Abstract
Objective
To quantify the economic burden of postpartum depression (PPD) that accrues to commercially insured households in the year following childbirth.
Methods
Administrative claims data from OptumHealth Care Solutions (2009–2016) were used to identify households that included women identified with PPD per the algorithm and propensity score–matched comparison households of women who were not identified with PPD or a history of depression after childbirth. Study outcomes included direct total all-cause medical and pharmaceutical costs during the first year following childbirth and number of outpatient visits at the household level stratified by household member.
Results
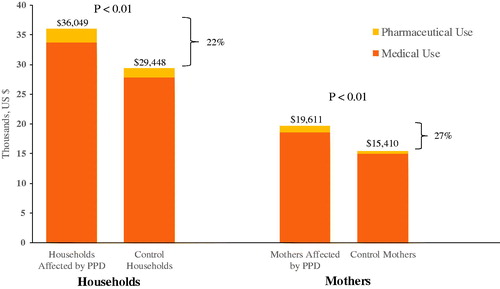
Households affected by PPD as identified by the algorithm (N = 7769) incurred 22% higher mean total all-cause medical and pharmaceutical spending than unaffected matched controls (N = 41,308) during the first year following childbirth ($36,049 versus $29,448, p < 0.01) and an average of 16 more outpatient visits than unaffected households (p < .01). Costs accrued by mothers comprised the largest share (>50%) of total all-cause spending. Mothers identified with PPD had significantly higher annual mean direct total all-cause medical and pharmaceutical spending than their matched controls without PPD ($19,611 versus $15,410, p < .01), driven primarily by an average of 11 more outpatient visits than unaffected mothers (p < .01).
Conclusions
Households affected by PPD as identified by the algorithm incurred higher mean total all-cause medical and pharmaceutical spending during the first year following childbirth than did their matched controls identified without PPD, but not all costs were attributable to maternal treatment for PPD. These findings contribute to a better understanding of the potential economic burden associated with PPD and demonstrated costs may extend beyond the mother to members of the household.
Introduction
Postpartum depression (PPD), a common but underdiagnosed medical complication of childbirthCitation1,Citation2, is a serious disorder that is characterized by a variety of symptoms that can include depressed mood, anhedonia, anxiety, irritability, sleep disturbance, feeling overwhelmed, and thoughts of self-harm or suicidal ideationCitation3. Timing of depression onset that qualifies as a “postpartum episode” and duration of the “postpartum period” differs greatly among experts and sources (e.g. onset during pregnancy to up to 4 weeks postpartum reported by the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition [DSM-5]Citation4 to any depressive disorder within the first postpartum year reported by the World Health Organization [WHO])Citation1,Citation5–8. The prevalence estimates for depression symptoms among women queried between 2 and 9 months postpartum were 9.7%-23.5% (average of 13.2%) based on an analysis of the Pregnancy Risk Assessment Monitoring System (PRAMS) data from 31 sites by researchers at the Centers for Disease Control (CDC)Citation9. Additionally, among 16 continuously reporting sites, a small but statistically significant increase in depression diagnosis (0.22%) was observed from 2012 to 2018. Notably, these estimates include women who may have experienced depression before or during pregnancy, but were counted as cases of PPD due to the presence of depressive symptoms during this postpartum window. Untreated PPD may have lasting adverse health consequences for new mothers and their families; in some studies, less than half of the women suffering from this condition were diagnosed and received treatmentCitation10,Citation11. It is estimated, that approximately 3–5% of women with PPD experience remission of their symptoms, indicating nearly 95% of women with PPD are not successfully treatedCitation11. Consequences associated with depression during the postnatal period (variably defined) include lack of attachment and bonding with the infantCitation2,Citation12,Citation13, significant impairment in maternal function including breastfeedingCitation14,Citation15, maternal health issuesCitation16, increased risk of unintentional injury for the infantCitation17, emotional and behavioral issues that emerge or persist during childhoodCitation18,Citation19, delays in the child’s cognitive and language developmentCitation20, and depressive symptoms for the partnerCitation21–23. Additionally, the presence of maternal PPD is associated with higher health resource utilization and economic burden among children of mothers with PPDCitation24.
While the individual and familial psychosocial and developmental consequences of PPD are clear, there is currently limited information about the economic impact of PPD. A recent research based on a cost-of-illness model found that the economic burden of perinatal mood and anxiety disorders in the US was high, with untreated disorders costing about $14 billion for the 2017 birth cohort from conception to 5 years postpartumCitation25. While this research combined published estimates of direct healthcare costs and indirect societal costs, it focused only on longer term economic burden of untreated perinatal mood and anxiety disorders. There remains little information regarding the economic impact of treated or untreated PPD on households immediately after childbirth and during the child’s first year of life when consequences are likely to impact the family and societyCitation26,Citation27. A small prospective cohort study conducted in 2001 in women initially hospitalized for childbirth in Minnesota and subsequently interviewed by phone at weeks 5 and 11 postpartum found that women with depression incurred 90% higher health services expenditures than women without depressionCitation28.
The purpose of the current study is to estimate the economic burden of PPD that accrued to households in the year following childbirth. The economic burden is measured by direct healthcare costs captured by individual-level administrative claims. The nature of the administrative claims healthcare data allows for evaluation of costs separately for each household member covered under the same insurance plan. We hypothesized that households affected by maternal PPD would have substantially higher spending when compared to matched, unaffected households. These analyses provide a detailed picture of the economic burden of illness not only for the mother affected by PPD but also the burden of illness borne by the household.
Methods
Study design and data source
This was a retrospective, observational cohort study based on US administrative claims data from OptumHealth Care Solutions, Inc. database. The data used in this study are from de-identified medical and pharmacy insurance claims spanning 1 January 2009 to 31 March 2016. The direct healthcare cost data include information on medical diagnoses and procedures, payment amounts, pharmaceutical use and costs, inpatient and outpatient visits, and patient demographics including age, health status, and size and composition of the household. Administrative claims are a readily available, accurate data source which shows payer paid amounts directly relevant to a large sample of commercially insured patients. All analyses were performed with SAS Enterprise Guide, Version 7.1 (SAS Institute, Cary, NC) and R (R version 3.4.3, RStudio version 1.0.143) was used in the propensity score matching process. Institutional Review Board approval to conduct this study was not necessary as this study used only de-identified administrative insurance claims.
Sample selection and construction
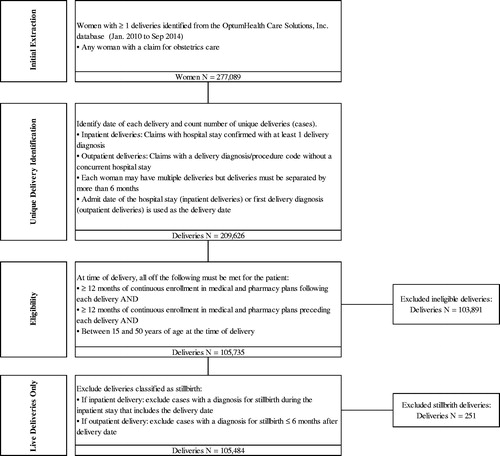
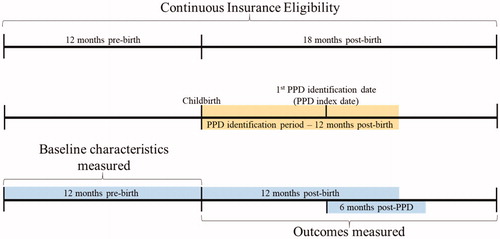
Eligibility criteria were the following: women aged 15–50 years who had a childbirth event (at least one inpatient medical claim with a diagnosis code or procedure code for a delivery, excluding stillbirths) during the period of 1 January 2010 through 30 September 2014 (); continuous health plan enrollment for 12 months before and 18 months after childbirth (). In this analysis, a PPD case identification algorithm was developed based on a combination of International Classification of Diseases, Clinical Modification, Ninth Revision (ICD-9-CM) diagnosis and procedure codes, National Drug Codes (NDCs), and Current Procedural Terminology (CPT) codes associated with depression and other mood disorders available in administrative claims records. Specifically, in the 12 months following childbirth, women with any of the following combinations of claims were identified as having PPD ():
Figure 3. Postpartum depression qualifying diagnoses and treatment combinations. *As is standard practice, at least two distinct outpatient visits were required when relying on outpatient visits alone as qualifying criteria. This requirement aims to reduce the potential for misidentifying patients based on the occasional erroneous inclusions of rule-out diagnoses on outpatient visit claims.
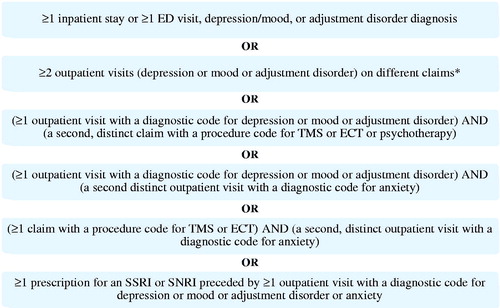
≥1 inpatient stay (IP) or ≥1 emergency department (ED) visit with a depression, mood, or adjustment disorder (pre-specified diagnoses)
≥2 distinct outpatient (OP) visits with pre-specified diagnoses
≥1 OP visit with pre-specified diagnoses and an additional claim with a procedure code for brain stimulation therapy or psychotherapy
≥1 OP visit with pre-specified diagnoses and an additional distinct OP visit with a diagnostic code for anxiety
≥1 claim with a procedure code for brain stimulation therapy and a second, distinct OP visit with a diagnostic code for anxiety
≥1 prescription for an SSRI or SNRI preceded by ≥1 OP visit with pre-specified diagnoses or anxiety
Mood and adjustment disorders were included to ensure a broad range of depressive symptoms were captured in the identification algorithm, as these may be underreported in claims databases (see Supplemental Appendix Table 1). Mood and adjustment disorders have been included in the criteria of other studies of PPD using administrative claims databasesCitation29–31. SSRI and SNRI prescription claims were used as additional criteria to identify PPD only if preceded by at least 1 OP visit with depression, mood or adjustment disorder, or anxiety diagnoses codes, as they are often used as first-line treatment for patients presenting with depression as a primary symptomCitation32–34. Additional antidepressants (TCAs and acyclics) and anxiolytics were included in estimates of PPD specific costs, but were not used for case identification (see Supplemental Appendix Table 3). Similar enrollment criteria and the same PPD case identification algorithm was used in a recent matched cohort study of healthcare resource utilization and costsCitation24. Cases of PPD identified by the algorithm were followed for an additional 6 months after their diagnosis date to collect associated costs, however, these results are not reported in this study. Notably, women with depression in the 12 months prior to pregnancy or during pregnancy could be included in the PPD group as women with a history of depression are at greater risk for developing PPDCitation35,Citation36. Evidence of bipolar disorder, schizophrenia, schizoaffective disorder, psychosis, other psychosis, or the filling of an antipsychotic drug prescription before childbirth were grounds for exclusion as patients with these diagnoses are at elevated risk for postpartum psychosis, a costlier condition than PPD, which could bias results.
Requiring continuous health plan enrollment for 12 months prior to childbirth reflects standard practice in claims analyses. Additionally, using 12 months prior to childbirth allows for the analysis to account for a mother’s and household’s characteristics and health spending beyond just those recorded during pregnancy and may more accurately capture annual levels of health spending at the household level.
Combinations of health resource use that qualified as identifiers of PPD are shown in .
The PPD index date was the date of the first insurance claim used to identify PPD.
Propensity score matching
To evaluate direct medical spending associated with PPD, a matched control group was developed. The matching algorithm used to select the matched control group followed an approach used to measure the societal burden of major depressive disorderCitation37 and used methodological tools appropriate for causal inferenceCitation38. Related literature also informed the matching approachCitation39–42.
The eligible prematched control cohort included all women who gave birth during the study data period and met the continuous enrollment criteria but did not include those women who met the criteria for PPD and those who had been diagnosed with or treated for depression, anxiety, or adjustment disorders in the year before childbirth.
To create the matched control cohort, 6-to-1 matching without replacement was conducted on the basis of a variety of underlying individual- and household-level characteristics (). Exact matches were required for insurance enrollment type (primary vs. secondary holder), the availability of work-loss data, region, and household composition (partner and/or infant covered on the same plan). Additionally, matched controls could differ from mothers in the PPD cohort in household-level non-mental healthcare spending by at most 1% of the standard deviation of its log transformation. The propensity score was then used to select “nearest neighbors” controls from the full set of potential controls for each woman in the PPD cohort. If 6 or fewer women in the control group met the matching criteria, then all potential controls were selected for the control cohort. Women in the PPD group for whom no match was identified (n = 137) were not included in the final PPD cohort for analysis. Excluded households differ from included households in that excluded households experienced exceptionally high non-mental healthcare spending largely associated with chronic conditions in the year prior to birth.
Table 1. Individual and household characteristics for matching.
All medical costs were adjusted for inflation and expressed in 2016 dollars.
Direct cost estimation
Total healthcare spending for both households affected by PPD and households not affected by PPD one year post childbirth was based on total all-cause medical and pharmaceutical costs paid by the insurer for the first year after childbirth.
Other considerations for direct cost estimation were the following: (1) cost stratification to distinguish direct medical spending for the care of the mother vs direct costs for the care of other household members and; (2) all-cause medical and pharmaceutical spending by month after childbirth and by month after the PPD index date for affected households and the control cohort.
Spending directly related to PPD via related diagnoses or treatments was also investigated. PPD related diagnoses or treatments, medical services, or pharmacologic therapy are defined in Supplemental Appendix A. Non-zero PPD-related medical and pharmaceutical costs were calculated for some households not affected by PPD because mothers in these households accrued costs associated with the same PPD-related diagnoses, medical services, or pharmacologic therapy but did not have the combination of diagnoses and healthcare resource use to be identified as having PPD by the algorithm.
Statistical analyses
Descriptive analyses included difference-in-mean estimates between households affected by PPD and the control cohort for direct total all-cause healthcare cost and underlying covariates. The underlying framework, specifically the propensity score–matching algorithm, assumed that, conditioning the baseline covariates described above, non-PPD–related direct all-cause medical and pharmaceutical costs for household affected by PPD and control cohorts are independent of PPD identification; thus, any difference in outcomes can be attributed to the identification of PPD.
Results
Baseline characteristics
After the matching process, the resulting sample was comprised of 7769 households affected by PPD matched to 41,308 unaffected control households. Baseline characteristics of mothers before and after matching are given in . The average time from childbirth to PPD identification was approximately 4 months. This represents the first time following childbirth that the mother is identified as having PPD based on the established algorithm and may not reflect the first depression diagnosis ever for the mother. Of the mothers identified with PPD during the first year after childbirth, 50% were identified during the first 3 months after childbirth (). Approximately 48% (3704) of mothers identified as having PPD in the analysis had evidence of depression in the 12 month period prior to childbirth.
Figure 4. Time from Childbirth to Postpartum Depression Diagnosis or Possible Treatmenta,b. aFrequency and cumulative distribution function (CDF) of number of weeks to PPD identification for mothers affected by PPD. No diagnoses in the first two weeks following childbirth. PPD identification begins in week 3 following childbirth. bAny point (week) on the CDF represents the fraction of mothers who have been identified as being affected with PPD by this week following childbirth. Percentages are out of 7769 mothers identified as being affected with PPD at 1 year following childbirth.
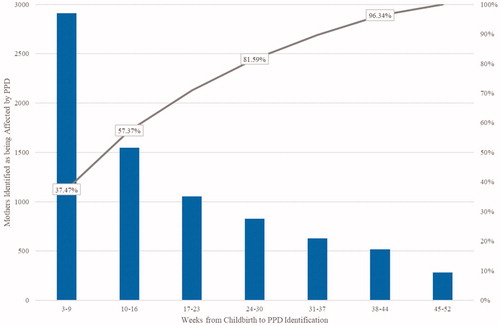
Table 2. Baseline characteristics before and after matching.
Costs and resource use
Household costs
In the first year after delivery, households affected by PPD experienced 22% higher levels of total all-cause medical and pharmaceutical insurer spending ($36,049 vs. $29,448 p < .01) than their matched controls (). PPD-related spending accounted for approximately 21% of the difference in total all-cause medical and pharmaceutical spending between households affected by PPD and the control cohort. Medical spending accounted for approximately 89% of the difference in total all-cause spending between the two groups; pharmaceutical spending comprised the remaining 11%. In part, higher total costs among households affected by PPD were driven by more all-cause outpatient visits on average in the PPD cohort compared with the control cohort 1 year after delivery (57 vs. 41, p < .01).
Mothers’ costs
Of the individuals within the evaluated households, mothers had the largest share (>50%) of total all-cause spending. Similar to the household-level analysis, mothers affected by PPD incurred higher total all-cause medical and pharmaceutical insurer spending than the matched cohort of mothers not affected by PPD at 1 year after delivery ($19,611 vs. $15,410 p < .01) (). Approximately 31% of the difference in total all-cause spending between mothers affected by PPD and their matched controls was accounted for by PPD-related medical or pharmaceutical spending.
Mothers affected by PPD had higher levels of total all-cause spending in each of the 12 months following childbirth than did matched control mothers unaffected by PPD. More than 95% of the costs in the first month are accrued during the first week after childbirth for mothers affected and unaffected by PPD and primarily reflect costs associated with delivery.
Infants’ costs and other dependent children’s costs
In evaluating the difference in costs borne by infants (n = 6293), the analysis found no statistically significant difference in average total all-cause medical and pharmaceutical costs between infants in households affected by PPD and their matched controls. On average, total all-cause medical and pharmaceutical costs for infants in households affected by PPD and the control cohort did not differ significantly over the first year after delivery ($10,662 vs. $9,461, p = .16).
Average per-child total all-cause spending for other dependent children of mothers affected by PPD was not significantly greater than the average per-child spending in the matched control group ($2243 vs. $1889, p = .10).
Partners’ costs
When costs incurred by the partners (n = 5280) in households affected by PPD and the control cohort were evaluated, there were significant differences in average total all-cause medical and pharmaceutical insurer spending and inpatient and outpatient visits during the first year following childbirth. The average total all-cause costs for partners in households affected by PPD were $3483, whereas those of the matched controls was $2663 (p < .01). Approximately 30% higher insurer spending was associated with partners in households affected by PPD.
Healthcare resource utilization
Households affected by PPD had significantly more outpatient visits and inpatient days than their matched controls in the year after delivery. PPD-affected households made an average of 57 outpatient visits, whereas matched controls made an average of 41 outpatient visits in the year after childbirth (p < .01). The average number of inpatients days during the year after childbirth was 7 for PPD-affected households and 6 for matched controls (p < .01). Mothers affected by PPD had an average of 11 more outpatient visits per year after childbirth than did matched controls.
Discussion
Our study estimated the effect of PPD as identified by the defined algorithm on healthcare insurer spending, suggesting that households of mothers affected by PPD faced significantly higher average health costs than the households of mothers unaffected by PPD in the first year following childbirth. Our findings represent a unique contribution to the evidence base on the burden of PPD since healthcare costs attributed to PPD have not been previously studied in a comprehensive manner, especially those that might be attributed to the partner and other dependent children in the household. Recent literature has noted that the economic burden of perinatal mood and anxiety disorders in the US was highCitation25, however like other studiesCitation24, was limited to only mother and child economic outcomes. Including household cost comparisons widens the perspective of the large economic PPD incurs and will help inform future cost estimates and analyses. The findings in this study suggest that the economic burden as measured by direct healthcare costs associated with PPD extends beyond mothers. Moreover, the findings from this study also suggest that indirect costs attributable to PPD, in the form of time spent utilizing the healthcare system, are also incurred. Differences are concentrated in the number of outpatient visits reflecting potential productivity costs in work days lost.
After observable differences in demographic and health characteristics between households affected by PPD and those unaffected by PPD were controlled for, we found that PPD was associated with higher average levels of health spending for the mother and the partner in the household. While other sources of payment namely out-of-pocket costs are present, the largest share of the costs to the household are borne by the insurer. Total all-cause and PPD-related spending for mothers was driven by medical spending relative to pharmaceutical spending: mothers affected by PPD had a significantly higher number of outpatient visits than their controls had.
The increased healthcare costs that accrued after PPD identification is in part due to increased resource utilization directly related to PPD (e.g. treatment). Consistent with differences in total all-cause medical spending, the analysis also identified differences in resource use (inpatient days and outpatient visits) between cohort affected by PPD and control cohorts for the mother, the mother's partner, other household members excluding the infant, and for the household as a whole. Notably, only 31% of the difference in total all-cause spending between mothers affected by PPD and their matched controls was accounted for by PPD-related medical or pharmaceutical spending, while the remainder was due to differences in unrelated spending. A recent study found that 51% of spending for mental health treatment following a PPD diagnosis was on medication only, contrasting our results that when considering overall healthcare resource utilization (both mental healthcare and non-mental healthcare) pharmaceutical spending accounted for only 11% of the difference in spending between PPD households and unaffected householdsCitation43. Infant resource use was not significantly different between households affected by PPD and the control cohort. This finding is consistent with mothers and other household members seeking psychological treatment during follow-up and also seeking further non-mental healthcare contrasted with the limited need for additional infant medical services.
There are several limitations of this study. The analysis relies on accurate reporting of diagnoses and costs, particularly reliable ICD-9-CM diagnosis codes. To the extent that inaccurate reporting of psychiatric conditions by physicians is reflected on the claims, there may be some contamination in the control cohort. Additionally, due to the range of definitions of PPD used in the literature and by health care providers, households identified as being affected by PPD are restricted to those identified by the algorithm used in this analysis. To the extent that this algorithm undercounts households affected with PPD, the results of the analysis may underestimate the true differences in health care spending between households with and without PPD diagnosis.
The analysis is restricted to claims from large self-insured employers; therefore, it does not reflect the costs associated with PPD for those women on public insurance or for the uninsured. The study excludes from the control cohort those women with pre-existing depression, anxiety, or mood disorders; therefore, differences in spending associated with PPD also reflect spending associated with psychiatric conditions that first manifested prior to childbirth. In addition, the analysis is restricted to family members who were covered by the same insurance plan as the mothers. To the extent that other family members are covered by alternative insurance plans, the analysis will not fully capture the resource use and costs borne by these individuals.
Lastly, the data do not permit an analysis based on the severity of PPD. That is, estimates represent the average treatment effect of PPD on the sample and do not reflect heterogeneous effects of the severity of PPD on health spending for the households. Analyses evaluating costs with more granular PPD-related data (e.g. Edinburgh Postnatal Depression Scale [EPDS] scores) would give a more complete picture of health spending for PPD sufferers.
The limitations of the propensity score matching analysis stem from unobservable attributes that are both correlated with PPD diagnosis and health spending. If this type of attribute exists, the estimates will be biased; that is, health spending could be wrongly attributed directly to PPD. As well as limitations, our study does have several strengths in its design. The use of administrative claims data from OptumHealth provides an accurate reflection of the amount paid by commercial insurers across a large sample of insured patients. The matched design, applying similar matching criteria used in a recent studyCitation24, allowed us to accurately capture the economic burden of PPD holding other household factors equal. Additionally, our study included costs beyond just mental health specific treatment costs.
Conclusions
PPD as identified by the algorithm was associated with higher average levels of health spending for the mother, the partner, and other children in the household. In general, higher total costs among PPD households were driven by more all-cause outpatient visits. While it is known that PPD has a substantial humanistic burden on a householdCitation26, knowledge of the household economic burden was quite limited beyond costs incurred by the mother. These findings represent a unique contribution to the evidence base on the burden of PPD by suggesting that the economic burden as measured by direct healthcare costs associated with PPD extends beyond mothers.
Transparency
Declaration of funding
Sage Therapeutics provided funding for this study.
Declaration of financial/other relationships
MYH and AEL are employees of Sage Therapeutics. KC, DG, AC, and PEG are employees of Analysis Group, and Analysis Group received consulting fees from Sage Therapeutics. CNE has been a consultant/advisory board member, investigator and speaker with funding from Sage Therapeutics. CNE serves on the advisory board for Asarina Pharma. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
MYH, AC, and KC conceptualized the study. KC and DG conducted the data analysis. PEG, AEL, and CNE provided critical feedback on the study design and findings. KC and MYH developed the manuscript with input from all authors. All authors reviewed and approved the final manuscript.
Supplemental Material
Download MS Word (24.1 KB)Acknowledgements
The authors acknowledge medical writing support from Eric Morris of Analysis Group and research support from Erica Birk, Gabi Greenberg, and Dean Young, all formerly of Analysis Group.
References
- Ko JY, Rockhill KM, Tong VT, et al. Trends in postpartum depressive symptoms - 27 States, 2004, 2008, and 2012. MMWR Morb Mortal Wkly Rep. 2017;66(6):153–158.
- Thurgood S, Avery DM, Williamson L. Postpartum depression (PPD). Am J Clin Med. 2009;6(2):17–22.
- Mayo Clinic Web Site. Postpartum depression 2018. [updated September 1, 2018; cited 2020 March 3]. Available from: https://www.mayoclinic.org/diseases-conditions/postpartum-depression/symptoms-causes/syc-20376617?p=1.
- American Psychiatric Association. Depressive disorders. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: APA; 2013.
- Wisner KL, Moses-Kolko EL, Sit DK. Postpartum depression: a disorder in search of a definition. Arch Womens Ment Health. 2010;13(1):37–40.
- Stewart DE, Robertson E, Dennis C-L, et al. Postpartum depression: literature review of risk factors and interventions. Toronto Public Health. 2003.
- The American College of Obstetricians and Gynecologists Committee Opinion no. 630. Screening for perinatal depression. Obstet Gynecol. 2015;125(5):1268–1271.
- Abbasi S, Chuang CH, Dagher R, et al. Unintended pregnancy and postpartum depression among first-time mothers. J Womens Health (Larchmt). 2013;22(5):412–416.
- Bauman BL, Ko JY, Cox S, et al. Vital signs: postpartum depressive symptoms and provider discussions about perinatal depression – United States, 2018. MMWR Morb Mortal Wkly Rep. 2020;69(19):575–581.
- Goodman JH, Tyer-Viola L. Detection, treatment, and referral of perinatal depression and anxiety by obstetrical providers. J Womens Health (Larchmt). 2010;19(3):477–490.
- Cox EQ, Sowa NA, Meltzer-Brody SE, et al. The perinatal depression treatment cascade: baby steps toward improving outcomes. J Clin Psychiatry. 2016;77(9):1189–1200.
- Ohoka H, Koide T, Goto S, et al. Effects of maternal depressive symptomatology during pregnancy and the postpartum period on infant-mother attachment. Psychiatry Clin Neurosci. 2014;68(8):631–639.
- Kerstis B, Aarts C, Tillman C, et al. Association between parental depressive symptoms and impaired bonding with the infant. Arch Womens Ment Health. 2016;19(1):87–94.
- Wouk K, Stuebe AM, Meltzer-Brody S. Postpartum mental health and breastfeeding practices: an analysis using the 2010–2011 pregnancy risk assessment monitoring system. Matern Child Health J. 2017;21(3):636–647.
- Gagliardi L, Petrozzi A, Rusconi F. Symptoms of maternal depression immediately after delivery predict unsuccessful breast feeding. Arch Dis Child. 2012;97(4):355–357.
- Safadi RR, Abushaikha LA, Ahmad MM. Demographic, maternal, and infant health correlates of post-partum depression in Jordan. Nurs Health Sci. 2016;18(3):306–313.
- Yamaoka Y, Fujiwara T, Tamiya N. Association between maternal postpartum depression and unintentional injury among 4-month-old infants in Japan. Matern Child Health J. 2016;20(2):326–336.
- Woolhouse H, Gartland D, Mensah F, et al. Maternal depression from pregnancy to 4 years postpartum and emotional/behavioural difficulties in children: results from a prospective pregnancy cohort study. Arch Womens Ment Health. 2016;19(1):141–151.
- van der Waerden J, Galera C, Larroque B, et al. Maternal depression trajectories and children's behavior at age 5 years. J Pediatr. 2015;166(6):1440–1448.e1.
- Quevedo LA, Silva RA, Godoy R, et al. The impact of maternal post-partum depression on the language development of children at 12 months. Child Care Health Dev. 2012;38(3):420–424.
- Vismara L, Rollè L, Agostini F, et al. Perinatal parenting stress, anxiety, and depression outcomes in first-time mothers and fathers: a 3- to 6-months postpartum follow-up study. Front Psychol. 2016;7:938–938.
- Nishimura A, Fujita Y, Katsuta M, et al. Paternal postnatal depression in Japan: an investigation of correlated factors including relationship with a partner. BMC Pregnancy Childbirth. 2015;15(1):128.
- Kerstis B, Engström G, Sundquist K, et al. The association between perceived relationship discord at childbirth and parental postpartum depressive symptoms: a comparison of mothers and fathers in Sweden. Ups J Med Sci. 2012;117(4):430–438.
- Moore Simas TA, Huang MY, Packnett ER, et al. Matched cohort study of healthcare resource utilization and costs in young children of mothers with postpartum depression in the United States. J Med Econ. 2020;23(2):174–110.
- Luca DL, Margiotta C, Staatz C, et al. Financial toll of untreated perinatal mood and anxiety disorders among 2017 births in the United States. Am J Public Health. 2020;110(6):888–896.
- Moore Simas TA, Huang MY, Patton C, et al. The humanistic burden of postpartum depression: a systematic literature review. Curr Med Res Opin. 2019;35(3):383–393.
- SAGE Therapeutics Inc. Technical study report: a systematic literature review of postpartum depression [Data on File]. 2017.
- Dagher RK, McGovern PM, Dowd BE, et al. Postpartum depression and health services expenditures among employed women. J Occup Environ Med. 2012;54(2):210–215.
- Dietz PM, Williams SB, Callaghan WM, et al. Clinically identified maternal depression before, during, and after pregnancies ending in live births. Am J Psychiatry. 2007;164(10):1515–1520.
- Kozhimannil KB, Trinacty CM, Busch AB, et al. Racial and ethnic disparities in postpartum depression care among low-income women. PS. 2011;62(6):619–625.
- Doktorchik C, Patten S, Eastwood C, et al. Validation of a case definition for depression in administrative data against primary chart data as a reference standard. BMC Psychiatry. 2019;19(1):9.
- American College of Obstetricians and Gynecologists. Use of psychiatric medications during pregnancy and lactation. 2008. (reaffirmed 2012).
- Kendig S, Keats JP, Hoffman MC, et al. Consensus bundle on maternal mental health: perinatal depression and anxiety. J Obstet Gynecol Neonatal Nurs. 2017;46(2):272–281.
- Molyneaux E, Howard LM, McGeown HR, et al. Antidepressant treatment for postnatal depression. Cochrane Database Syst Rev. 2014;9:CD002018.
- Fitelson E, Kim S, Baker AS, et al. Treatment of postpartum depression: clinical, psychological and pharmacological options. Int J Womens Health. 2010;3:1–14.
- National Institute of Mental Health. Perinatal Depression 2020. [cited 2020 Jun 1]. Available from: https://www.nimh.nih.gov/health/publications/perinatal-depression/index.shtml.
- Greenberg PE, Fournier AA, Sisitsky T, et al. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J Clin Psychiatry. 2015;76(2):155–162.
- Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25(1):1–21.
- Akazawa M, Halpern R, Riedel AA, et al. Economic burden prior to COPD diagnosis: a matched case-control study in the United States. Respir Med. 2008;102(12):1744–1752.
- Guo A, Grabner M, Palli SR, et al. Treatment patterns and health care resource utilization associated with dalfampridine extended release in multiple sclerosis: a retrospective claims database analysis. Clinicoecono Outcomes Res. 2016;8:177–186.
- Juurlink DN, Herrmann N, Szalai JP, et al. Medical illness and the risk of suicide in the elderly. Arch Intern Med. 2004;164(11):1179–1184.
- Sicras A, Rejas J, Navarro R, et al. Treating patients with fibromyalgia in primary care settings under routine medical practice: a claim database cost and burden of illness study. Arthritis Res Ther. 2009;11(2):R54.
- Sherman LJ, Ali MM. Diagnosis of postpartum depression and timing and types of treatment received differ for women with private and medicaid coverage. Womens Health Issues. 2018;28(6):524–529.