?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective: Autoinjectors are a convenient and efficient way to self-administer subcutaneous injections of biopharmaceuticals. Differences in device mechanical design can affect the autoinjector functionality and performance. This study investigates the performance differences of two single-spring-actuated autoinjectors.
Methods: We compare the performance between Emgality (120 mg/mL) and Aimovig (140 mg/mL) autoinjector devices from an engineering point of view at two test conditions: room (25 C) and storage (5 C
) temperatures. We employ a novel experimental procedure to simultaneously acquire the force and acoustic signals during operation, and high-speed imaging during the needle insertion and drug injection.
Results: We perform 18 quantitative comparisons between Emgality and Aimovig, and we observe that 14 of these have statistically significant differences. For both test conditions, Emgality requires an 8 N activation force while Aimovig requires 14 N activation force, and the needle of Emgality has an insertion depth of 5 mm while Aimovig has an insertion depth of 7 mm. The injection speeds are significantly affected by temperature. Emgality has an injection speed of 0.40 mL/s and 0.28 mL/s at room and storage temperature condition, respectively; while Aimovig has an injection speed of 0.24 mL/s and 0.16 mL/s at those conditions. Lastly, confirmation “click” sound of Emgality occurs 0.75–1.53 s after dose completion, while in Aimovig, the confirmation “click” sound occurs 0.26-0.46 s before dose completion.
Conclusions: This study revealed performance differences between Emgality and Aimovig autoinjector devices, despite the fact that the delivery principle of these single-spring-actuated autoinjectors are the same. These differences may result in different risk of intramuscular injection and premature device removal, both of which need to be further verified in clinical trials.
1. Introduction
Drug delivery by subcutaneous (SQ) injection is an effective alternative to intravenous delivery for protein-based productsCitation1. Self-administered autoinjectors provide convenience to users as a cost-efficient way for subcutaneous injection of biopharmaceuticals. Traditionally, the injection costs at hospitals or infusion centers constitute ∼50% of the total treatment costCitation2.
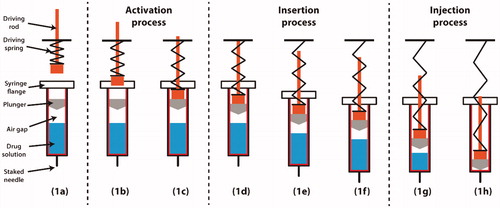
shows a typical configuration of a single-spring-actuated autoinjector. The glass syringe is prefilled with the drug product, with a plunger (elastomeric ‘stopper’ or piston) inside and a stainless-steel needle secured at the bottom. An air gap or bubble exists in the syringe due to the manufacturing process. In the vertical, tip-down orientation shown below, the air gap is located between the plunger and the drug product. A plastic driving rod is loaded using a pre-compressed spring and set on top of the syringe. Upon actuation (), the driving rod is accelerated due to the release of the spring energy and moves downwards to impact the plunger. When the driving rod impacts the plunger, the plunger experiences a sudden acceleration. This large impulse force can exceed the static friction force between the plunger and syringe wall, resulting in sliding motion between the plunger and syringe, and compression of the air gap. The needle insertion process (), which takes place over a few milliseconds, starts when the friction force between the plunger and the syringe wall accelerates the syringe, and the pressure builds up inside the syringe. At the designed insertion depth, the syringe is stopped by a barrier at the flange or shoulder of the syringe. After the syringe reaches the target position, the driving rod and the plunger are pushed downwards by the remaining spring force, and the drug product is dispensed through the needle into the subcutaneous tissue. The injection process () is completed when the plunger reaches the bottom of the syringe barrel. Upon completion of the injection, a confirmation “click” sound is made to indicate the completion of dose delivery. After injection completion, the needle is either retracted from the skin in some devices or covered by a safety guard in others (after the needle is manually pulled out from the skin) to prevent needle stick injuries. Available autoinjector in the market may also consist of two driving springs. For such autoinjectors (e.g. Emerade), one driving spring is used for needle insertion, while another spring is used for pushing the plunger and deposition of the medication. In this study, we focus on the single-spring-actuated autoinjector as it is the most popular design on the market owing to its simple mechanisms and low cost (e.g. TALTZ, Enbrel, Humira, EpiPen).
Figure 1. Typical operation procedure of a spring-actuated autoinjector. (a) Configuration and terminology. (b) Driving rod accelerated by a pre-compressed spring. (c) Driving rod impact onto the plunger. (d) Sliding motion of plunger and air gap compression. (e) Needle insertion into the skin. (f) Completion of needle insertion. (g) Injection of drug product. (h) Completion of drug delivery.
Many design parameters of single-spring-actuated autoinjectors have been previously analyzedCitation3–5. It has been shown that a stronger spring helps shorten the injection duration but increases the probability of syringe breakage and cavitation in the liquidCitation3, which may lead to device failure and protein aggregationCitation6. Meanwhile, studies on user preference and experiences of subcutaneous injection using prefilled syringes also have been carried outCitation7,Citation8. Guerlain et al.Citation9 studied the usability and patient preference of four epinephrine autoinjectors and found that the design aspects of autoinjector may affect the correctness of autoinjector usage. Hey-Hadavi et al.Citation10 compared the disposable pen injector with a reusable pen injector for the administration of recombinant human growth hormone (rhGH). They concluded that both devices have similar performance, while nearly two-thirds of patients preferred disposable autoinjectors. Xiao et al.Citation11 utilized a motion capture system to track needle tip movement. They analysed the performance of autoinjector during self-administration, based on which they found that the autoinjector is easy to use even for rheumatoid arthritis patients. Schneider and LangeCitation12 measured the injection forces of three autoinjectors and concluded that although mechanical testing revealed significant differences between these three, these differences were not directly perceived by users regarding ease of injection in simulated injection studies. Brand-Schieber et al.Citation13 developed an autoinjector (DFN-11) to deliver 3 mg sumatriptan and reported that patients made no mistake and had no difficulty of operating such an autoinjector.
Although the working principles of the single-spring-actuated, routinely used autoinjectors available in the market are similarCitation14,Citation15, their design of the mechanical mechanism do vary. Such mechanical differences may result in different user experiences. A detailed comparison of such differences between autoinjectors from an engineering perspective and their effect on user experience is not established and would be valuable for any future autoinjector designs and improvements.
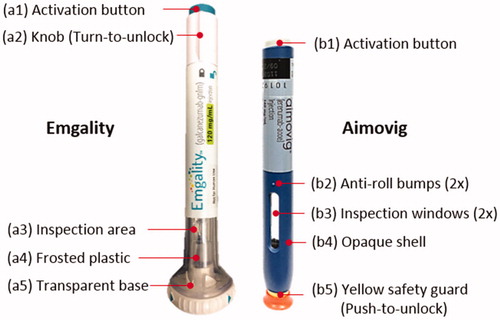
To address this gap, we compare two types of autoinjector devices (), Emgality (galcanezumab-gnlm, Eli Lilly and Company) and Aimovig (erenumab-aooe, Amgen Inc.). Both Emgality and Aimovig are recently-marketed calcitonin gene-related peptide (CGRP) antagonists for migraine prevention. Since such drug requires routine injection, both companies launched self-administered, spring-actuated, prefilled single-dose disposable autoinjectors. Emgality leverages an Eli Lilly designed device, while Aimovig uses the “SureClick” device designed from SHL Group. As mentioned above, the goal of this study is to reveal the performance difference of autoinjectors due to different mechanical designs. To make the comparison meaningful, it is necessary to select autoinjectors that have similar drug type, drug concentration, injection volume, and design principles while still have inherently distinct mechanical designs. Emgality and Aimovig meet those requirements and therefore offer an appropriate case and the opportunity to conduct this study. (Note that both drugs are also available in prefilled syringes, which is outside of the scope of this study).
2. Research design and methods
2.1. Materials and test matrix
The Emgality devices were assembled at Eli Lilly using commercial glass syringes filled with Emgality drug product (120 mg/mL, Lot. # IWS21082019) approximately 30 months before the device testing. For the entire shelf life, these devices were kept at standard 2–8 °C storage conditions. The Aimovig devices (Lot. #1103650 with an expiration date of September 2020) were obtained from the market. The Aimovig devices were kept at 2–8 °C storage conditions for the entire shelf life prior to this study. Both sets of devices were stored in the same refrigerator at 2–8 °C before testing. All tests were performed in September and October 2019, at both room temperature (∼25 °C) and storage temperature (∼5 °C) as shown in . For room temperature testing, the autoinjectors were taken out from the refrigerator in a batch and placed at room temperature for at least 2 h to allow full warm-up, since Instructions For User (IFU)Citation16,Citation17 of Emgality and Aimovig both indicate the device should be out of the fridge for at least 30 min before dosing. Yet, in some cases, users do not allow for enough time for the device to warm-up before drug administration. We accounted for this scenario by testing samples at storage temperature, i.e. the autoinjectors were taken out from the refrigerator one at a time and tested within 3 min. Testing at storage temperature corresponds to the worst-case scenario from an autoinjector performance standpoint.
Table 1. Samples information and test matrix.
Spring stiffness, drug product viscosity, drug product density, and needle dimensions were quantified. Springs from five samples (randomly selected after injection test) were taken out and compressed against a precision scale (1 gr resolution, Model V11P6, Chaus) using a manual translational micro-stage (0.01-inch resolution, Model PT1, Thorlabs) to measure the spring stiffness. We also measured the viscosity of both drugs at both temperature conditions using a viscometer (2% of reading accuracy, m-VROC, RheoSense). Viscosity measurements were repeated five times. The weight of each autoinjector before and after firing was measured to calculate the amount of drug released. Given the volume of the drug product in each autoinjector is 1 mL, the density of drug products is then calculated. Spring stiffness, viscosity, and additional experimental parameters are listed in .
2.2. Experimental setup
A photo and a schematic drawing of the experimental setup are shown in ,b). Two sets of specially designed fixtures, consisting of an upper housing and two bottom plates, were designed and 3D-printed in-house: one for Aimaovig device and one for Emgality device. The top housing was bolted onto an optical post (410-RC, Newport) to secure the position of the autoinjector. At the same time, two bottom plates were used to elevate the autoinjector device from the optical table. The bottom plates also kept Aimovig device unlocked by pushing against the yellow safety guard. The Integrated Electronics Piezo-Electric (IEPE) array microphone was mounted separately next to the autoinjector (∼30 mm distance). The load cell was mounted on a magnetic track linear stage and faced towards the activation button on the autoinjector device. An LED light source and a light diffuser were used to achieve uniform background illumination. The total time of autoinjector exposure to the LED light was limited to less than 10 s, and the light was turned off manually after each testing to avoid heating of the drug product. A high-speed camera was positioned in front of the sample and synchronized via a delay generator. The delay generator and data acquisitions of load cell and microphone were synchronized by a modular data acquisition (DAQ) system via the Data Acquisition Toolbox in MATLAB (R2018b, MathWorks, Inc.). The microphone and the load cell were sampled at 10,240 Hz (). The trigger signal () was also sent to a high-speed camera to capture the insertion and injection processes, respectively (see supplementary document for details). While the audio, force, and video sequences were captured, the load cell carried by the linear stage started moving towards the activation button on the unlocked autoinjector device at 5 mm/s (). After the device activation, the linear stage retracted back to its initial position. All tests were conducted with injections made into air in order to allow for optical access. The details of the testing instrumentation are listed in . All the testing instrumentations are calibrated and carefully tested before each experiment.
Figure 3. (a) Photo of the test section. (b) Schematic drawing of the experimental setup. (c) Synchronization of the testing instrument. The arrows are coded in chronological order.
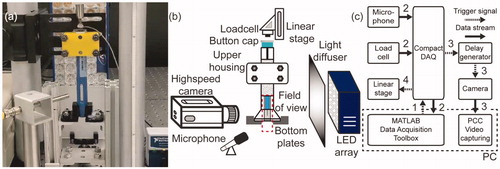
Figure 4. (a) and (c) are post-processed video sequences of the needle insertion process for Emgality and Aimovig devices, respectively. The time step between adjacent images is 0.25 ms. (b) and (d) are post-processed video sequences of the drug injection process at room temperature for Emgality and Aimovig devices, respectively. The time step between adjacent images is 500 ms.
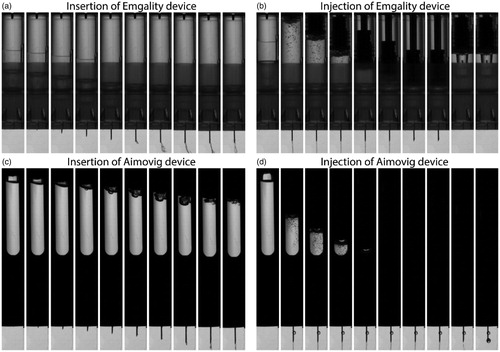
Table 2. Details of the testing instrument.
2.3. Sample video sequences and image processing methods
The insertion process was recorded by the high-speed camera at 20,000 frames per second (fps). The drug injection process was captured at a frame rate of 200 fps. The image resolution was calibrated to be 43.1 μm/pixel. Example video sequences are shown in .
The video sequences were analyzed using an in-house image processing toolkit implemented in MATLAB (see supplementary document for details). The image processing and tracking code have been extensively tested and carefully examined for accuracy and consistency. A list of all measured quantities from the load cell, microphone, and image analysis is shown in , along with their estimated measurement uncertainties. These quantities explain the primary kinematic performance of an autoinjector.
Table 3. List of measured quantities.
3. Results
We report nine quantitative measurement results at two test conditions in this section. Specifically, appearance and safety features are presented in Section 3.1. Measurements of the activation force are described in Section 3.2. The needle kinematics (lateral movement and insertion depth) during insertion are presented in Section 3.4. Results for the drug injection process (injection speed and duration) are presented in Section 3.5. Findings related to confirmation “click” sound measurements to evaluate premature device removal are also presented in Section 3.5.
Within each test group, the data spread is indicated by the interquartile ranges (IQRs). For all test groups, outliers are identified if they lie outside 1.5 × IQR from the 1st and 3rd quartilesCitation18,Citation19. For all following analysis, the statistical significance between groups is characterized by the p-values from Tukey–Kramer testsCitation20, which are shown between room temperature and storage temperature, and between Emgality and Aimovig devices in the boxplots (–Citation8) for reader’s reference.
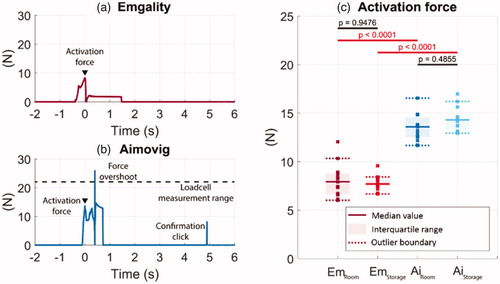
Figure 5. Example force profiles at the activation button from one tested sample during the autoinjector operation for (a) Emgality and (b) Aimovig devices at room temperature. The dashed line in (b) indicates the load cell measurement range. (c) Activation forces measured for all samples. In (c), solid lines indicate the group medians, shaded regions indicate the interquartile ranges, and the dashed lines indicate the data range (excluding outliers). p-Values are listed in c to represent the statistical significance between groups, and the red color (online version only) represents p-value smaller than the significant level, .0001.
3.1. Before activation: design features
3.1.1. Drug inspection and needle concealment
The syringe is made visible in both devices to allow users to inspect the drug product before injection for precipitation, aggregation, or discoloration. The shell of the Emgality device is made of transparent material so that the drug product can be inspected from any angle (∼360°, ). The Aimovig device has an opaque shell (), and two narrow windows (, each ∼33° wide) that allow partial visual inspection. The needle is not directly visible before activation in both devices to help reduce user stress. For an Emgality device, the lower part of the inner shell is frosted () such that view of the needle is obscured, while for an Aimovig device, the needle is concealed by the opaque shell.
3.1.2. Safety feature and injection site inspection
There are unlocking mechanisms as safety features in both devices to prevent unintended activation of drug delivery. For the Emgality device, users need to twist the knob () to unlock the device after needle shield removal, prior to device actuation. To unlock the Aimovig device, the user needs to press the yellow safety guard () against the skin at the chosen injection site after needle shield removal. Besides, the Emgality device has a transparent base (), allowing for a full inspection of the injection site when the device is in contact with the skin.
3.1.3. Anti-roll feature and device stability
Both devices are designed with anti-roll features. For the Emgality device, the autoinjector remains stationary when placed on a flat surface owing to the linear sides of the base and fin on the lock knob (). For the Aimovig device, two bumps on the side () stops the autoinjector from rolling. In addition, the base of the Emgality device also gives ∼10 times larger skin contact area (∼1000 mm2) compared with Aimovig (∼100 mm2). While for the Aimovig device, due to the limited device-skin contact area, the users are instructed to follow the “stretch method” or “pinch method” as described in the user manual (Amgen Inc., 2019, IFU step #2 F) to create an injection site.
3.2. Activation: force profile
As the load cell moves toward the activation button, the contact force increases until it suddenly drops due to device activation. Representative force measurements from the load cell are shown in . The maximum value of the force before activation is, therefore, the device activation force that the user needs to exert on the button. The time that corresponds to this maximum force is referenced as time = 0 s for analysis in the following sections; all other times are measured relative to this activation event. The measured activation forces are shown in with statistics, including median, interquartile range within each group, and p-values between groups. The medians for the Emgality device are below 8 N at both temperature conditions, which are consistently smaller than for Aimovig device with medians of 13 N (room temperature) and 15 N (storage temperature).
The force profiles measured on the Aimovig device consistently exhibit an overshoot right after the sudden force drop due to device activation, as shown in . This short force pulse is possibly a result of the reacting force from the main driving spring. The magnitude of this force overshoot usually exceeds the measurement range of the current load cell (22.2 N, dashed line in ), and therefore, its peak magnitude is not captured reliably.
3.3. Insertion: needle kinematics
From the time sequences, the maximum needle lateral movement, the initial dispensing depth (insertion depth when drug product starts to dispense), the peak penetration depth, and the final penetration depth are compared between test groups, as shown in .
Figure 6. (a) Maximum needle lateral movement, (b) initial dispensing depth, (c) peak penetration depth, and (d) final penetration depth. Solid lines indicate the group medians, shaded regions indicate the interquartile ranges, and the dashed lines indicate the data range (excluding outliers). p-Values are listed in a, b, c, and d to represent the statistical significance between groups, and the red color (online version only) represents p-value smaller than the significant level, .0001.
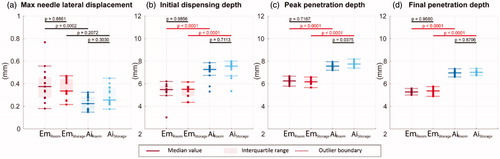
The medians for the maximum needle lateral movement of the Emgality device are about 0.35–0.4 mm at both test conditions, and the medians for Aimovig device are about 0.2–0.25 mm at both test conditions; however, such differences between Emgality and Aimovig are not statistically significant as indicated by p values (.0002 and .2072 for room and storage temperature condition, respectively). The medians of initial dispensing depth for Emgality are around 5.5 mm, while for Aimovig, the medians are around 7.3 mm. Due to material compliance of the syringe carriers, slight penetration overshoots (peak penetration deeper than final penetration) are observed for both devices with small variations within each group (<0.3 mm). For Emgality, the medians of the peak penetration are around 6.2 mm, while for Aimovig, the medians are around 7.6 mm. When compared with the final penetration depths, the relative overshoot for Emgality and Aimovig are around 0.7 and 0.3 mm, respectively. This penetration overshoot can only be captured by high-speed imaging due to its short duration (<2 ms), and its effect on drug delivery is unclear. Both devices show consistent performance on penetration with narrow interquartile ranges (<0.4 mm) for all test groups. Between two devices, Emgality shows shallower final penetration with medians around 5.3 mm compared to Aimovig device with medians around 7.0 mm. Based on the p-values between room temperature and storage temperature for each device, the change in temperature does not alter the needle insertion kinematics for either device.
3.4. Injection: flow rate and injection duration
The comparison of the injection flow rate between test groups is shown in . The change in device temperature significantly affects the injection flow rate. For the Emgality device, the median flow rate is around 0.40 mL/s at room temperature and 0.28 mL/s at storage temperature. For the Aimovig device, the flow rate at room temperature is around 0.24 and 0.16 mL/s at storage temperature. The flow rate variations at both conditions are around 0.02 mL/s for the Emgality device, which is about half the variation values of 0.04 mL/s for the Aimovig device.
Figure 7. (a) Drug injection flow rate. (b) Drug injection duration. (c) The time duration between the device activation and injection completion clicks. Solid lines indicate the group medians, shaded regions indicate the interquartile ranges, and the dashed lines indicate the data range (excluding outliers). p-Values are listed in a, b, and c to represent the statistical significance between groups, and the red color (online version only) represents p-value smaller than the significant level, .0001.
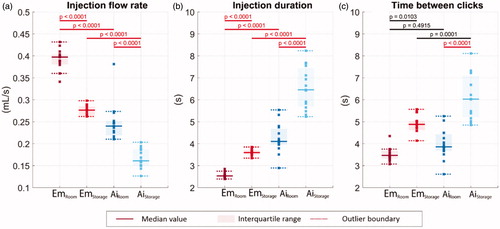
The results of injection duration for all test groups are shown in . The Emgality device is able to complete the drug delivery under 4 s at both test conditions. The increase in injection duration median at storage temperature (3.6 s) is about 40% when it is compared with room temperature (2.5 s). While for Aimovig device, the increase in injection duration median at storage temperature (6.5 s) is about 60% when it is compared with room temperature (4.1 s). For the Aimovig device, the spreads of injection duration are as large as 0.72 and 1.74 s at room and storage temperature, respectively (as indicated by the shaded areas). For the Emgality device, those values are relatively smaller (0.10 and 0.22 s for room and storage temperature, respectively).
Shortly after pressing the activation button, both Emgality and Aimovig devices make an activation “click” sound due to internal part motion, which serves as an audio signal to the users that the device has been activated and the injection has begun. There is also a designed confirmation “click” sound on both devices around injection completion. It serves as a confirmation signal that the device is ready to be removed from the injection site. The time durations between the activation “click” and the confirmation “click” recorded from the microphone are shown in , which shows a qualitative agreement with the injection duration obtained from image analysis shown in . The difference between are quantitatively analysed in Section 3.5.
3.5. Injection completion: needle retraction and post-click
After injection completion, the Emgality device has an automatic mechanism to retract the needle and syringe back into the device to avoid potential post-injection needle stick injuries. For the Aimovig device, there is no auto-retraction of the needle; instead, the yellow safety guard () automatically extends from the device after the user removes the device from the injection site to avoid post-injection needle stick injuries.
Both devices produce a sound to indicate the completion of the injection. For the Emgality device, the retraction of the needle and syringe creates a secondary sound, which happens after the injection is completed. For the Aimovig device, as the plunger approaches the bottom of the syringe barrel, the device generates a confirmation “click” sound. The chronological order in which the activation “click”, true drug product injection completion, and confirmation “click” take place is different for Emgality and Aimovig devices.
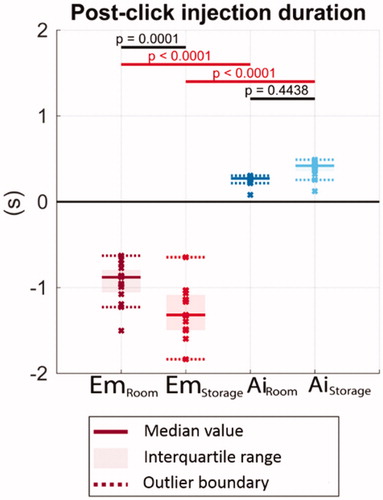
The results of the post-click injection duration (time duration between confirmation “click” and injection completion from high-speed imaging) are shown in . A positive value indicates the “click” is heard prematurely before the dose is entirely delivered. A negative value indicates that the “click” only occurs after delivery completion. At storage temperature, the Emgality device makes confirmation “click” sound about 1.3 s after completion of drug delivery. In contrast, the confirmation “click” sound in the Aimovig device occurs 0.4 s prematurely – before the actual injection completion.
Figure 8. The remaining injection duration after the completion clicks. Solid lines indicate the group medians, shaded regions indicate the interquartile ranges, and the dashed lines indicate the data range (excluding outliers). p-Values are listed to represent the statistical significance between groups, and the red color (online version only) represents p-value smaller than the significant level, .0001.
4. Discussion
4.1. Potential impacts on user experience
The Emgality autoinjector is more stable when placed on the skin as its contact area is ten times larger than Aimovig. In addition, compared to Aimovig’s opaque housing, the transparent nature of the Emgality autoinjector shell allows easier inspection of both the drug product and the injection site, while it still obscures the needle to help reduce needle phobia user stress.
Both devices have consistent performance in terms of activation force at both test conditions, and the users are not likely to experience variations for the force exerted on the activation button between monthly doses. However, for Aimovig users, there exists a sudden overshoot () of the button reacting force upon activation, and the consequence of this force overshoot is unclear. Note that this activation force is far less compared to emergency used autoinjectors, e.g. EpiPen, Auvi-Q. The epinephrine autoinjector is an emergency-use autoinjector to treat Anaphylaxis. Therefore, the user may not be the patient, and it is necessary to have strong spring release mechanism so that the operator/helper could have clear feedback. Furthermore, such autoinjector is required to inject into the muscle layer even through clothing, a firm hold with a strong activation force could increase the insertion depth of the deviceCitation21. However, Emgality/Aimovig are subcutaneous injections and used on a monthly basis, typically at home, and patients usually operate themselves. There is no need for Emgality/Aimovig to have a large activation force for activation.
The lateral needle movement during the insertion of the Emgality device is larger than the Aimovig device while statistically not significant. This might be due to the different supporting structures for the syringe in the device: Emgality uses a syringe clip (with shocker absorber material inside) at the syringe flange to hold the syringe, while Aimovig syringes are supported at the shoulder by a plastic carrier. It is unclear whether this lateral movement during insertion is relevant to the overall injection experience since it happened only within 5 ms. However, after insertion, the needle of the autoinjector device may still have lateral movement during the injection (∼10 s timescale) due to the hand movement of the userCitation11. Such lateral movement during the injection (after insertion), which is not quantified in this study, may have an influence on the pain the user experiences.
A fast injection speed (short injection duration) may reduce the stress the user experiences during self-administration. Emgality has consistently faster injection speed (shorter injection duration) compared to Aimovig at both room and storage temperature test conditions. Since the needle geometry between the two groups is the same, this injection difference is attributed to two factors: (1) Viscosity difference: for Emgality, the viscosity is 4.5 cP at room temperature (5.5 cP at storage temperature); while for Aimovig, the viscosity is 7.4 cP at room temperature (9.1 cP at storage temperature). (2) Spring force difference: for Emgality, the spring stiffness is 205.9 N/m; while for Aimovig, the spring stiffness is 325.4 N/m. We were unable to measure the compression distance of spring inside the device. While given that these two devices have a similar length, same glass syringe dimension, and same filling volume, it is safe to assume they have similar spring compression distance. According to the Poiseuille’s Law (Section S4 in Supplementary Material), the injection speed is linearly increased with the pressure difference (due to spring force) and linearly decreased with viscosity. Therefore, although Aimovig utilized a higher spring force, due in part to the higher viscosity of its drug product, Aimovig still needs 43–50% more time to finish injection, compared to Emgality.
In addition, for consistent user injection experience between doses, a small variation in the injection speed (or injection duration) is desired. Emgality is likely to give a more consistent user experience than Aimovig since the variation of injection speed (or injection duration) in Emgality is one order of magnitude smaller than Aimovig device. As discussed above, the injection speed (or injection duration) mainly depends on viscosity and spring force. As shown in , the variability of viscosity between the two groups is the same, while the variability of spring stiffness in Emgality is one order of magnitude smaller than Aimovig. This variation difference of injection speed (or injection duration) might be due in part to the difference of spring stiffness variability between the two test groups.
The injection speed (or injection duration) is directly related to the injection force, a parameter that has been extensively studied in manual syringes and autoinjectors. Many studies claimed the importance of injection force during autoinjector designsCitation22–25. While the study of insulin autoinjector from Schneider and LangeCitation12 suggested that the mean injection force of autoinjector is less likely to have any influence on the users’ perceived ease of injection. It is unclear if the same conclusion ofCitation12 can be drawn for autoinjector that carries CGRP antagonists, and further investigation is needed to answer this question.
4.2. Risk of intramuscular injection
The drugs of both Emgality and Aimovig are monoclonal antibodies (mAbs) and need to be injected into the subcutaneous (SQ) layer monthly. This is because the SQ layer provides the required path for lymphatic absorption rate and bioavailability, ensuring the approved efficacy of these drugs. On the other hand, the intramuscular (IM) layer, has a greater blood supply and absorbs parental drugs faster compared to the SQ layer, which is not desirable (nor has been tested) for these types of drugs. In contrast, intramuscular epinephrine autoinjectors used for treating anaphylaxis require a fast-acting delivery. The opposite is needed for these drugsCitation26,Citation27. Therefore, it is undesirable for Emgality and Aimovig to inject into IM layer as IM injection results in shorter duration of the efficacy of these drugs.
To evaluate the risk of inadvertent IM injection of Emgality and Aimovig, the needle’s final penetration depths for these two devices are compared with the reported SQ thickness for users of different body mass indices and injection locations in . For BMI ≥19.4, the data of dermal and SQ thickness are fromCitation17,Citation18. While for SQ and dermal thickness when BMI is within 15 ∼ 19.4, we performed linear extrapolation using SQ and dermal thickness values when BMI is within 19.4 ∼ 24.9 and 25 ∼ 30 fromCitation17,Citation18. This extrapolation is based on the assumption that the dermal thickness is less sensitive over BMI, while SQ thickness linearly increases with BMICitation28,Citation29. In the IFU for both devicesCitation16,Citation17, the users are instructed that the autoinjectors can be used at the back-of-arm, abdomen, and thigh. To ensure the drug product is delivered to the SQ tissue, it is desired for each horizontal line (final penetration depth) to stay within the shaded bar regions without running across the black whiskers, which indicate the variations in the IM-SQ boundary positions. Furthermore, due to larger variation in the IM-SQ boundary compared to the dermal-SQ boundary, it is less risky to have IM injection if the needle tip stays farther from IM-SQ boundary (closer to the dermal-SQ boundary).
Figure 9. Comparison between insertion depth and skin layer thickness. Horizontal solid lines represent the mean final penetration depth at room temperature, and horizontal dash-dotted lines represent the two standard deviations (5–95 percentile range if normal distribution is assumed) of the insertion depth. Lower and upper (shown as Red and Blue in the online version) lines correspond to the Emgality and Aimovig device, respectively. The coloured vertical bars represent the SQ space for a particular BMI range. SQ and dermal thickness for BMI > 19.4 patients are obtained fromCitation30,Citation31. For SQ and dermal thickness when BMI is within 15 ∼ 19.4, we performed linear extrapolation using SQ and dermal thickness values when BMI is within 19.4 ∼ 24.9 and 25 ∼ 30, assuming SQ thickness increases linearly with BMI while dermal thickness is not sensitive to BMI. The 0 mm on the vertical axis represents the skin surface that is in contact with the autoinjector base. The black whiskers at the bottom and top of each bar indicate the variation in dermal-SQ and IM-SQ boundaries for that group, respectively.
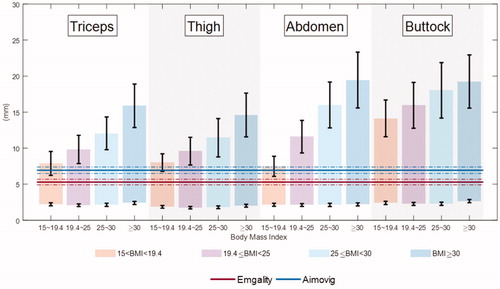
Based on , we found that the needle tip of both Emgality and Aimovig reached the SQ layer during insertion; while the injection depth of Emgality is closer at the dermal-SQ boundary compared to Aimovg device. Furthermore, when BMI the needle tip of the Aimovig device starts to overlap with IM-SQ boundary of Arm, thigh, and abdomen. These suggest that the Aimovig device presents a higher risk of IM injection for BMI
patients at injection sites of Arm, thigh, and abdomen; while the risk of IM injection for the Emgality device is consistently low for all injection sites. The probability of IM injection can be analysedCitation30; however, we do not perform such an analysis herein due to the lack of raw data on SQ layer thickness distribution.
4.3. Risk of premature device removal
There usually exists a confirmation “click” sound in autoinjector devices, informs the patient when the device should be removed from the skin after injection. It is critical to quantify if this confirmation sound “click” happens correctly. If this confirmation sound occurs before the completion of the injection, patients may remove the device prematurely, and this removal is unrecoverable. We refer to this undesired consequence as premature device removal.
Both Emgality and Aimovig generate a “click” sound mechanically to indicate the completion of the injection. indicate that only the Emgality device presents reliable confirmation “click” sound. The Emgality device always makes a “click” sound after the dose completion, while on the opposite, the Aimovig device always makes a “click” sound before the dose completion. Although the time span between the “click” sound and the end of the actual drug delivery is only 0.2–0.4 s, premature device removal might happen with the Aimovig device. The consequence of premature device removal in Aimovig may affect the accuracy of dosing, which demands further clinical investigation.
4.4. Limitations of the current study
There are certain limitations in the current study. First, only two types of autoinjectors for migraine prevention were compared since there existed only these two groups in the market when this study was performed. We noticed a third autoinjector AJOVY for migraine prevention launched on 03/2020; however, the study was completed before this date, and therefore we did not consider Ajovy as an additional test group. Second, only 15 samples were used for each test group due to the difficulty in obtaining enough samples from the market. Although the p-values from Tukey–Kramer tests between test groups showed clear separation between Emgality and Aimovig for most of the measurements, additional samples may give us more confidence on the conclusion that the effect of temperature on needle kinematics and post-click confirmations of both devices is insignificant. Third, only one projection of the needle lateral movement during insertion was captured by the single-viewed camera, the needle lateral movement in the depth direction of the camera view was ignored, further measurement of 3 D needle movement (such as 3 D electromagnetic motion capture systemCitation11) during insertion and injection may be needed to completely quantify the lateral movement of the autoinjector.
Furthermore, all samples in the current study were tested in air and vertically positioned for optical access and simplification, while during actual administration, the device position angle may vary, and the interaction between the device and the user skin may change the performance. This simplification may affect needle kinematics and injection duration. For example, the lateral constraint from the skin may reduce the lateral movement of the needle for both Emgality and Aimovig devices. The tissue friction and elastic deformation may reduce insertion velocity and penetration depth. The interstitial backpressure at the injection site may reduce the injection flow rate and prolong the injection duration for both devices. Other quantities, such as activation force, “Post-click” confirmation, are not likely to be affected. Nevertheless, future studies may need to incorporate synthetic matrix as an injection substrate, e.g. a gel layer or an injection pad, to simulate the “in vivo” skin conditionCitation11.
Lastly, this study did not involve any patients or users for simulated injections or clinical trials. The claims in Section 4.1 4.2 and 4.3 should be interpreted with further clinical evidence.
5. Conclusions
We compared two types of autoinjectors, Emgality and Aimovig, both of which are used to deliver injectable monoclonal antibody medications for the prevention of migraine headaches. Several kinematic performance differences were found between these two autoinjector devices, and potential effects on the user experience were discussed.
The major findings of this study are depicted in . The Emgality device shows a consistently smaller activation force than the Aimovig device. The Emgality device has a larger lateral needle movement during insertion while statistically insignificant. Both devices start dispensing drug products within 0.5 mm from their designed final injection depths, and the penetration overshoot is roughly about 10–20%. The Emgality device has a shorter injection duration and delivers the full dose within 4 s under both room and storage temperature conditions, while the Aimovig device delivers the full dose within 10 s. While both devices incorporate a confirmation “click” to inform the user of dose completion, the relative timing for the confirmation ‘click’ with respect to actual injection completion is different between these two devices. Emgality device produces dose completion “click” sound up to 1.3 s after full dose delivery, while Aimovig device produces dose completion “click” sound up to 0.4 s before full dose delivery. The difference in performance between room temperature and storage temperature tests revealed that only the injection flow rate and duration are significantly affected for both devices, and the increase in variability at the storage temperature was observed for the Aimovig device.
Figure 10. Overall comparison of performances between test groups. For each test category, bar lengths are normalized by the largest median among the four test groups. Texts in each bar show the median ± 0.5 interquartile range for that test group. The bars for the post-click injection durations for the Emgality device are not shown due to their negative values. p-Values are listed to represent the statistical significance between groups, and the red color (online version only) represents p-value smaller than the significant level, .0001.
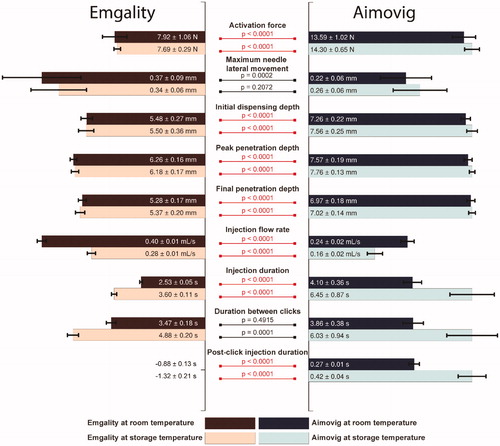
Our measurements and analysis suggest that although the design principle of Emgality and Aimovig are the same, detailed mechanical design differences resulted in seven statically significant kinematic performance differences between the two. These mechanical differences may result in different risks of intramuscular injection and premature device removal. These two consequences, although need to be further verified on clinical trials, may need to be considered in any future design and improvement of autoinjector device.
Transparency
Declaration of funding
This work was sponsored by Eli Lilly and Company.
Declaration of financial/other relationships
TG and JE are research assistants, ZD is a postdoctoral research associate, and AMA and PPV are professors in Mechanical Engineering at Purdue University. JCV, KHD, GHS, and DSC are employees of Eli Lilly and Company. Peer reviewers on this manuscript have received an honorarium from CMRO for their review work but have no other relevant financial relationships to disclose
Author contributions
JCV, KHD, GHS, DSC, AMA, and PPV contributed in the conception and design of the current study. TG, ZD, and JE executed the experiments and data analysis. TG, ZD, JCV, KHD, AMA, and PPV conducted the interpretation of the data and the drafting and revisions of the manuscript. GHS and DSC provided critical inputs for the final version of the paper.
Supplemental Material
Download PDF (492.4 KB)Acknowledgements
No assistance in the preparation of this article is to be declared.
References
- Collins DS, Kourtis LC, Thyagarajapuram NR, et al. Optimizing the bioavailability of subcutaneously administered biotherapeutics through mechanochemical drivers. Pharm Res. 2017;34(10):2000–2011. Springer New York LLC.
- Jones GB, Collins DS, Harrison MW, et al. Subcutaneous drug delivery: an evolving enterprise. Sci Transl Med. 2017;9(405):eaaf9166.
- Veilleux JC, Shepherd JE. Pressure and stress transients in autoinjector devices. Drug Deliv Transl Res. 2018;8(5):1238–1253.
- Cilurzo F, Selmin F, Minghetti P, et al. Injectability evaluation: an open issue. AAPS PharmSciTech. 2011;12(2):604–609.
- Burckbuchler V, Mekhloufi G, Giteau AP, et al. Rheological and syringeability properties of highly concentrated human polyclonal immunoglobulin solutions. Eur J Pharm Biopharm. 2010;76(3):351–356.
- Randolph TW, Schiltz E, Sederstrom D, et al. Do not drop: mechanical shock in vials causes cavitation, protein aggregation, and particle formation. J Pharm. Sci. 2015;104(2):602–611.
- Dammerman R, Mead J. User acceptance of a prefilled auto‐injector device for erenumab in patients with migraine. 61st Annual Science Meet American Headache Society. Philadelphia, PA: Pennsylvania Convention Center; 2019. p. P266LB.
- Stauffer VL, Sides R, Lanteri-Minet M, et al. Comparison between prefilled syringe and autoinjector devices on patient-reported experiences and pharmacokinetics in galcanezumab studies. Patient Prefer Adher. 2018;12:1785–1795.
- Guerlain S, Hugine A, Wang L. A comparison of 4 epinephrine autoinjector delivery systems: usability and patient preference. Ann Allergy Asthma Immunol. 2010;104(2):172–177.
- Hey-Hadavi J, Pleil A, Deeb LC, et al. Ease of use and preference for a new disposable self-injection pen compared with a reusable pen for administering recombinant human growth hormone: A multicenter, 2-Month, single-arm, open-label clinical trial in patient-caregiver dyads. Clin Ther. 2010;32(12):2036–2047.
- Xiao X, Li W, Clawson C, et al. Evaluation of performance, acceptance, and compliance of an auto-injector in healthy and rheumatoid arthritic subjects measured by a motion capture system. Patient Prefer Adher. 2018;12:515–526.
- Schneider AE, Lange J. Pen devices for self-injection: contrasting measured injection force with users’ perceived ease of injection. Expert Opin Drug Deliv. 2018;15(2):115–125.
- Brand-Schieber E, Munjal S, Kumar R, et al. Human factors validation study of 3 mg sumatriptan autoinjector, for migraine patients. Med Devices. 2016;9:131–137.
- French DL, Collins JJ. Advances in parenteral injection devices and aids. In Nema S, Ludwig JD, editors. Pharmaceutical dosage forms-parenteral medications. Boca Raton, FL: CRC Press; 2016. p. 85–99.
- Thompson I, Lange J. Pen and autoinjector drug delivery devices. In Sterile product development. New York, NY: Springer; 2013. p. 331–356.
- Eli Lilly and Company. Highlights of prescribing information for Emgality. 2019. Available from: https://pi.lilly.com/us/emgality-uspi.pdf
- Amgen Inc. Highlights of prescribing information for Aimovig. 2019. Available from: https://www.pi.amgen.com/∼/media/amgen/repositorysites/pi-amgen-com/aimovig/aimovig_pi_hcp_english.ashx
- McGill R, Tukey JW, Larsen WA. Variations of box plots. Am Stat. 1978;32(1):12–16.
- Velleman PF, Hoaglin DC. Applications, basics, and computing of exploratory data analysis. Pacific Grove, CA: Duxbury Press; 1981.
- Kutner MH. Applied linear statistical models. 5th ed. In Nachtsheim C, Neter J, Li W, editors;. New York, NY: McGraw-Hill/Irwin; 2005.
- Frew AJ. What are the “ideal” features of an adrenaline (epinephrine) auto-injector in the treatment of anaphylaxis? Eur J Allergy Clin Immunol. 2011;66(1):15–24.
- Watt RP, Khatri H, Dibble ARG. Injectability as a function of viscosity and dosing materials for subcutaneous administration. Int J Pharm. 2019;554:376–386.
- Van Der Burg T. Injection force of SoloSTAR compared with other disposable insulin pen devices at constant volume flow rates. J Diabetes Sci Technol. 2011;5(1):150–155.
- Clarke A, Spollett G. Dose accuracy and injection force dynamics of a novel disposable insulin pen. Expert Opin Drug Deliv. 2007;4(2):165–174.
- Ignaut DA, Opincar M, Lenox S. FlexPen and KwikPen prefilled insulin devices: a laboratory evaluation of ergonomic and injection force characteristics. J Diabetes Sci Technol. 2008;2(3):533–537.
- Simons FER, Gu X, Simons KJ. Epinephrine absorption in adults: intramuscular versus subcutaneous injection. J Allergy Clin Immunol. 2001;108(5):871–873.
- Dreborg S, Kim L, Tsai G, et al. Epinephrine auto-injector needle lengths: can both subcutaneous and periosteal/intraosseous injection be avoided? Ann Allergy Asthma Immunol. 2018;120(6):648–653.e1.
- Van Mulder TJS, de Koeijer M, Theeten H, et al. High frequency ultrasound to assess skin thickness in healthy adults. Vaccine. 2017;35(14):1810–1815.
- Derraik JGB, Rademaker M, Cutfield WS, et al. Effects of age, gender, BMI, and anatomical site on skin thickness in children and adults with diabetes. PLOS One. 2014;9(1):e86637.
- Hirsch L, Byron K, Gibney M. Intramuscular risk at insulin injection sites-measurement of the distance from skin to muscle and rationale for shorter-length needles for subcutaneous insulin therapy. Diabetes Technol Ther. 2014;16(12):867–873.
- Gibney MA, Arce CH, Byron KJ, et al. Skin and subcutaneous adipose layer thickness in adults with diabetes at sites used for insulin injections: implications for needle length recommendations. Curr Med Res Opin. 2010;26(6):1519–1530.