Abstract
Objective: To assess the efficacy and safety of the sodium–glucose cotransporter 2 inhibitor ertugliflozin across racial groups in patients with type 2 diabetes mellitus (T2DM).
Methods: Pooled analysis of data from randomized, double-blind studies in the ertugliflozin phase III development program. Seven placebo- and comparator-controlled studies were used to assess safety (N = 4859) and three placebo-controlled studies were used to assess efficacy (N = 1544). Least-squares (LS) mean change from baseline was calculated for glycated hemoglobin (HbA1c), body weight and systolic blood pressure (SBP). Safety evaluation included overall and prespecified adverse events (AEs).
Results: At Week 26, ertugliflozin provided a greater reduction in HbA1c, body weight and SBP versus placebo in all racial subgroups. The placebo-adjusted LS mean change (95% confidence interval) from baseline in HbA1c was −0.8% (−1.0, −0.7) and −1.0% (−1.1, −0.8) with ertugliflozin 5 mg and 15 mg, respectively, in the White subgroup, −0.7% (−1.2, −0.2) and −0.8% (−1.3, −0.3) in the Black subgroup, and −0.8% (−1.1, −0.5) and −1.0% (−1.3, −0.8) in the Asian subgroup. The incidences of overall AEs, serious AEs and AEs leading to discontinuation from study medication were similar between the ertugliflozin 5 mg, 15 mg and non-ertugliflozin groups within each racial subgroup. The incidence of female genital mycotic infection (GMI) was higher with ertugliflozin than non-ertugliflozin across all racial subgroups. The incidence of male GMI was higher with ertugliflozin than non-ertugliflozin in the White sub-group; however, there were few male GMI events in the non-White subgroups.
Conclusions: In patients with T2DM, treatment with ertugliflozin improved HbA1c, body weight and SBP across all racial subgroups. Ertugliflozin had a generally similar safety profile across racial subgroups and was generally well tolerated.
Clinicaltrials.gov identifiers: NCT01986855, NCT01999218, NCT01958671, NCT02099110, NCT02036515, NCT02033889, and NCT02226003.
1. Introduction
Disparities have been observed in the prevalence of diabetes and its associated comorbidities worldwide with a higher incidence of diabetes in racial minority groups compared with non-minority groupsCitation1. This is emphasized by differences in comparative prevalence of diabetes between countries and regions, with age-adjusted prevalence rates of 11.0% in North America/Caribbean, 10.8% in the Middle East/Northern Africa, 10.1% in Southeast Asia, 8.6% in the Western Pacific regions, 7.6% in South/Central America, 6.8% in Europe and 4.4% in AfricaCitation2. The pathophysiology of T2DM may vary with race with possible differences in insulin sensitivity, β-cell function and hepatic insulin extraction between racial groupsCitation3. Racial differences in the pathophysiology of T2DM, together with intrinsic and extrinsic (socioeconomic and lifestyle) differences between racial groups, may impact the response to antidiabetic treatment. Therefore, identifying the potential impact of race on the efficacy and safety of an antidiabetic medication is important from a therapeutic perspective.
Ertugliflozin is a selective sodium–glucose cotransporter 2 (SGLT2) inhibitor that has been approved as an adjunct to diet and exercise to improve glycemic control in adults with T2DMCitation4,Citation5. SGLT2 inhibitors lower blood sugar by blocking renal glucose reabsorption, and thereby increasing urinary glucose excretion in an insulin-independent mannerCitation6. No clinically meaningful racial differences were observed in the pharmacokinetics and pharmacodynamics of ertugliflozin in clinical pharmacology studies carried out in healthy subjectsCitation7,Citation8. VERTIS (eValuation of ERTugliflozin effIcacy and Safety) was a global, phase III clinical development program. The results of the VERTIS studies showed that ertugliflozin improved glycemic control, body weight and blood pressure, alone and in combination with metformin and/or sitagliptinCitation9–16. Here we present the results of an analysis that was conducted to assess the efficacy and safety of ertugliflozin in racial subgroups using pooled data from the VERTIS program.
2. Methods
These analyses included pooled data from multinational, randomized, double-blind, phase III clinical studies of ertugliflozin 5 mg and 15 mgCitation9–15. Pooled data were analyzed due to the small numbers of patients in some racial subgroups in the individual studies. Two pooled data sets were utilized – for efficacy analyses, the placebo pool was used, and for safety analyses, the broad pool was used ().
Table 1. Phase III VERTIS clinical studies included in the pooled analyses.
The placebo pool includes three randomized, double-blind, multicenter, placebo-controlled phase III studies (VERTIS MONO, VERTIS MET and VERTIS SITA2), which had similar study designs and enrollment criteriaCitation9,Citation10,Citation13. The efficacy analysis of the placebo pool used data from Week 26, which was the primary efficacy time point for studies in this pool. Two other placebo-controlled studies were excluded from the pooled data for efficacy analysis; the VERTIS RENAL study was excluded due to the inclusion of patients with chronic kidney diseaseCitation14 and the VERTIS SITA study was excluded as ertugliflozin was coadministered with sitagliptinCitation12. However, the safety data from these two studies were included in the safety analysis to provide a robust population appropriate for safety assessment.
The broad pool includes safety data from seven placebo-controlled or active comparator studies comprising the three placebo-controlled studies in the placebo pool and four phase III randomized, double-blind, multicenter studies: VERTIS SUCitation15, VERTIS SITACitation12, VERTIS FACTORIALCitation11 and VERTIS RENALCitation14. Data were collected for each patient from the time of randomization through each study’s primary time point (Week 26, or Week 52 for VERTIS SU) and, for studies with a predefined extension period (all except VERTIS SITA), up to a prespecified data cut-off date (prior to completion of the extension period) to support regulatory submissions. The safety analysis for the broad pool represents mean durations of exposure to ertugliflozin 5 mg, ertugliflozin 15 mg and non-ertugliflozin, of 356 days, 355 days and 355 days, respectively.
2.1. Study design, treatment and assessments
Detailed study design, inclusion criteria, and exclusion criteria have been previously reported for the individual studies included in these analysesCitation9–15. Participants were aged ≥18 years with a diagnosis of inadequately controlled T2DM (glycated hemoglobin [HbA1c] between 7.0% and 11%, depending on the study). Details on background therapy and active comparators for the individual studies are listed in . Informed consent was obtained from participants in each study, and all studies included in this analysis were conducted in accordance with principles of Good Clinical Practice and approved by the appropriate institutional review boards and regulatory agencies.
Patients received ertugliflozin 5 mg, ertugliflozin 15 mg or non-ertugliflozin (placebo or active comparator) once daily with or without sitagliptin, depending on the study. Patients received glycemic rescue therapy if they exceeded the protocol-specified glycemic thresholds. Patients in each study received counseling on diet and were asked to maintain a routine exercise program with consistent physical activity throughout the study.
The efficacy endpoints were change from baseline to Week 26 in HbA1c, body weight and systolic blood pressure (SBP). Race was self-reported by patients at screening. Throughout the studies, body weight measurements were taken in duplicate with a standardized digital scale at approximately the same time of day after voiding. Sitting blood pressure (BP) was measured in triplicate with an automated oscillometric BP measuring device at each study center.
Safety endpoints included the incidences of adverse event (AE) summary measures including overall AEs and serious AEs (SAEs), and pre-specified AEs of interest for SGLT2 inhibitors (urinary tract infection [UTI], genital mycotic infection [GMI, by gender], volume depletion and symptomatic hypoglycemia).
2.2. Statistical analysis
2.2.1. Efficacy analysis
These analyses were conducted on pooled data from all randomized patients in the placebo pool who received ≥1 dose of study medication and who had a baseline measurement and at least one post-baseline measurement. Efficacy analyses excluded results obtained after initiation of glycemic rescue therapy.
The subgroups analyzed for change from baseline in HbA1c, body weight and SBP were White, Black, Asian and Other. A repeated measures analysis of covariance (RMANCOVA) model was used. The RMANCOVA model was adjusted for treatment, time, study, baseline estimated glomerular filtration rate (eGFR), baseline value of the response variable and the interaction of time by treatment. A separate model was applied for each subgroup to test differences between treatments within racial subgroups. No formal statistical comparisons were made between racial subgroups for any of the endpoints due to the exploratory nature of the efficacy analyses, lack of multiplicity control and the small size of some of the subgroups.
2.2.2. Safety analysis
Safety analyses were conducted on pooled data from all randomized patients in the broad pool who received ≥1 dose of study medication and included data obtained after initiation of glycemic rescue therapy, except the analysis of symptomatic hypoglycemia. The analysis of symptomatic hypoglycemia was assessed in the placebo pool (to avoid confounding issues with the heterogeneity of active control treatments in the broad pool) and excluded data obtained after initiation of glycemic rescue.
3. Results
3.1. Patient population
The placebo pool included 1544 patients; 1134 (73.4%) patients self-reported as White, 102 (6.6%) Black, 233 (15.1%) Asian and 75 (4.9%) Other (). In the seven studies comprising the broad pool (N = 4859), 3732 (76.8%) patients self-reported as White, 241 (5.0%) Black, 648 (13.3%) Asian and 238 (4.9%) Other (). In the broad pool, the majority of patients in the White subgroup were enrolled from Europe (∼50%) and North America (∼33%), patients in the Black subgroup were enrolled from North America (∼70%) and South Africa (∼29%), and patients in the Asian subgroup were enrolled from Asia (∼70%) and North America (∼24%).
Table 2. Baseline demographics and disease characteristics of patients included in the pooled efficacy (placebo pool) and safety analyses (broad pool).
Baseline demographics and characteristics were generally similar across treatment groups in the placebo and broad pools, with no meaningful differences noted across treatment groups by racial subgroup. In the overall placebo pooled population comprising all racial subgroups, 52.6% of the population were male, the mean (standard deviation, SD) age was 57.3 (9.6) years and baseline eGFR was 88.9 (18.5) mL/min/1.73 m2. The duration of T2DM was 7.5 (5.9) years and the baseline HbA1c was 8.1 (0.9) %. Baseline body weight was 87.9 (20.0) kg and body mass index (BMI) was 31.5 (5.8) kg/m2. SBP at baseline was 130.4 (13.6) mmHg. Baseline demographics and characteristics for patients across all racial subgroups in the broad pool were generally similar compared with the placebo pool except for a lower eGFR in the broad pool (85.3 [22.2] mL/min/1.73 m2), as the broad pool included ∼12% of patients with baseline moderate renal impairment. In the broad pool, 2647 (70.9%) patients in the White subgroup, 186 (77.2%) patients in the Black subgroup, 420 (64.8%) patients in the Asian subgroup and 136 (57.1%) patients in the Other subgroup had hypertension at baseline. No meaningful differences were noted across racial subgroups, except for a lower proportion of males in the Black and Other subgroups, a lower body weight and BMI in the Asian subgroup, and a longer duration of T2DM in the Black subgroup ().
3.2. Efficacy
3.2.1. Glycated hemoglobin
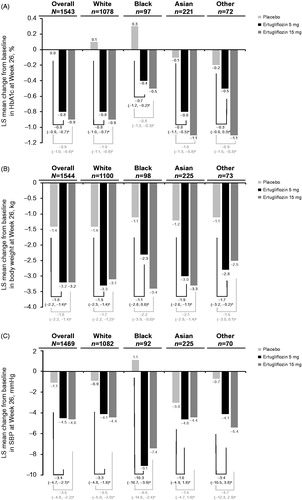
At Week 26, treatment with ertugliflozin 5 mg or 15 mg reduced HbA1c from baseline compared with placebo across all racial subgroups in the placebo pool (). The placebo-adjusted reductions in HbA1c for each racial subgroup were generally similar to the effect observed in the overall population.
3.2.2. Body weight
At Week 26, treatment with ertugliflozin 5 mg or 15 mg reduced body weight from baseline compared with placebo across all racial subgroups in the placebo pool (). The placebo-adjusted reductions in body weight for each racial subgroup were generally similar to the effect observed in the overall population.
3.2.3. Systolic blood pressure
At Week 26, treatment with ertugliflozin 5 mg or 15 mg reduced SBP from baseline compared with placebo across all racial subgroups in the placebo pool (). The placebo-adjusted reductions in SBP for each racial subgroup were generally similar to the effect observed in the overall population, with the exception of the Black subgroup where greater placebo-adjusted differences were seen.
3.3. Safety
In the broad pool, the incidences of overall AEs, SAEs and discontinuations due to an AE were generally similar across the ertugliflozin and non-ertugliflozin groups within each racial subgroup (). The incidence of death was low across the ertugliflozin and non-ertugliflozin groups in each racial subgroup. Within each racial subgroup, the incidence of UTI was not notably different across the ertugliflozin and non-ertugliflozin groups. There was a higher incidence of female GMI in the ertugliflozin groups than in the non-ertugliflozin group within each racial subgroup. Although there was an increased incidence of male GMI in the ertugliflozin groups compared with the non-ertugliflozin group in the White subgroup, there were too few events in the non-White subgroups to draw meaningful conclusions. There was no notable difference in the incidence of volume depletion AEs across treatment groups in the White subgroup; there were few events in the non-White subgroups. In the placebo pool, there was no notable difference in the incidence of symptomatic hypoglycemia across treatment groups in the White subgroup; there were few events in the non-White subgroups.
Table 3. Summary of AEs and pre-specified AEs.
4. Discussion
The results from these analyses demonstrated that ertugliflozin was generally effective and well tolerated in patients with T2DM across racial subgroups in the VERTIS phase III program. Efficacy data were pooled from three similarly designed phase III studies (VERTIS MONO, VERTIS MET and VERTIS SITA2) to provide a larger sample size for the assessment of efficacy across racial subgroups. Safety data were generally assessed in the broad pool, which comprised seven VERTIS studies including the subset of three placebo pool studies. This provided a robust population appropriate for safety assessment.
The results reported here demonstrate that ertugliflozin reduced HbA1c, body weight and SBP compared with placebo in all racial subgroups studied. Efficacy results were generally similar across racial subgroups. Both doses of ertugliflozin were effective in improving glycemic control across racial subgroups and the effects were similar to those observed in the overall population, with an incremental HbA1c lowering with ertugliflozin 15 mg compared with ertugliflozin 5 mg. The reduction in body weight across treatment groups in each racial subgroup was similar to that observed in the overall population despite a lower baseline body weight in the Asian subgroup.
Reduction in SBP across treatment groups in each racial subgroup was generally similar to that in the overall population except for the Black subgroup that had a larger placebo-adjusted reduction with both doses of ertugliflozin. In the placebo pooled population, the subgroup of Black patients also had a higher baseline SBP than other racial subgroups. The results of the National Health and Nutrition Examination Survey showed the prevalence of hypertension among non-Hispanic Black adults (40.3%) was greater than non-Hispanic White (27.8%), non-Hispanic Asian (25.0%) and Hispanic (27.8%) adultsCitation17. Black patients are also more salt-sensitive and generally have a better response to diuretics and calcium channel blockers than White patientsCitation18. The antihypertensive effect of ertugliflozin in Black patients may in part be related to its osmotic diuretic and natriuretic effects and the associated plasma volume reductionCitation6. However, the SBP findings in the Black subgroup in this current study should be interpreted with caution due to the small patient numbers and wide confidence intervals in observed results. A larger study in patients with hypertension would be required to explore the SBP reduction in this subgroup further. A recent placebo-controlled study of another SGLT2 inhibitor, empagliflozin, explored the effects on HbA1c, body weight and SBP in Black patients with T2DM and hypertensionCitation19. The SBP lowering effects of empagliflozin increased over the course of the 24 week study with a similar placebo-subtracted BP effect at Week 24 to standard antihypertensive agents. These data suggest that SGLT2 inhibitors may have beneficial antihypertensive effects for Black patients and hypertension.
The pharmacokinetic profile of ertugliflozin is similar in healthy subjects and in patients with T2DMCitation20. Ertugliflozin exhibits a linear pharmacokinetics and dose proportionalityCitation7, with an absolute oral bioavailability of approximately 100%Citation8, a low susceptibility to dietary effects on absorptionCitation21 and a low propensity for drug–drug interactionsCitation22. Glucuronidation by UGT1A9 and UGT2B7 is the dominant pathway of the metabolic disposition of ertugliflozin with a minor contribution from oxidative phosphorylationCitation7,Citation23. Genetic polymorphisms in the enzymes that metabolize ertugliflozin do not have a clinically meaningful impact on the pharmacokinetics of ertugliflozinCitation24. No clinically meaningful racial differences were observed in the pharmacokinetics and pharmacodynamics of ertugliflozin in clinical pharmacology studies carried out in healthy subjectsCitation25,Citation26. On the basis of these studies, no racial differences in the clinical efficacy and safety of ertugliflozin were expected in the phase III studies of patients with T2DM. The results of this current analysis are consistent with these clinical pharmacology studies.
Safety results from the broad pool analysis indicated that both ertugliflozin 5 mg and 15 mg were generally well tolerated in all racial subgroups. There were no notable differences in the incidence of AEs or SAEs across treatment groups in each racial subgroup. Within each racial subgroup, the incidence of UTI was not notably different across the ertugliflozin and non-ertugliflozin groups. There was a higher incidence of female GMI in the ertugliflozin groups than in the non-ertugliflozin group within each racial subgroup. In the Asian sub-group, the incidence of GMI was generally lower with ertugliflozin compared with other racial subgroups. It is not anticipated that there would be a difference in the risk of GMIs in Asian females receiving SGLT2 inhibitors relative to other racial subgroups. A possible explanation for the lower incidence in Asian females compared with non-Asian females may be in part due to cultural reporting bias which was given as a possible explanation for similar findings in a study with canagliflozin in Asian patients with T2DMCitation27. There was an increased incidence of male GMI in the ertugliflozin groups compared with the non-ertugliflozin group in the White subgroup; there were too few events of male GMI in the non-White subgroups to draw meaningful conclusions, although it is not anticipated that the risk of male GMI would differ by race. The higher incidence of GMI with ertugliflozin compared with non-ertugliflozin is consistent with previously published safety data for ertugliflozin and other SGLT2 inhibitors, as GMI is a class effect common to SGLT2 inhibitorsCitation28.
Limitations of this analysis are its exploratory nature and use of multiple statistical evaluations without control for multiplicity. It was therefore not possible to make formal statistical comparisons between racial subgroups for any of the endpoints. Another limitation is the low proportion of Black patients (6.6%) compared with other racial subgroups. The precision of treatment effect may be limited because of the small group size in certain racial subgroups, and the results should therefore be interpreted with caution. The racial subgroups included in this analysis may represent a mix of nationalities with cultural, physiological and genetic heterogeneity as well as immigrants residing in different countries from their ancestry. As such, the results from the current analysis may not accurately represent all patients of a particular race. Another limitation is the short-term nature of these studies; longer-term outcomes will be assessed in the ongoing VERTIS CV trialCitation29.
5. Conclusions
In conclusion, treatment with ertugliflozin generally improved glycemic control, body weight and SBP across all racial subgroups. Ertugliflozin had a generally similar safety profile across racial subgroups and was generally well tolerated.
Transparency
Declaration of funding
This study was sponsored by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co. Inc., Kenilworth, NJ, USA in collaboration with Pfizer Inc., New York, NY, USA.
Declaration of financial/other relationships
I.G., S.H., J.L., S.P., A.P. and L.W. have disclosed that they are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co. Inc., Kenilworth, NJ, USA and may own stock in Merck & Co. Inc., Kenilworth, NJ, USA.
A.H., J.P.M., L.T. and S.G.T. have disclosed that they are employees of Pfizer Inc., New York, NY, USA and own stock in Pfizer Inc., New York, NY, USA.
CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All authors critically reviewed the draft manuscript and approved the final version of the manuscript for publication.
Acknowledgements
Editorial support was provided by Marion James, PhD, of Engage Scientific Solutions (Horsham, UK) and was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co. Inc., Kenilworth, NJ, USA, in collaboration with Pfizer Inc., New York, NY, USA.
Data availability statement
Upon request, and subject to certain criteria, conditions and exceptions (see https://www.pfizer.com/science/clinical-trials/trial-data-and-results for more information) Pfizer will provide access to individual de-identified participant data from Pfizer-sponsored global interventional clinical studies conducted for medicines, vaccines and medical devices (1) for indications that have been approved in the US and/or EU or (2) in programs that have been terminated (i.e. development for all indications has been discontinued). Pfizer will also consider requests for the protocol, data dictionary and statistical analysis plan. Data may be requested from Pfizer trials 24 months after study completion. The de-identified participant data will be made available to researchers whose proposals meet the research criteria and other conditions, and for which an exception does not apply, via a secure portal. To gain access, data requestors must enter into a data access agreement with Pfizer.
References
- Golden SH, Brown A, Cauley JA, et al. Health disparities in endocrine disorders: biological, clinical, and nonclinical factors – an Endocrine Society scientific statement. J Clin Endocrinol Metab. 2012;97(9):E1579–E1639.
- International Diabetes Federation. IDF diabetes atlas. 8th ed. Brussels (Belgium): International Diabetes Federation; 2017 [cited 2020 May 4]. Available from: https://www.idf.org/e-library/epidemiology-research/diabetes-atlas/134-idf-diabetes-atlas-8th-edition.html
- Moller JB, Dalla Man C, Overgaard RV, et al. Ethnic differences in insulin sensitivity, beta-cell function, and hepatic extraction between Japanese and Caucasians: a minimal model analysis. J Clin Endocrinol Metab. 2014;99(11):4273–4280.
- European Medicines Agency. Steglatro (ertugliflozin) summary of product characteristics. Hoddesdon (UK): Merck Sharp & Dohme Ltd; 2018.
- US Food and Drug Administration. Steglatro (ertugliflozin) prescribing information. Whitehouse Station (NJ): Merck Sharp & Dohme Corp; 2017.
- Heerspink HJ, Perkins BA, Fitchett DH, et al. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134(10):752–772.
- Kalgutkar AS, Tugnait M, Zhu T, et al. Preclinical species and human disposition of PF-04971729, a selective inhibitor of the sodium-dependent glucose cotransporter 2 and clinical candidate for the treatment of type 2 diabetes mellitus. Drug Metab Dispos. 2011;39(9):1609–1619.
- Raje S, Callegari E, Sahasrabudhe V, et al. Novel application of the two-period microtracer approach to determine absolute oral bioavailability and fraction absorbed of ertugliflozin. Clin Transl Sci. 2018;11(4):405–411.
- Terra SG, Focht K, Davies M, et al. Phase III, efficacy and safety study of ertugliflozin monotherapy in people with type 2 diabetes mellitus inadequately controlled with diet and exercise alone. Diabetes Obes Metab. 2017;19(5):721–728.
- Rosenstock J, Frias J, Pall D, et al. Effect of ertugliflozin on glucose control, body weight, blood pressure and bone density in type 2 diabetes mellitus inadequately controlled on metformin monotherapy (VERTIS MET). Diabetes Obes Metab. 2018;20(3):520–529.
- Pratley RE, Eldor R, Raji A, et al. Ertugliflozin plus sitagliptin versus either individual agent over 52 weeks in patients with type 2 diabetes mellitus inadequately controlled with metformin: the VERTIS FACTORIAL randomized trial. Diabetes Obes Metab. 2018;20(5):1111–1120.
- Miller S, Krumins T, Zhou H, et al. Ertugliflozin and sitagliptin co-initiation in patients with type 2 diabetes: The VERTIS SITA randomized study. Diabetes Ther. 2018;9(1):253–268.
- Dagogo-Jack S, Liu J, Eldor R, et al. Efficacy and safety of the addition of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sitagliptin: the VERTIS SITA2 placebo-controlled randomized study. Diabetes Obes Metab. 2018;20(3):530–540.
- Grunberger G, Camp S, Johnson J, et al. Ertugliflozin in patients with stage 3 chronic kidney disease and type 2 diabetes mellitus: the VERTIS RENAL randomized study. Diabetes Ther. 2018;9(1):49–66.
- Hollander P, Liu J, Hill J, et al. Ertugliflozin compared with glimepiride in patients with type 2 diabetes mellitus inadequately controlled on metformin: the VERTIS SU randomized study. Diabetes Ther. 2018;9(1):193–207.
- Liu J, Tarasenko L, Terra SG, et al. Efficacy of ertugliflozin in monotherapy or combination therapy in patients with type 2 diabetes: a pooled analysis of placebo-controlled studies. Diab Vasc Dis Res. 2019;16(6):415–423.
- Fryar CD, Ostchega Y, Hales CM, et al. Hypertension prevalence and control among adults: United States, 2015–2016. NCHS Data Brief. 2017;(289):1–8.
- Lindhorst J, Alexander N, Blignaut J, et al. Differences in hypertension between blacks and whites: an overview. Cardiovasc J Afr. 2007;18(4):241–247.
- Carnethon MR, Pu J, Howard G, et al. Cardiovascular health in African Americans: a scientific statement from the American Heart Association. Circulation. 2017;136(21):e393–e423.
- Sahasrabudhe V, Terra SG, Hickman A, et al. The effect of renal impairment on the pharmacokinetics and pharmacodynamics of ertugliflozin in subjects with type 2 diabetes mellitus. J Clin Pharmacol. 2017;57(11):1432–1443.
- Sahasrabudhe V, Fediuk DJ, Matschke K, et al. Effect of food on the pharmacokinetics of ertugliflozin and its fixed-dose combinations ertugliflozin/sitagliptin and ertugliflozin/metformin. Clin Pharmacol Drug Dev. 2019;8(5):619–627.
- Dawra VK, Cutler DL, Zhou S, et al. Assessment of the drug interaction potential of ertugliflozin with sitagliptin, metformin, glimepiride, or simvastatin in healthy subjects. Clin Pharmacol Drug Dev. 2019;8(3):314–325.
- Miao Z, Nucci G, Amin N, et al. Pharmacokinetics, metabolism, and excretion of the antidiabetic agent ertugliflozin (PF-04971729) in healthy male subjects. Drug Metab Dispos. 2013;41(2):445–456.
- Liang Y, Sahasrabudhe V, Tensfeldt T, et al. Meta-analysis of non-compartmental pharmacokinetic parameters to evaluate the effect of UGT1A9 polymorphism on ertugliflozin exposure. J Pharmacokinet Phar. 2018;45(S1):W-066.
- Li Y, Mu Y, Shi H, et al. Pharmacokinetic properties of single and multiple doses of ertugliflozin, a selective inhibitor of SGLT2, in healthy Chinese subjects. Clin Pharmacol Drug Dev. 2019;9(1):97–106.
- Li Y, Nucci G, Yamamoto Y, et al. Comparison of pharmacokinetic (PK) and pharmacodynamic (PD) Results of ertugliflozin (ERTU) between Japanese and western healthy subjects. Clin Pharmacol Ther. 2019;105:S1.
- Ji L, Han P, Liu Y, et al. Canagliflozin in Asian patients with type 2 diabetes on metformin alone or metformin in combination with sulphonylurea. Diabetes Obes Metab. 2015;17(1):23–31.
- Dave CV, Schneeweiss S, Patorno E. Comparative risk of genital infections associated with sodium–glucose co-transporter-2 inhibitors. Diabetes Obes Metab. 2019;21(2):434–438.
- Cannon CP, McGuire DK, Pratley R, et al. Design and baseline characteristics of the eValuation of ERTugliflozin effIcacy and Safety CardioVascular outcomes trial (VERTIS-CV). Am Heart J. 2018;206:11–23.
- Liu J, Patel S, Cater NB, et al. Efficacy and safety of ertugliflozin in East/Southeast Asian patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2020;22(4):574–582.