as were CAR9–52 (18.6% vs. 5.6%; OR, 4.04; 95% CI: 2.13, 7.67)Citation37.
The efficacy of smoking cessation pharmacotherapies including varenicline in subjects with severe/very severe COPD was evaluated in a retrospective study of 472 smokers who received behavioral counselling with pharmacotherapyCitation38. CO-confirmed CAR9–24 were significantly higher for varenicline (12- or 24-week treatment) versus NRT patch (high or standard dose) (61.0% vs. 44.1%; OR, 1.98; 95% CI: 1.25, 3.12), but not for varenicline (12- or 24-week treatment) versus bupropion (61.0% vs. 60.0%; OR, 1.43; 95% CI: 0.49, 2.2).
Smokers with cardiovascular disease (CVD)
The efficacy of varenicline in smokers with stable CVD was evaluated in a 12-week, double-blind, placebo-controlled RCTCitation39. CAR9–12 were higher for varenicline compared with placebo (47.0% vs. 13.9%; OR, 6.11; 95% CI: 4.18, 8.93) as were CAR9–52 (19.2% vs. 7.2%; OR, 3.14; 95% CI: 1.93, 5.11). Varenicline was also evaluated in a double-blind, placebo-controlled RCT of 302 smokers hospitalized for acute coronary syndrome. In this study, treatment began while participants were hospitalized and continued after discharge. Varenicline showed higher CARs versus placebo at 24 weeks (35.8% vs. 25.8%)Citation40 and 52 weeks (31.1% vs. 21.2%)Citation41.
Smokers with psychiatric disorders history
Evaluation of the efficacy of varenicline in smokers with psychiatric illnesses, a population disproportionately affected by the tobacco epidemicCitation42, has been complicated by concerns over its safety (see safety section below). To address concerns, regulatory authorities required Pfizer and GlaxoSmithKline (the manufacturers of varenicline and bupropion, respectively) to conduct a large safety and efficacy study (EAGLES) in smokers with and without a history of psychiatric disorders.
The EAGLES psychiatric cohort (N = 4116) included smokers who met DSM-IV-TR criteria for mood (N = 2882), anxiety (N = 782), psychotic (N = 386) and borderline personality (N = 24) disordersCitation33. CAR9–12 were significantly higher for varenicline compared with bupropion (). Varenicline maintained significantly higher CARs compared with all active treatments and placebo through follow-up (weeks 9–24) (Supplementary Table 1)Citation33. CARs in the psychiatric history cohort were lower than those observed in the non-psychiatric cohort across all treatment arms, including placebo. These results confirm the findings of earlier trials, including two small RCTs in smokers with schizophrenia or schizoaffective disorder (N = 9Citation43 and N = 128Citation44) and a trial in smokers with stable major depressive disorder (N = 525)Citation45, all of which showed higher abstinence rates with varenicline versus placebo. In a meta-analysis of the two studies in smokers with schizophrenia/schizoaffective disorder, abstinence rates at the end of treatment (week 12) were higher for varenicline versus placebo (OR 1.81; 95% CI: 0.41, 3.20)Citation46.
Alternative treatment paradigms: extending use, increasing the dose, varenicline pre-loading and flexible quit date/reduce-to-quit approach
While standard dose varenicline has demonstrated superior efficacy versus placebo, NRT and bupropion, overall abstinence rates are less than 50% at the end of treatment and tend to decline over time as quitters relapse to smoking. Several studies have examined whether abstinence rates can be further improved via modifications to the standard varenicline dosing regimen. In addition, alternative approaches for smokers unwilling or unable to quit immediately have been evaluated. Key studies are summarized in and discussed below.
Table 1. Extended use and other alternative treatment paradigms.
Extending varenicline use (maintenance of abstinence)
The extended use of varenicline to maintain abstinence and reduce relapse was evaluated in a trial (N = 1210) in which smokers who achieved abstinence at the end of 12 weeks of open-label varenicline treatment were randomized to receive an additional 12 weeks of varenicline or placeboCitation47. Sustained abstinence rates between 3 months and 1 year were 43.6% (varenicline) versus 36.9% (placebo) (OR, 1.34; 95% CI: 1.06, 1.69). The benefit of extended treatment was most notable among participants who failed to quit smoking by the initial TQDCitation48.
In a similarly designed study of smokers with bipolar disorder or schizophrenia, participants who achieved ≥2 weeks of continuous abstinence after 12 weeks’ varenicline treatment and cognitive behavioral therapy (CBT) were randomized to receive additional treatment (varenicline plus CBT, or placebo plus CBT) for up to 52 weeks with follow-up through week 76. CAR12‒76 were higher for varenicline versus placebo (30.0% vs. 11.0%; OR, 3.4; 95% CI: 1.02, 13.6)Citation49.
Treatment extension beyond the 12 weeks’ regulatory approved treatment periodCitation27 should therefore be offered to smokers who have successfully stopped smoking at the end of 12 weeks, but who quit only later in their cessation attempt or still feel vulnerable to temptations to smoke, to further increase the likelihood of long-term abstinence.
Varenicline pre-loading
Hajek et al.Citation50 hypothesized that if varenicline reduced the rewarding properties associated with cigarette smoking, ‘pre-loading’ the drug prior to TQD could help weaken this association and enhance efficacy. Participants (N = 101) received either varenicline for 4 weeks prior to the TQD, or placebo for 3 weeks followed by varenicline for 1 week. Both groups then received varenicline for 12 weeks. The effect on early quit rates (CARs at week 4) did not reach statistical significance (varenicline, 49.1% vs. placebo, 33.3%), but there was a statistically significant improvement in self-reported abstinence at 12 weeks (47.2% vs. 20.8%). In a second similarly designed study (N = 60), CAR9–12 were higher following 4 weeks versus 1 week of varenicline pre-load, but the differences were not statistically significant (53.0% vs. 40.0%), except among women (67.0% vs. 35.0%)Citation51.
Results of these preliminary trials need further confimation before extended pre-quit becomes a recommended approach.
Flexible quit date
A further variation to the standard treatment paradigm included in the varenicline prescribing information affords smokers the option to choose a flexible quit date following varenicline titration, but within the first month of treatment (i.e. between day 8 and day 35 of treatment)Citation27. With this approach, smokers do not start their quit attempt until they feel ready to quit within the given time frame. In a placebo-controlled study that evaluated this paradigm (N = 659), CAR9–12 were significantly higher with varenicline versus placebo (53.1% vs. 19.3%; OR, 5.9; 95% CI: 3.7, 9.4), as were CAR9–24Citation52. However, there has been no direct test of the flexible versus standard, fixed TQD approaches and the demographic and clinical characteristics of smokers selecting one or the other may differ.
‘Reduce-to-quit’
Varenicline prescribing information also includes a ‘reduce-to-quit’ approach, which is gradual smoking reduction with a goal of eventually quitting completelyCitation27. This approach has been evaluated in smokers unwilling or unable to quit immediately. In a study of 1510 participantsCitation53 (including 210 Japanese participants who were also analyzed separatelyCitation54) smokers were asked to reduce their smoking rate by at least 50% after 4 weeks of varenicline, with a further 50% reduction from week 4 to week 8, and a goal of total abstinence at week 12. Participants were to continue varenicline treatment until week 24. With this paradigm, CAR15–24 were significantly higher for varenicline versus placebo (32.1% vs. 6.9%; RR, 4.6; 95% CI: 3.5, 6.1). Similar results were observed for CAR21–24 and CAR21–52Citation53 and for the Japanese sub-populationCitation54.
No trials have directly compared the ‘reduce-to-quit’ approach with the standard, fixed TQD approach. However, reduction prior to complete abstinence should be offered to smokers not willing or able to quit abruptly.
Low-dose varenicline
In addition to the 1 mg BID dosing recommended in varenicline prescribing information, lower doses are recommended in those who cannot tolerate the standard dose. Lower doses were evaluated in several RCTsCitation29,Citation55–57. In studies that included a fixed dose of 0.5 mg BID for a 12-week treatment period, varenicline resulted in significantly higher CAR9–12 than placebo (55.5% vs. 39.5%, p = .01Citation29; 44.0% vs. 11.6%, p < .001Citation55) and lower reported rates of nausea than the standard dose. However, only one study observed a significant difference in CAR9–52 with low-dose varenicline versus placeboCitation55. In a study where participants were allowed to select their own dose (0.5–2.0 mg/day; mean modal dose of 1.35 mg/day), CAR9–12 and CAR9–52 were significantly higher for varenicline versus placeboCitation57. Meta-analyses showed ORs of 2.08 (95% CI: 1.56, 2.78) for low-dose varenicline versus placebo (CAR9–52), and 1.25 (95% CI: 1.00, 1.55) in a comparison of standard dose versus low-dose vareniclineCitation36. In summary, varenicline 0.5 mg BID is efficacious and can reduce nausea compared with the standard 1 mg BID dose.
High-dose varenicline
Varenicline dosing 0.1–10 mg QD covers the effective exposure rangeCitation25. Standard varenicline dosing was based on balancing effectiveness with tolerability, specifically nauseaCitation55,Citation56. Increasing doses were associated with an increased occurrence of nausea, but otherwise appeared to be safe as demonstrated in Phase 1 clinical trials and dose escalating studiesCitation23,Citation58, where nausea and vomiting were the limiting factors and no adverse effects remained or developed after discontinuation of drug use.
A few studies have evaluated the benefit of increasing the dose of varenicline. In one trial, participants started varenicline treatment 3 weeks before their TQDCitation59. Participants who did not reduce smoking by >50%, did not have a reduction in smoking enjoyment, and did not experience strong nausea, received additional tablets (varenicline or placebo) starting on Day 12. The varenicline dose could be increased up to 5 mg/day prior to the TQD, and was then maintained for 3 weeks after which time it was reduced to 2 mg/day. Increasing the dose of varenicline had no effect on urges to smoke post-TQD or on smoking cessation rates at 1, 4 or 12 weeks post-TQD versus placebo. Two studies reported increased abstinence rates with varenicline doses of 3 mg/day, but they did not include an interpretable comparison groupCitation60,Citation61.
Re-treatment with varenicline
The efficacy of retreatment with varenicline in smokers who had previously taken the drug but failed to quit smoking, or who relapsed after a period of abstinence, has been demonstrated in one RCTCitation62. Overall, 498 participants with ≥1 prior quit attempt using varenicline for ≥2 weeks were randomized to varenicline or placebo: CAR9–12 were 45.0% and 11.8% (OR, 7.08; 95% CI: 4.34, 11.55) for varenicline and placebo, respectively. Similarly, CAR9–24 and CAR9–52 were significantly higher for varenicline than placebo (CAR9–24: 28.9% vs. 7.8%; OR, 5.83; 95% CI: 3.25, 10.44; CAR9–52: 20.1% vs. 3.3%, OR, 9.00; 95% CI: 3.97, 20.41). Consistent with these findings, post-hoc analyses of EAGLES found no evidence of a moderator effect of prior treatment on the success of a new quit attempt with vareniclineCitation63.
Combination therapies
Combining varenicline with NRT
Varenicline and NRT act primarily on α4β2 and α6β2* nAChRs, but with different binding affinities and functional potencies. In terms of observable effects on smokers, both drugs appear to support abstinence primarily by alleviating the discomfort of nicotine withdrawalCitation64,Citation65, while varenicline, but not NRT, also attenuates the rewarding effects from smokingCitation18,Citation21.
Potential benefits of combining varenicline with NRT have not been completely clarified. Two RCTs of similar design (N = 117Citation66; N = 341Citation67) in which varenicline was provided with nicotine or placebo patches starting from TQD, showed no significant effect on abstinence rates at 12 weeks with the addition of NRT. In another RCT (N = 446), participants received nicotine or placebo patches for 2 weeks prior to TQD and for a further 12 weeks, and varenicline 1 week before the TQD, and for a further 12 weeksCitation68. Abstinence rates at 24 weeks post-TQD for varenicline plus active patch versus varenicline plus placebo patch were 32.9% vs. 19.2%, respectively [OR, 2.06; 95% CI: 1.33, 3.21]. While encouraging, results are difficult to interpret unequivocally as only the nicotine arm received NRT pre-loading, an intervention that has been shown by itself to increase quit ratesCitation69.
It is possible that NRT products other than patches, such as short-acting nicotine gum, inhaler, lozenge or e-cigarettes, which allow ad-lib dosing and provide a degree of sensory-motor replacement for cigarettes—features that varenicline does not provide—may be better candidates for combination treatmentCitation70. Varenicline treatment combined with e-cigarettes could be an effective treatment paradigm, as suggested in a small pilot study that evaluated e-cigarette use in conjunction with other smoking cessation treatmentsCitation70.
Combining varenicline with bupropion
Combining a partial nAChR receptor agonist with a norepinephrine and dopamine uptake inhibitor such as bupropion may result in a synergistic effect on the central effects from smoking.
Two studies suggest that the combination of varenicline and bupropion can improve short-term abstinence rates, but the effect seems to diminish over time. In one trial, 506 smokers received varenicline plus bupropion, or varenicline plus placeboCitation71. There was a significant effect of combination treatment on abstinence at 12 weeks (53.0% vs. 43.2%; OR, 1.49, 95% CI: 1.05, 2.12) and 26 weeks (36.6% vs. 27.6%, OR, 1.52; 95% CI: 1.04, 2.22) versus varenicline monotherapy, but not at 52 weeks, although the treatment difference still favored combination therapy (30.9% vs. 24.5%; OR, 1.39; 95% CI: 0.93, 2.07).
In a more complex trialCitation72, smokers used placebo patch while smoking. Those who did not reduce their smoking rate by >50% within 1 week (N = 222) were randomized to receive varenicline plus bupropion, or varenicline plus placebo. There was a significant increase in abstinence for weeks 8–11 post-TQD observed with combination therapy versus varenicline alone (39.8% vs. 25.9%; OR, 1.89; 95% CI: 1.07, 3.35), although this was observed only in men. Six-month abstinence rates for the overall population were not provided, but men continued to show a significant combined treatment effect at this time point whereas women did not.
However, in a more recent study (N = 385) that reported CARs at 6 and 12 months, varenicline plus bupropion showed no superiority over varenicline aloneCitation73.
Predictors of abstinence
Identification of particular characteristics that predict a subject’s response to different smoking cessation treatments would allow physicians to optimize existing treatment options. However, current data are limited. With the present knowledge it does not seem possible to increase efficacy by matching smokers to treatment.
Degree of dependence
Degree of dependence is one of the best predictors of outcome in smoking cessation in general and particularly among untreated or placebo-treated smokers. In a pooled analysis of 10 double-blind, placebo-controlled RCTs of varenicline, the abstinence rate decreased as dependence increased, but varenicline was equally efficacious relative to placebo across the whole spectrum of dependence (as measured by the Fagerström Test for Cigarette Dependence [FTCD])Citation74. In line with these findings, EAGLES also found an effect of degree of dependence on abstinence, but no interaction with any treatmentCitation63.
Sex
In general, women appear to have less success quitting smoking than men in smokers’ clinics and in smoking cessation clinical trials with bupropionCitation75. However, this has not always been observed in population studiesCitation76. In varenicline trials, women have poorer quit rates on placebo compared with men, but similar outcomes on active treatmentCitation77, suggesting that varenicline has greater effect in women than men. Similarly, results of a recent meta-analysis suggested that the comparative efficacy of varenicline versus bupropion or NRT is greater in women than in menCitation78. In contrast, EAGLES demonstrated no effect of sex on the efficacy of vareniclineCitation63.
Other characteristics
The large size of EAGLES permitted analyses of effects of multiple smoker characteristics (e.g. demographic, smoking history and psychiatric characteristics) on efficacy across treatments. While the results showed that lower quit rates at weeks 9–24 were associated with certain characteristics, such as younger age at start of smoking, previous use of NRT, living in the US and poorer mental health status, there were no treatment by characteristic interactionsCitation63.
Reduction in craving and reward
As predicted by its pharmacological properties, varenicline reduces both the urge to smoke and the rewarding properties of cigarettes. In pivotal registration studies varenicline reduced cravings and the rewarding properties of the first cigarette smoked after the TQD significantly more than bupropion and placebo, in abstinent and non-abstinent participantsCitation18. Similarly, an analysis of eight RCTs found that varenicline significantly reduced the urge to smoke versus placebo throughout the 12-week treatment periodCitation19. In a placebo-controlled study designed to evaluate the effect of varenicline on craving and reward, varenicline reduced abstinence-induced and cue-provoked craving versus placebo, the rewarding properties of a first full cigarette after abstinence, and also altered smoking behaviorCitation21.
Safety
General safety
Randomized controlled trials
Varenicline was shown to have an acceptable safety and tolerability profile in the two pivotal registration RCTs in generally healthy smokersCitation32. The most frequently occurring adverse event (AE) was nausea (28.8% vs. 9.1% for placebo). The majority of all nausea events were considered to be transient and mild in severity. Rates of discontinuation due to AEs were 9.5% for varenicline and 8.2% for placebo. Other trials in generally healthy smokers reported similar safety profilesCitation29,Citation33,Citation55,Citation57,Citation79, including EAGLES, in which the most frequently-reported general AEs in the non-psychiatric cohort for varenicline versus placebo were nausea (24.5% vs. 6.3%), headache (11.7% vs. 9.5%), insomnia (9.6% vs. 7.3%), nasopharyngitis (8.7% vs. 7.3%) and abnormal dreams (8.4% vs. 3.9%)Citation33.
Meta-analyses of randomized controlled trials
In a recent Cochrane ReviewCitation36 the most frequent AE for varenicline-treated smokers was mild to moderate nausea, at rates generally between 24% and 29% in the majority of studies. Other frequent AEs included insomnia, abnormal dreams and headache. Meta-analyses of these four AEs for varenicline versus placebo gave RRs of 3.27 (95% CI: 3.00, 3.55; 32 studies; 14,963 participants) for nausea; 1.49 (95% CI: 1.35, 1.65; 29 studies; 14,447 participants) for insomnia; 2.12 (95% CI: 1.88, 2.38; 26 studies; 13,682 participants) for abnormal dreams; and 1.17 (95% CI: 1.07, 1.29; 25 studies; 13,835 participants) for headache, with all differences being statistically significantCitation36.
Safety topics of special interest
The safety topics that have been of greatest concern, because of a potential association with varenicline use, are CV and neuropsychiatric events.
Cardiovascular disease (CVD)
Randomized controlled trials
In a multicenter, double-blind, placebo-controlled RCT in 714 smokers with stable CVD, there was no significant difference in CVD mortality, all-cause mortality, CV events or the incidence of serious AEs in smokers treated with varenicline or placeboCitation39. However, some non-fatal CV events were numerically more frequent with varenicline versus placebo. The results of this study prompted a warning to be added to varenicline prescribing information.
The CV safety of varenicline, bupropion and NRT was also explored as an adjunct to EAGLES: CV events were captured and adjudicated during the main study (12 weeks’ treatment, 12 weeks’ non-treatment follow-up) and for an additional 28-week non-treatment extension for a total of 52 weeksCitation80. The incidence of major adverse CV events (MACE), or an expanded list of significant CV events (MACE+) during treatment and follow-up, was very low (<0.5% for MACE; <0.8% for MACE+) in all treatment groups and no differences between active treatment groups and placebo were observed. Furthermore, no treatment differences were observed in time to CV events or in vital sign measures such as blood pressure and heart rate. It should be noted that the EAGLES population was not enriched for those with CVD, in fact only 8.0% of participants had high CV risk and 21.7% had medium CV risk as measured by their 10-year Framingham Risk Score for total coronary heart disease.
Meta-analyses of randomized controlled trials
Some meta-analyses concerning the CV safety of varenicline have suggested CV risk to marginally increase in smokers who receive varenicline versus placeboCitation81,Citation82 while others have reported no differencesCitation83,Citation84, even when focusing on smokers with previous CVD or predisposing conditions (Supplementary Table 2). This disparity has been attributed to the limited size and duration of varenicline RCTs and to a low incidence of CV events within such trialsCitation85, as well as differences in methodology among the analyses. A recent, large meta-analysis that included 38 RCTs (N = 12,706) of smokers found that CV events were rare overall and there were no differences in serious CV AEs between varenicline- and placebo-treated smokers (RR, 1.03; 95% CI: 0.72, 1.49)Citation85. Furthermore, similar findings were observed when the analysis was stratified by smokers with and without CVD (RR, 1.04; 95% CI: 0.57, 1.89; RR, 1.03; 95% CI: 0.64, 1.64, respectively).
Observational studies
Several observational studies have evaluated the occurrence of major CV events in smokers treated with varenicline or bupropion, none of which showed an increased risk for CV events with varenicline. In a national cohort study of Danish smokers aged ≥18 years who were prescribed varenicline (N = 17,926) or bupropion (N = 17,926) between 2007 and 2010, Kaplan-Meier curves for major CV events occurring up to 24 months after the start of treatment were similarCitation86. At 6 months, log rank tests showed no differences between treatments for any major CV event, or for specific events including acute coronary syndrome, ischemic stroke or CV death.
In a US study of Medicare claims data for patients aged ≥65 years who filled a prescription for varenicline (N = 74,824, with total on drug follow-up of 9530 person-years) or bupropion (N = 14,133; 2063 person-years) hazard ratios showed no difference in risk for any CV outcomes between the two pharmacotherapiesCitation87.
A retrospective cohort study based on the QResearch database in England, which was designed to investigate the association of varenicline use with the occurrence of serious adverse CV and neuropsychiatric events, concluded that adult smokers prescribed varenicline (N = 51,450) did not have an associated increased risk of documented CV events versus smokers prescribed NRT (N = 106,759)Citation88.
Neuropsychiatric events
Shortly after the launch of varenicline in 2006, post-marketing (pharmacovigilance) reports of neuropsychiatric AEs emerged. These events included suicidal ideation and behavior, completed suicide, changes in mood, psychosis, aggression and hostility, in smokers with and without previous psychiatric conditions, prompting drug label warnings in many countries. Following these warnings, a large body of evidence evaluating neuropsychiatric AEs associated with the use of varenicline has accumulated. Some of the key studies are described below.
Randomized controlled trials
EAGLES compared varenicline and bupropion with an active (NRT patch) and placebo control in smokers with and without a history of clinically stable neuropsychiatric diseaseCitation33 (). The primary safety endpoint was a composite measure of 16 neuropsychiatric symptom categories (moderate or severe intensity) that included 261 individual event termsCitation33. The trial found no significant increase in incidence of neuropsychiatric AEs with varenicline relative to placebo (the primary comparison), NRT patch or bupropion, in either cohort (varenicline vs. placebo: non-psychiatric cohort, 1.3% vs. 2.4%; risk difference (RD), −1.28; 95% CI: −2.40, −0.15; psychiatric cohort, 6.5% vs. 4.9%; RD, 1.59; 95% CI: −0.42, 3.59). There were more neuropsychiatric AEs in the psychiatric cohort than in the non-psychiatric cohort (5.8% vs. 2.1%, respectively); the increase was seen across all active treatment groups as well as for placebo. Furthermore, there were no treatment differences in the number of neuropsychiatric AEs that were considered serious or that lead to discontinuation. Within each cohort the number of participants reporting suicidal ideation or behavior on the Columbia Suicide Severity Rating Scale was similar across treatment groups although the numbers across all treatments were greater in the psychiatric cohort than in the non-psychiatric cohort. A completed suicide occurred in a placebo-treated smoker with no psychiatric history. Average Hospital Anxiety and Depression Scale scores improved similarly across all treatment groups (∼2 points in the non-psychiatric cohort and 3 points in the psychiatric cohort) during treatment. The findings demonstrate that clinically significant neuropsychiatric AEs occurring during smoking cessation are not attributable to the medication used. However, the authors concluded that clinicians should continue to monitor all patients who are using these medications for potential changes, especially among patients with a current or past psychiatric disorder.
Table 2. Neuropsychiatric adverse events reported in the EAGLES randomized controlled safety trial: incidence of the primary composite endpoint and selected components.
The results of EAGLES confirmed prior studies comparing varenicline and placebo in smokers with major depressive disorderCitation45 and stable schizophrenia or schizoaffective disorderCitation44. Smokers in these studies showed no worsening of disease as assessed by psychiatric scales and no differences in neuropsychiatric AEs between treatment groups.
Meta-analyses of randomized controlled trials
Meta-analyses of varenicline RCTs () consistently demonstrate that varenicline does not increase the risk of neuropsychiatric AEs versus placeboCitation89–95. The largest meta-analysis conducted to date which included 39 placebo-controlled trials (10,761 participants)Citation92 found no increased risk for suicide or suicide attempt, suicidal ideation, depression, irritability or aggression, and reported a reduced risk of anxiety in varenicline- versus placebo-treated smokers (ORs ranging from 0.58 to 1.67, 95% CIs included 1).
Table 3. Meta-analyses of randomized controlled trials examining neuropsychiatric adverse events related to varenicline.
A 2016 meta-analyses of neuropsychiatric serious AEs in varenicline- versus placebo-treated smokers (including EAGLES)Citation36 reported RRs for depression (0.94; 95% CI: 0.77, 1.14; 36 studies; 16,189 participants), and suicidal ideation (0.68; 95% CI: 0.43, 1.07; 24 studies; 11,193 participants). Lower rates were observed with varenicline versus placebo, but did not reach statistical significance.
Observational studies
Several large retrospective observational cohort studies, looking at a variety of endpoint measures, have investigated whether varenicline is associated with an increased risk of neuropsychiatric AEs, using NRT or bupropion as a comparatorCitation88,Citation96–102 (Supplementary Table 3). Four of these studiesCitation98–101 are included in varenicline prescribing information. Two looked at psychiatric hospitalizations and reported no differences between varenicline and NRT patch usersCitation100,Citation101. Another study looked at psychiatric AEs diagnosed during an emergency department visit or inpatient admission and reported no differences between varenicline and bupropion usersCitation98. The final study looked at fatal and non-fatal self-harm and showed no evidence of a higher risk in smokers prescribed varenicline versus NRTCitation99.
Three other large observational studies were published subsequently. One study analyzed data in the QResearch database to look at the incidence of fatal and non-fatal self-harm and depression within 6 months of treatment with varenicline or NRT and found no increased risk for these events versus NRTCitation88. Another study compared inpatient discharge diagnosis for seven separate psychiatric conditions within 30 days of treatment initiation in smokers using varenicline (N = 11,774) versus NRT (N = 23,548) and reported no between-treatment differencesCitation96. A secondary endpoint looking at outpatient visits for these same disorders found no differences except for schizophrenia. The third study which utilized a within-subject design based on the population of SwedenCitation97, looked at hospital admission or unplanned outpatient specialist visits for incident psychosis, mood or anxiety conditions; and emergency inpatient or outpatient hospital visits or death due to intentional self-harm. An increased risk of new (within-subject) incident psychiatric conditions during varenicline treatment versus periods of non-treatment were noted (HR, 1.18; 95% CI: 1.05, 1.31). Further analysis isolated this effect to an increased risk of incident anxiety (HR, 1.23; 95% CI: 1.01, 1.51) and mood conditions (HR, 1.31; 95% CI: 1.06, 1.63) among smokers with pre-existing psychiatric disorders.
Despite the limitations of cohort studies, data from approximately 269,000 smokers receiving varenicline do not support an increased risk of neuropsychiatric AEs.
Clinical implications
Considering current efficacy and safety data, the following are evidence-based recommendations for practitioners when prescribing varenicline.
Discussion and conclusions
There is a now a wealth of evidence on which to recommend that smokers making a quit attempt should be offered varenicline as a first-line treatment, in combination with behavioral counseling, consistent with the US Surgeon General’s recommendationsCitation103.
Varenicline has proven to be more effective than placebo, bupropion and NRT in the general populationCitation11,Citation12,Citation33 and in populations with comorbid conditions, such as those with psychiatric diseaseCitation33,Citation44,Citation45,Citation104, CVDCitation39,Citation40 and COPDCitation37. In addition, efficacy results have been replicated in numerous geographical areasCitation28–31,Citation33.
Data suggest that in addition to the standard administration of varenicline, several alternative treatment paradigms/quit approaches may prove beneficial. Those that have already received regulatory approval include extended varenicline treatment as maintenance therapy in people who have succeeded in quittingCitation47,Citation48, lower daily doses, and reduce-to-quit or flexible quit date approaches in those unwilling or unable to quit abruptlyCitation52,Citation53. While currently not included in the prescribing information, varenicline pre-loadingCitation50,Citation51,Citation105 has demonstrated improved efficacy, particularly in highly dependent smokers. These additions to the practitioner’s arsenal may be considered depending on the history and preference of the individual, and present opportunities to use varenicline more flexibly, to thereby maximize its efficacy, and to extend its clinical reach.
Varenicline has an acceptable safety/tolerability profile. The most frequently reported AE is nausea; varenicline treatment has also been associated with sleep disturbance and abnormal dreams. Most AEs are transient and mild in severity. Despite earlier case reports, there is now a large body of evidence that does not support the notion of increased risk of serious neuropsychiatric AEsCitation33,Citation44,Citation45 with varenicline in comparison with smokers quitting with placebo or the other approved medications. The rate of serious neuropsychiatric AEs is higher in smokers attempting to quit who have a psychiatric history compared to those without such a history, but this is observed across all treatments including placebo. Consequently, varenicline may be offered to smokers with psychiatric conditions who seek help with stopping smoking. Likewise, there is no evidence that use of varenicline is associated with increased risk of CV eventsCitation80.
There are a number of areas in which future research could broaden the application of varenicline. As discussed in the current article, studies that further investigate varenicline pre-loading and combination therapy with NRT or bupropion could confirm the potential benefits of these approaches. Additional research on the combination of varenicline specifically with short-acting NRT forms (gum and lozenges) or e-cigarettes also warrants further investigation. In addition, other research questions may be important that were beyond the scope of the current article. Varenicline may be a promising treatment for post-smoking cessation nicotine use in individuals who continue to use nicotine replacement products for yearsCitation106. Finally, varenicline has shown some potential for indications beyond smoking cessation, including for alcohol use disorder and addiction treatment for other drugs such as cocaineCitation107–109. Additional research could also confirm these uses.
Transparency
Declaration of funding
Editorial support was provided by Alexandra Bound, PhD; Michelle Jenvey, PhD; and Katy Beck, PhD of Engage Scientific and was funded by Pfizer.
Declaration of financial/other relationships
ST has received support from Pfizer for advisory boards and lectures. HR was a paid consultant to Pfizer in connection with the development of this manuscript. IB declares having received honoraria for consulting and presentations from Pfizer in the last 3 years. PH received research funding from and provided consultancy to Pfizer and other manufacturers of stop-smoking medications. KF has received consulting and speaking fees from many companies that develop or market pharmacological and behavioral treatments for smoking cessation. He currently receives consulting fees from the Snuscommission that is funded by the Swedish snus manufacturer’s association and is a member of an Advisory Panel for Swedish Match in the company’s work with the FDA to obtain a Modified Risk Tobacco Product license. CE has no declarations of conflict of interest. CA, TM and CR are employees and stockholders of Pfizer. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors were involved in the drafting of the paper and revising it critically for intellectual content. Furthermore, all authors provided final approval of the version to be published and agree to be accountable for all aspects of the work.
Figure 1
Download MS Word (86 KB)Table 3
Download MS Word (25.6 KB)Table 2
Download MS Word (19.2 KB)Table 1
Download MS Word (30.5 KB)Acknowledgements
Editorial support was provided by Alexandra Bound, PhD; Michelle Jenvey, PhD; and Katy Beck, PhD of Engage Scientific and was funded by Pfizer.
References
- Lim SS, Vos T, Flaxman AD, et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2224–2260.
- Hughes JR, Stead LF, Lancaster T. Anxiolytics for smoking cessation. Cochrane Database Syst Rev. 2000;(4):CD002849.
- Fernö O, Lichtneckert SJA, Lundgren CEG. A substitute for tobacco smoking. Psychopharmacologia. 1973;31(3):201–204.
- Fagerström KO. A comparison of psychological and pharmacological treatment in smoking cessation. J Behav Med. 1982;5(3):343–351.
- Ferry LH, Burchette RJ. Efficacy of bupropion for smoking cessation in nondepressed smokers. J Addict Dis. 1994;13:249.
- Hughes J, Stead L, Lancaster T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 2004;(4):CD000031.
- Silagy C, Lancaster T, Stead L. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2004;(3):CD000146.
- Cohen C, Bergis OE, Galli F, et al. SSR591813, a novel selective and partial alpha4beta2 nicotinic receptor agonist with potential as an aid to smoking cessation. J Pharmacol Exp Ther. 2003;306(1):407–420.
- Coe JW, Vetelino MG, Bashore CG, et al. In pursuit of alpha4beta2 nicotinic receptor partial agonists for smoking cessation: carbon analogs of (-)-cytisine. Bioorg Med Chem Lett. 2005;15(12):2974–2979.
- Coe JW, Brooks PR, Vetelino MG, et al. Varenicline: an alpha4beta2 nicotinic receptor partial agonist for smoking cessation. J Med Chem. 2005;48(10):3474–3477.
- Gonzales D, Rennard SI, Nides M, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):47–55.
- Jorenby DE, Hays JT, Rigotti NA, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):56–63.
- Cahill K, Ussher MH. Cannabinoid type 1 receptor antagonists for smoking cessation. Cochrane Database Syst Rev. 2011;(3):CD005353.
- Goniewicz ML, Delijewski M. Nicotine vaccines to treat tobacco dependence. Hum Vaccin Immunother. 2013;9(1):13–25.
- Brunzell DH. Preclinical evidence that activation of mesolimbic alpha 6 subunit containing nicotinic acetylcholine receptors supports nicotine addiction phenotype. Nicotine Tob Res. 2012;14(11):1258–1269.
- Rollema H, Chambers LK, Coe JW, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52(3):985–994.
- Rollema H, Hurst RS. The contribution of agonist and antagonist activities of alpha4beta2* nAChR ligands to smoking cessation efficacy: a quantitative analysis of literature data. Psychopharmacology (Berl). 2018;235(9):2479–2505.
- West R, Baker CL, Cappelleri JC, et al. Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology (Berl). 2008;197(3):371–377.
- Foulds J, Russ C, Yu CR, et al. Effect of varenicline on individual nicotine withdrawal symptoms: a combined analysis of eight randomized, placebo-controlled trials. Nicotine Tob Res. 2013;15(11):1849–1857.
- Ravva P, Gastonguay MR, Faessel HM, et al. Pharmacokinetic-pharmacodynamic modeling of the effect of varenicline on nicotine craving in adult smokers. Nicotine Tob Res. 2015;17(1):106–113.
- Brandon TH, Drobes DJ, Unrod M, et al. Varenicline effects on craving, cue reactivity, and smoking reward. Psychopharmacology (Berl). 2011;218(2):391–403.
- Obach RS, Reed-Hagen AE, Krueger SS, et al. Metabolism and disposition of varenicline, a selective alpha4beta2 acetylcholine receptor partial agonist, in vivo and in vitro. Drug Metab Dispos. 2006;34(1):121–130.
- Faessel HM, Smith BJ, Gibbs MA, et al. Single-dose pharmacokinetics of varenicline, a selective nicotinic receptor partial agonist, in healthy smokers and nonsmokers. J Clin Pharmacol. 2006;46(9):991–998.
- Burstein AH, Fullerton T, Clark DJ, et al. Pharmacokinetics, safety, and tolerability after single and multiple oral doses of varenicline in elderly smokers. J Clin Pharmacol. 2006;46(11):1234–1240.
- Faessel HM, Obach RS, Rollema H, et al. A review of the clinical pharmacokinetics and pharmacodynamics of varenicline for smoking cessation. Clin Pharmacokinet. 2010;49(12):799–816.
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41.
- Chantix (varenicline tartrate) prescribing information [Internet]. Pfizer Inc.; 2018. [updated 2018 June; cited 2019 Jan 30]. Available from: http://labeling.pfizer.com/showlabeling.aspx?id=557
- Bolliger CT, Issa JS, Posadas-Valay R, et al. Effects of varenicline in adult smokers: a multinational, 24-week, randomized, double-blind, placebo-controlled study. Clin Ther. 2011;33(4):465–477.
- Nakamura M, Oshima A, Fujimoto Y, et al. Efficacy and tolerability of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, in a 12-week, randomized, placebo-controlled, dose-response study with 40-week follow-up for smoking cessation in Japanese smokers. Clin Ther. 2007;29(6):1040–1056.
- Tsai ST, Cho HJ, Cheng HS, et al. A randomized, placebo-controlled trial of varenicline, a selective alpha4beta2 nicotinic acetylcholine receptor partial agonist, as a new therapy for smoking cessation in Asian smokers. Clin Ther. 2007;29(6):1027–1039.
- Wang C, Xiao D, Chan KP, et al. Varenicline for smoking cessation: a placebo-controlled, randomized study. Respirology. 2009;14(3):384–392.
- Nides M, Glover ED, Reus VI, et al. Varenicline versus bupropion SR or placebo for smoking cessation: a pooled analysis. Am J Health Behav. 2008;32(6):664–675.
- Anthenelli RM, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with and without psychiatric disorders (EAGLES): a double-blind, randomised, placebo-controlled clinical trial. Lancet. 2016;387(10037):2507–2520.
- Mills EJ, Wu P, Lockhart I, et al. Comparisons of high-dose and combination nicotine replacement therapy, varenicline, and bupropion for smoking cessation: a systematic review and multiple treatment meta-analysis. Ann Med. 2012;44(6):588–597.
- Cahill K, Stevens S, Perera R, et al. Pharmacological interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database Syst Rev. 2013;5:CD009329.
- Cahill K, Lindson-Hawley N, Thomas KH, et al. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2016;(5):CD006103.
- Tashkin DP, Rennard S, Hays JT, et al. Effects of varenicline on smoking cessation in patients with mild to moderate COPD: a randomized controlled trial. Chest. 2011;139(3):591–599.
- Jimenez Ruiz CA, Ramos Pinedo A, Cicero Guerrero A, et al. Characteristics of COPD smokers and effectiveness and safety of smoking cessation medications. Nicotine Tob Res. 2012;14(9):1035–1039.
- Rigotti NA, Pipe AL, Benowitz NL, et al. Efficacy and safety of varenicline for smoking cessation in patients with cardiovascular disease: a randomized trial. Circulation. 2010;121(2):221–229.
- Eisenberg MJ, Windle SB, Roy N, et al. Varenicline for smoking cessation in hospitalized patients with acute coronary syndrome. Circulation. 2016;133(1):21–30.
- Windle SB, Dehghani P, Roy N, et al. Smoking abstinence 1 year after acute coronary syndrome: follow-up from a randomized controlled trial of varenicline in patients admitted to hospital. CMAJ. 2018;190(12):E347–E354.
- Peckham E, Brabyn S, Cook L, et al. Smoking cessation in severe mental ill health: what works? an updated systematic review and meta-analysis. BMC Psychiatry. 2017;17(1):252.
- Weiner E, Buchholz A, Coffay A, et al. Varenicline for smoking cessation in people with schizophrenia: a double blind randomized pilot study. Schizophr Res. 2011;129(1):94–95.
- Williams JM, Anthenelli RM, Morris CD, et al. A randomized, double-blind, placebo-controlled study evaluating the safety and efficacy of varenicline for smoking cessation in patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry. 2012;73(05):654–660.
- Anthenelli RM, Morris C, Ramey TS, et al. Effects of varenicline on smoking cessation in adults with stably treated current or past major depression: a randomized trial. Ann Intern Med. 2013;159(6):390–400.
- Ahmed S, Virani S, Kotapati VP, et al. Efficacy and safety of varenicline for smoking cessation in schizophrenia: a meta-analysis. Front Psychiatry. 2018;9:428
- Tonstad S, Tønnesen P, Hajek P, et al. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA. 2006;296(1):64–71.
- Hajek P, Tønnesen P, Arteaga C, et al. Varenicline in prevention of relapse to smoking: effect of quit pattern on response to extended treatment. Addiction. 2009;104(9):1597–1602.
- Evins AE, Cather C, Pratt SA, et al. Maintenance treatment with varenicline for smoking cessation in patients with schizophrenia and bipolar disorder: a randomized clinical trial. JAMA. 2014;311(2):145–154.
- Hajek P, McRobbie HJ, Myers KE, et al. Use of varenicline for 4 weeks before quitting smoking: decrease in ad lib smoking and increase in smoking cessation rates. Arch Intern Med. 2011;171(8):770–777.
- Hawk LW, Jr., Ashare RL, Lohnes SF, et al. The effects of extended pre-quit varenicline treatment on smoking behavior and short-term abstinence: a randomized clinical trial. Clin Pharmacol Ther. 2012;91(2):172–180.
- Rennard S, Hughes J, Cinciripini PM, et al. A randomized placebo-controlled trial of varenicline for smoking cessation allowing flexible quit dates. Nicotine Tob Res. 2012;14(3):343–350.
- Ebbert JO, Hughes JR, West RJ, et al. Effect of varenicline on smoking cessation through smoking reduction: a randomized clinical trial. JAMA. 2015;313(7):687–694.
- Nakamura M, Abe M, Ohkura M, et al. Efficacy of varenicline for cigarette reduction before quitting in Japanese smokers: a subpopulation analysis of the Reduce to Quit trial. Clin Ther. 2017;39(4):863–872.
- Oncken C, Gonzales D, Nides M, et al. Efficacy and safety of the novel selective nicotinic acetylcholine receptor partial agonist, varenicline, for smoking cessation. Arch Intern Med. 2006;166(15):1571–1577.
- Nides M, Oncken C, Gonzales D, et al. Smoking cessation with varenicline, a selective alpha4beta2 nicotinic receptor partial agonist: results from a 7-week, randomized, placebo- and bupropion-controlled trial with 1-year follow-up. Arch Intern Med. 2006;166(15):1561–1568.
- Niaura R, Hays JT, Jorenby DE, et al. The efficacy and safety of varenicline for smoking cessation using a flexible dosing strategy in adult smokers: a randomized controlled trial. Curr Med Res Opin. 2008;24(7):1931–1941.
- Faessel HM, Gibbs MA, Clark DJ, et al. Multiple-dose pharmacokinetics of the selective nicotinic receptor partial agonist, varenicline, in healthy smokers. J Clin Pharmacol. 2006;46(12):1439–1448.
- Hajek P, McRobbie H, Myers Smith K, et al. Increasing varenicline dose in smokers who do not respond to the standard dosage: a randomized clinical trial. JAMA Intern Med. 2015;175(2):266–271.
- Jiménez-Ruiz CA, Barrios M, Peña S, et al. Increasing the dose of varenicline in patients who do not respond to the standard dose. Mayo Clin Proc. 2013;88(12):1443–1445.
- Karam-Hage M, Kypriotakis G, Robinson JD, et al. Improvement of smoking abstinence rates with increased varenicline dosage: a propensity score-matched analysis. J Clin Psychopharmacol. 2018;38(1):34–41.
- Gonzales D, Hajek P, Pliamm L, et al. Retreatment with varenicline for smoking cessation in smokers who have previously taken varenicline: A randomized, placebo-controlled trial. Clin Pharmacol Ther. 2014;96(3):390–396.
- West R, Evins AE, Benowitz NL, et al. Factors associated with the efficacy of smoking cessation treatments and predictors of smoking abstinence in EAGLES. Addiction. 2018;113(8):1507–1516.
- Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2008;(1):CD000146.
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database Syst Rev. 2011;(2):CD006103.
- Hajek P, Smith KM, Dhanji AR, et al. Is a combination of varenicline and nicotine patch more effective in helping smokers quit than varenicline alone? A randomised controlled trial. BMC Med. 2013;11(1):140.
- Ramon JM, Morchon S, Baena A, et al. Combining varenicline and nicotine patches: a randomized controlled trial study in smoking cessation. BMC Med. 2014;12(1):172.
- Koegelenberg CF, Noor F, Bateman ED, et al. Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: a randomized clinical trial. JAMA. 2014;312(2):155–161.
- Preloading Investigators. Effects on abstinence of nicotine patch treatment before quitting smoking: parallel, two arm, pragmatic randomised trial. BMJ. 2018;361:k2164.
- Hajek P, Corbin L, Ladmore D, et al. Adding E-cigarettes to specialist stop-smoking treatment: City of London Pilot Project. J Addict Res Ther. 2015;6:244.
- Ebbert JO, Hatsukami DK, Croghan IT, et al. Combination varenicline and bupropion SR for tobacco-dependence treatment in cigarette smokers: a randomized trial. JAMA. 2014;311(2):155–163.
- Rose JE, Behm FM. Combination treatment with varenicline and bupropion in an adaptive smoking cessation paradigm. AJP. 2014;171(11):1199–1205.
- Cinciripini PM, Minnix JA, Green CE, et al. An RCT with the combination of varenicline and bupropion for smoking cessation: clinical implications for front line use. Addiction. 2018;113(9):1673–1682.
- Fagerström K, Russ C, Yu CR, et al. The Fagerstrom Test for Nicotine Dependence as a predictor of smoking abstinence: a pooled analysis of varenicline clinical trial data. Nicotine Tob Res. 2012;14(12):1467–1473.
- Scharf D, Shiffman S. Are there gender differences in smoking cessation, with and without bupropion? Pooled- and meta-analyses of clinical trials of Bupropion SR. Addiction. 2004;99(11):1462–1469.
- Jarvis MJ, Cohen JE, Delnevo CD, et al. Dispelling myths about gender differences in smoking cessation: population data from the USA, Canada and Britain. Tob Control. 2013;22(5):356–360.
- McKee SA, Smith PH, Kaufman M, et al. Sex differences in varenicline efficacy for smoking cessation: a meta-analysis. NICTOB. 2016;18(5):1002–1011.
- Smith PH, Weinberger AH, Zhang J, et al. Sex differences in smoking cessation pharmacotherapy comparative efficacy: a network meta-analysis. Nicotine Tob Res. 2017;19(3):273–281.
- Tonstad S. Smoking cessation efficacy and safety of varenicline, an alpha4beta2 nicotinic receptor partial agonist. J Cardiovasc Nurs. 2006;21(6):433–436.
- Benowitz NL, Pipe A, West R, et al. Cardiovascular safety of varenicline, bupropion, and nicotine patch in smokers: a randomized clinical trial. JAMA Intern Med. 2018;178(5):622–631.
- Singh S, Loke YK, Spangler JG, et al. Risk of serious adverse cardiovascular events associated with varenicline: a systematic review and meta-analysis. CMAJ. 2011;183(12):1359–1366.
- Ware JH, Vetrovec GW, Miller AB, et al. Cardiovascular safety of varenicline: patient-level meta-analysis of randomized, blinded, placebo-controlled trials. Am J Ther. 2013;20(3):235–246.
- Prochaska JJ, Hilton JF. Risk of cardiovascular serious adverse events associated with varenicline use for tobacco cessation: systematic review and meta-analysis. BMJ. 2012;344:e2856–e2856.
- Mills EJ, Thorlund K, Eapen S, et al. Cardiovascular events associated with smoking cessation pharmacotherapies: a network meta-analysis. Circulation. 2014;129(1):28–41.
- Sterling LH, Windle SB, Filion KB, et al. Varenicline and adverse cardiovascular events: a systematic review and meta-analysis of randomized controlled trials. J Am Heart Assoc. 2016;5(2):e002849.
- Svanström H, Pasternak B, Hviid A. Use of varenicline for smoking cessation and risk of serious cardiovascular events: nationwide cohort study. BMJ. 2012;345:e7176.
- Graham DJ, By K, McKean S, et al. Cardiovascular and mortality risks in older Medicare patients treated with varenicline or bupropion for smoking cessation: an observational cohort study. Pharmacoepidemiol Drug Saf. 2014;23(11):1205–1212.
- Kotz D, Viechtbauer W, Simpson C, et al. Cardiovascular and neuropsychiatric risks of varenicline: a retrospective cohort study. Lancet Respir Med. 2015;3(10):761–768.
- Wu Q, Gilbody S, Peckham E, et al. Varenicline for smoking cessation and reduction in people with severe mental illnesses: systematic review and meta-analysis. Addiction. 2016;111(9):1554–1567.
- Roberts E, Eden Evins A, McNeill A, et al. Efficacy and tolerability of pharmacotherapy for smoking cessation in adults with serious mental illness: a systematic review and network meta-analysis. Addiction. 2016;111(4):599–612.
- Kishi T, Iwata N. Varenicline for smoking cessation in people with schizophrenia: systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci. 2015;265(3):259–268.
- Thomas KH, Martin RM, Knipe DW, et al. Risk of neuropsychiatric adverse events associated with varenicline: systematic review and meta-analysis. BMJ. 2015;350:h1109–h1109.
- Gibbons RD, Mann JJ. Varenicline, smoking cessation, and neuropsychiatric adverse events. AJP. 2013;170(12):1460–1467.
- Huang Y, Li W, Yang L, et al. Long-term efficacy and safety of varenicline for smoking cessation: a systematic review and meta-analysis of randomized controlled trials. J Public Health. 2012;20(4):355–365.
- Tonstad S, Davies S, Flammer M, et al. Psychiatric adverse events in randomized, double-blind, placebo-controlled clinical trials of varenicline: a pooled analysis. Drug Saf. 2010;33(4):289–301.
- Cunningham FE, Hur K, Dong D, et al. A comparison of neuropsychiatric adverse events during early treatment with varenicline or a nicotine patch. Addiction. 2016;111(7):1283–1292.
- Molero Y, Lichtenstein P, Zetterqvist J, et al. Varenicline and risk of psychiatric conditions, suicidal behaviour, criminal offending, and transport accidents and offences: population based cohort study. BMJ. 2015;350:h2388–h2388.
- Pasternak B, Svanstrom H, Hviid A. Use of varenicline versus bupropion and risk of psychiatric adverse events. Addiction. 2013;108(7):1336–1343.
- Thomas KH, Martin RM, Davies NM, et al. Smoking cessation treatment and risk of depression, suicide, and self harm in the Clinical Practice Research Datalink: prospective cohort study. BMJ. 2013;347:f5704–f5704.
- Meyer TE, Taylor LG, Xie S, et al. Neuropsychiatric events in varenicline and nicotine replacement patch users in the Military Health System. Addiction. 2013;108(1):203–210.
- FDA Drug Safety Communication: Safety review update of Chantix (varenicline) and risk of neuropsychiatric adverse events [Internet]. U.S. Food and Drug Administration; 2011 [updated 2011 Oct 24; cited 2014 Aug 31]. Available from: http://www.fda.gov/drugs/drugsafety/ucm276737.htm
- Gunnell D, Irvine D, Wise L, et al. Varenicline and suicidal behaviour: a cohort study based on data from the General Practice Research Database. BMJ. 2009;339:b3805–b3805.
- Clinical Practice Guideline Treating Tobacco Use and Dependence 2008 Update Panel, Liaisons, and Staff. A clinical practice guideline for treating tobacco use and dependence: 2008 update. A U.S. Public Health Service report. Am J Prev Med. 2008;35(2):158–176.
- Chengappa KN, Perkins KA, Brar JS, et al. Varenicline for smoking cessation in bipolar disorder: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry. 2014;75(07):765–772.
- Ashare RL, Tang KZ, Mesaros AC, et al. Effects of 21 days of varenicline versus placebo on smoking behaviors and urges among non-treatment seeking smokers. J Psychopharmacol. 2012;26(10):1383–1390.
- Tonnesen P, Mikkelsen K. Varenicline to stop long-term nicotine replacement use: a double-blind, randomized, placebo-controlled trial. Nicotine Tob Res. 2013;15(2):419–427.
- Plebani JG, Lynch KG, Yu Q, et al. Results of an initial clinical trial of varenicline for the treatment of cocaine dependence. Drug Alcohol Depend. 2012;121(1–2):163–166.
- Litten RZ, Ryan ML, Fertig JB, et al. A double-blind, placebo-controlled trial assessing the efficacy of varenicline tartrate for alcohol dependence. J Addict Med. 2013;7(4):277–286.
- Oon-Arom A, Likhitsathain S, Srisurapanont M. Efficacy and acceptability of varenicline for alcoholism: a systematic review and meta-analysis of randomized-controlled trials. Drug Alcohol Depend. 2019;205:107631.
- Evins AE, Benowitz NL, West R, et al. Neuropsychiatric safety and efficacy of varenicline, bupropion, and nicotine patch in smokers with psychotic, anxiety, and mood disorders in the EAGLES trial. J Clin Psychopharmacol. 2019;39(2):108–116.
