Abstract
Objective
In the phase 3 RELAY trial, ramucirumab/erlotinib demonstrated superior progression-free survival (PFS) over placebo/erlotinib in patients with EGFR-mutated metastatic NSCLC (median PFS 19.4 versus 12.4 months; HR = 0.59, 95% CI = 0.46–0.76; p < .0001). Safety was consistent with established profiles for ramucirumab and erlotinib in NSCLC. Here, we present patient-reported outcomes.
Methods
Patients received oral erlotinib (150 mg daily) plus intravenous ramucirumab (10 mg/kg) or placebo Q2W until progressive disease or unacceptable toxicity. Patients completed the Lung Cancer Symptom Scale (LCSS) and EQ-5D questionnaires at baseline and every other cycle. Analyses included time to deterioration (TtD) for LCSS via Kaplan–Meier method and Cox models and changes from baseline using mixed-model repeated-measures regression analysis.
Results
Overall patient compliance for LCSS and EQ-5D was >95%. TtD did not differ between treatment arms for LCSS Total Score (HR = 0.962, 95% CI = 0.690–1.343) and Average Symptom Burden Index (HR = 1.012, 95% CI = 0.732–1.400). TtD of individual LCSS items (appetite loss, fatigue, cough, shortness of breath, pain, symptom distress, difficulties with daily activities, quality of life) indicated no difference between arms; however, patient-reported blood in sputum was worse for ramucirumab/erlotinib (HR = 1.987, 95% CI = 1.206–3.275). Results of LCSS mean changes from baseline were consistent with TtD, indicating no significant differences between treatment arms except for blood in sputum. Mean changes from baseline in EQ-5D index score (p = .94) and visual analogue scale (p = .95) revealed no overall differences in health status between treatment arms.
Conclusions
Patients’ overall quality of life and symptom burden did not differ with the addition of ramucirumab to erlotinib compared to placebo/erlotinib. These data support the clinical benefit of ramucirumab/erlotinib in untreated EGFR-mutated metastatic NSCLC.
Introduction
First-line treatment with tyrosine kinase inhibitors (TKIs) is the standard of care for patients with metastatic non-small-cell lung cancer (NSCLC) that exhibits epidermal growth factor receptor (EGFR)-activating mutations (exon 19 deletion or exon 21 L858R point mutation)Citation1–3. Patients treated with first-generation TKIs (erlotinib, gefitinib) exhibit median progression-free survival (PFS) of approximately one yearCitation4, while use of second-generation TKIs has resulted in median PFS of 11.0 months with afatinib and 14.7 months with dacomitinibCitation5,Citation6. For the approximately 50% of patients who develop Thr790Met resistance to first- or second-generation TKIs, the third generation TKI, osimertinib, would remain a treatment optionCitation7. Additionally, use of osimertinib for first-line therapy produced a median PFS of 18.9 monthsCitation8. Ultimately, patients become resistant to TKI treatment, so prolonging TKI-mediated tumor control with new targeted approaches is an ongoing need for patients with metastatic EGFR-mutated NSCLC.
Clinical evidence supports dual EGFR and VEGF inhibition to delay progression. RELAY, a worldwide, double-blind, placebo-controlled phase 3 trial, evaluated the efficacy and safety of erlotinib, a standard-of-care EGFR TKI, plus ramucirumab, a human IgG1 VEGFR2 antagonist, or placebo in untreated patients with metastatic NSCLC with EGFR-activating mutationsCitation9. RELAY met its primary endpoint; ramucirumab/erlotinib improved median PFS by 7 months (19.4 months versus 12.4 months) over placebo/erlotinib and reduced the hazard of disease progression or death by 41% (HR = 0.591, 95% CI = 0.461–0.760, p value <.0001). The ramucirumab arm was also favored over placebo across subgroups, including by EGFR mutation type (exon 19-del and 21_L858R). In addition, the ramucirumab arm exhibited an increase in duration of response relative to the placebo group (18.0 versus 11.1 months, HR = 0.619, 95% CI = 0.477–0.805, p = .0003).
Assessment of adverse events in RELAY showed that the safety profile of ramucirumab plus erlotinib among patients with advanced NSCLC was similar to the safety profiles of the individual drugs in the same setting. Established side effects of antiangiogenic drugs, such as proteinuria, hypertension and bleeding events, were observed more frequently among ramucirumab-treated patients. These antiangiogenic-related adverse events were typically low grade (1–2), except for hypertension (maximum of Grade 3), and were manageable with dose modifications and supportive care. In addition, some Grade 3 toxicities related to erlotinib (e.g. dermatitis acneiform and diarrhea) and some Grade 1 and 2 toxicities related to erlotinib (e.g. elevated levels of ALT and AST) occurred more frequently in the ramucirumab treatment arm, possibly due to the longer treatment exposure to erlotinib. In general, the additional toxicity observed with ramucirumab/erlotinib treatment did not prevent patients from continuing treatment. Based on these results, ramucirumab/erlotinib is approved as a first-line treatment for patients with EGFR-mutated metastatic NSCLC in Europe and included in the National Comprehensive Cancer Network (NCCN) and European Society for Medical Oncology (ESMO) guidelines.
Patient-reported outcome data (symptoms, function, health-related quality-of-life) can provide a more comprehensive evaluation of the impact of cancer therapy on the patient experience and quality of life. Prolonged PFS may not always have a positive association in cancer clinical trials, suggesting the need to measure patient-reported outcomes directly, rather than assuming that lengthened PFS is associated with positive quality of lifeCitation10.
For RELAY, patient-reported outcomes were part of the secondary outcome measures and disease-related symptoms and their impact were prospectively assessed by the Lung Cancer Symptom Scale (LCSS)Citation11,Citation12. Time to deterioration (TtD) of LCSS items has been used to compare treatment arms in NSCLC studiesCitation13,Citation14. A recent review has summarized the numerous advantages to TtD analysis, including its ability to accommodate missing data, death and response shift, and its ability to provide clinically interpretable resultsCitation15. In addition to LCSS, another secondary outcome measure was the EuroQol 5-dimension 5-level questionnaire (EQ-5D-5L) which was employed to measure the impact of treatment on patient-reported general health statusCitation16–18. Here, we present analyses of the prospectively collected, patient-reported outcomes data from RELAY.
Methods
Patients, study design and treatment
RELAY was conducted in 100 investigative centers in 13 countries; details of the trial have been published (ClinicalTrials.gov NCT02411448)Citation9. In brief, eligible patients met the following criteria: previously untreated Stage IV NSCLC; EGFR exon 19-del or 21_L858R mutation; Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0 or 1Citation19; measurable disease as assessed by Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1Citation20; and adequate hematological and organ function. Exclusion criteria included known EGFR T790M mutation and CNS metastases.
Patients were treated with either intravenous ramucirumab (10 mg/kg once every 2 weeks) or intravenous placebo (once every 2 weeks). Both treatment arms received oral erlotinib (150 mg/day). Treatment was discontinued upon progressive disease, unacceptable toxicity or withdrawal of consent. RELAY adhered to the principles of the Declaration of Helsinki and the Good Clinical Practice Guidelines of the International Conference on Harmonization. The institutional review board of each investigative site approved the trial, and all patients provided written informed consent.
The primary endpoint was investigator-assessed PFS (RECIST v1.1). Secondary endpoints were safety and toxicity, overall survival, overall response rate, disease control rate, duration of response, pharmacokinetics, immunogenicity, and patient-reported outcomes. Exploratory endpoints included time to deterioration in ECOG PS, second disease progression (PFS2) and biomarker analyses. This manuscript focuses on patient-reported outcomes and time to deterioration in ECOG PS.
Clinical outcomes assessments
Patient-reported outcomes were assessed using two validated questionnaires: the LCSS and the EQ-5D-5L. The LCSS data assess the impact of treatment on lung cancer–specific symptoms. It consists of six symptom-specific questions that address cough, dyspnea, fatigue, pain, hemoptysis and anorexia, and three summary items on symptom distress, interference with activity level and global quality of life. Patients recorded perceived degree of impairment on a 100 mm visual analogue scale (VAS), with scores reported from 0 to 100, 0 representing the best score. The LCSS Total Score was calculated as the mean of the 9 LCSS items. Average Symptom Burden Index (ASBI) was calculated as the mean of the 6 symptom items of the LCSS. Neither the LCSS Total Score nor the ASBI was computed if the patient had one or more missing values for the LCSS items.
The EQ-5D-5L was used to examine the impact of treatment on general health status, using five measures or “dimensions”: mobility, self-care, usual activities, pain or discomfort, and anxiety or depression. For each measure, patients recorded one of five levels of severity: having no problems, having slight problems, having moderate problems, having severe problems, and unable to do or having extreme problems. The Index Score, a summary score of the dimensions, was calculated from the United Kingdom valuation set of item weights to derive a score of 0 to 1, with 1 representing the best health statusCitation21. The Index Score was not computed for a patient if he/she had one or more missing values for the 5 items. In a second part of the EQ-5D-5L questionnaire, patients were asked to indicate their health status on the day of the interview on a VAS with endpoints of 0 to 100, where “0” corresponded to the worst health one could imagine and the highest rate (100) corresponds to the best health one could imagine.
Patients completed the LCSS and the EQ-5D-5L at baseline, every other cycle, and at the 30 day follow-up visit. The patient-reported outcome instruments were completed at the beginning of the clinic visit before any extensive contact with investigative staff occurred. ECOG PS was evaluated by the clinician at baseline, before every cycle, at the end of treatment and at the 30 day follow-up.
Statistical analyses
The TtD for each of the 9 LCSS items, ASBI and the LCSS total score was defined as the time from the date of randomization until the date of first deterioration, which was defined as the first ≥15 mm increase (worsening) from baseline. Analyses included patients with baseline data and at least one post-baseline assessment. Patients without deterioration were censored on the date of the patient’s last post-baseline LCSS assessment or randomization date, whichever was last. The TtD was estimated using the Kaplan–Meier methodCitation22 and univariate Cox Proportional Hazards ModelCitation23, and expressed as HR and 95% CI. The LCSS data were also summarized descriptively by baseline and cycle for actual measurement and change from baseline by treatment arm. For the LCSS (each item, ASBI and Total LCSS), mean change from baseline was estimated using mixed-model repeated-measures (MMRM) regression and included the independent variables treatment, visit, treatment by visit interaction and baseline, with a negative change indicating improvement.
The EQ-5D-5L summary statistics for the Index Score and VAS were calculated for each assessment period by treatment arm. Mean change from baseline was estimated for the EQ-5D-5L Index Score and the EQ VAS self-rated health score using longitudinal MMRM regression models and included independent variables treatment, visit, treatment by visit interaction and baseline. A negative change from baseline indicated improvement.
The TtD in ECOG PS was a pre-specified exploratory endpoint defined as time from randomization to the first observed ECOG PS ≥ 2; it was analyzed using the Kaplan–Meier method and compared using an unstratified log rank test.
Results
As of the data cutoff date for the primary analysis (23 January 2019), 29% of patients (64/224) in the ramucirumab/erlotinib arm and 19% of patients (43/225) in the placebo/erlotinib arm continued study treatment. The median duration of ramucirumab treatment was 11.0 months (IQR 4.2, 15.6), and the median duration of placebo treatment was 9.7 months (IQR 3.7, 15.6) at the time of data cutoff. The median duration of follow-up was 20.7 months.
Overall patient compliance for LCSS and EQ-5D-5L completion across all time points was high for both instruments: 95.7% ramucirumab/erlotinib arm, 96.7% placebo/erlotinib arm for the LCSS; 96.1% ramucirumab/erlotinib arm, 96.6% placebo/erlotinib arm for the EQ-5D-5L. With patient compliance rates for completion of the LCSS and EQ-5D-5L >95%, nearly all patients completed the measures at each applicable cycle while on the study, thus missing data were minimal. At the 30 day safety follow-up visit, compliance for the LCSS was 74.4% in the ramucirumab/erlotinib arm and 79.1% in the placebo/erlotinib arm, and compliance for the EQ-5D-5L was 74.4% in the ramucirumab/erlotinib arm and 79.7% in the placebo/erlotinib arm.
For the LCSS, a lower score indicates better symptoms or outcome. Baseline summary scores were similar between treatment arms and relatively low but reflecting some symptom burden among patients (). Excluding the symptom of blood in sputum, the range of mean baseline scores was 18.3 to 30.8 mm for the ramucirumab/erlotinib arm and 16.6 to 30.0 mm for the placebo/erlotinib arm on the 100 mm scale. Blood in sputum was generally not reported as a disease symptom of concern at baseline based on a mean score of 2.6 mm for ramucirumab/erlotinib arm patients and 1.6 mm for placebo/erlotinib arm patients.
Table 1. LCSS scores: baseline and change from baseline – difference between treatment arms.
Treatment arms were compared by examining change in symptom scores over time, assessed as TtD. Results are expressed in , forest plot. The TtD in the LCSS Total Score and the ASBI were similar between treatment arms: LCSS Total Score HR = 0.962; 95% CI = 0.690–1.343; ASBI HR = 1.012; 95% CI = 0.732–1.400. The TtD of individual LCSS items (appetite loss, fatigue, cough, shortness of breath, pain, symptom distress, difficulties with daily activities and quality of life) indicated no significant difference between arms since the HR 95% CIs all include 1.0. The exception is the symptom blood in sputum, which was worse for the ramucirumab/erlotinib arm (HR = 1.987; 95% CI = 1.206–3.275). Notably, the number of deterioration events was low for blood in sputum (n = 47 for ramucirumab/erlotinib; n = 23 for placebo/erlotinib), the lowest number of events reported among the LCSS items, and median TtD was not achieved in either arm. The TtD Kaplan–Meier plots for LCSS Total Score and ASBI are presented in . To assess the impact of treatment discontinuation due to adverse events on LCSS analyses, a sensitivity analysis was conducted with the combined endpoint of time to either deterioration in LCSS or treatment discontinuation due to AE. Adding treatment discontinuation due to AEs to the analysis did not alter the TtD LCSS results; the HR 95% CIs all include 1.0 except for blood in sputum.
Figure 1. Time to deterioration (TtD) in LCSS. A forest plot is shown to display the TtD hazard ratios for the LCSS data for the six symptom-specific items and three summary items, as well as the Total Score (mean of the nine LCSS items) and the Average Symptom Burden Index (ASBI; mean of the 6 symptom items). Abbreviations. HR, Hazard ratio; PboErl, Placebo + erlotinib treatment arm; RamErl, Ramucirumab + erlotinib treatment arm.
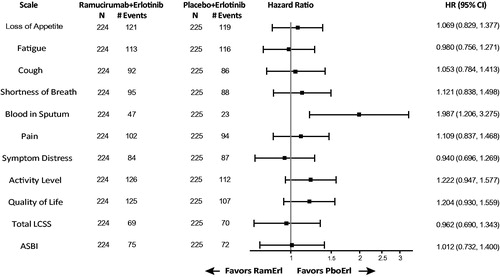
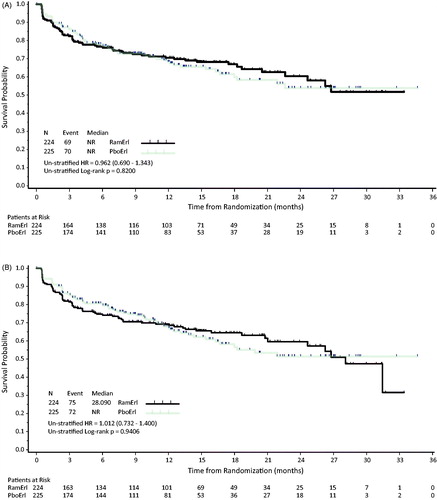
Figure 2. Kaplan–Meier estimates of time to deterioration for (A) Total LCSS and (B) ASBI. Abbreviations. HR, Hazard ratio; NR, Not reached; PboErl, Placebo + erlotinib treatment arm; RamErl, Ramucirumab + erlotinib treatment arm.
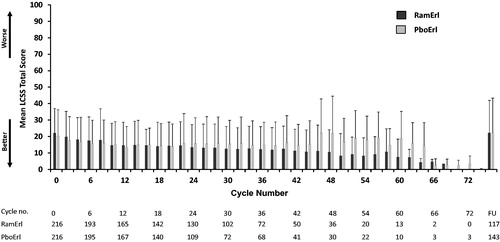
Mean LCSS Total Scores across the study were plotted for both treatment arms (). These mean scores were similar for the two treatment arms across the study treatment visits, including the 30 day follow-up.
Figure 3. LCSS Total Score by cycle. For the 100 point LCSS scale, the mean LCSS Total Score ± SD is shown for each cycle for the two treatment arms. Higher mean LCSS scores indicate worse symptom burden. Abbreviations. PboErl, Placebo + erlotinib treatment arm; RamErl, Ramucirumab + erlotinib treatment arm.
Comparing changes from baseline in LCSS items between treatment arms was also analyzed using MMRM analysis (). The mean change from baseline for each LCSS parameter was similar between arms except for blood in sputum. Consistent with the primary analysis of TtD, the blood in sputum item was significantly worse for the ramucirumab/erlotinib arm.
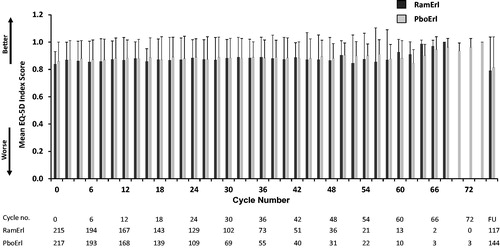
For the EQ-5D-5L, a higher score indicates better health status. Analysis of mean changes from baseline in the Index Score (least square mean = −0.01, SE = 0.01, p = .94) and VAS score (least square mean = 1.00, SE = 1.21, p = .95) revealed no overall differences in health status between treatment arms. The mean EQ-5D Index Score (±SD; ) and VAS scores (not shown) across treatment cycles were similar between treatment arms and within arms over time. The only exception to the consistent EQ-5D-5L scores was a decline in mean scores for both treatment arms at the 30 day follow-up assessment.
Figure 4. Mean EQ-5D Index Scores (±SD) across treatment cycles. Patients completed the EQ-5D at baseline (Cycle/Visit 0) and every other subsequent visit. The mean EQ-5D Index score for each treatment arm is shown over time, including the 30 day post-discontinuation follow-up visit. Abbreviations. PboErl, Placebo + erlotinib treatment arm; RamErl, Ramucirumab + erlotinib treatment arm.
The TtD in ECOG PS was a pre-specified exploratory endpoint. The vast majority (94%) of patients in both treatment arms maintained an ECOG PS of 0 or 1, so the TtD analysis to ECOG PS ≥ 2 was not feasible as planned at the time of data cutoff.
Discussion
In RELAY, first-line treatment with ramucirumab plus erlotinib demonstrated superior efficacy relative to placebo plus erlotinib in patients with EGFR-mutated metastatic NSCLC. The detailed analyses of patient-reported outcome data presented here support the clinical benefit of the RELAY regimen. Overall patient compliance rates for completion of the LCSS and EQ-5D-5L surveys were >95%. This high compliance rate was a strength of these analyses because substantial missing data lower the statistical power of the analyses and can introduce a population selection bias if the missing data are associated with treatment group assignment and not accounted for in analysesCitation15. Other strengths of the RELAY PRO data include its routine collection across the long duration of the trial and the use of both LCSS and EQ-5D-5L, two well established and thoroughly validated assessments.
Incremental improvement in PFS, such as that observed in RELAY, is clinically meaningful if it is associated with a preservation of quality of lifeCitation24. Prior to the start of treatment, baseline scores for RELAY patients showed no imbalance at baseline and generally did not indicate severe symptom burden or impaired quality of life among patients. These baseline results are consistent with general clinical characteristics (e.g. few comorbidities, non-smoking) of patients with EGFR-mutated metastatic NSCLC, the ECOG 0–1 performance status inclusion criterion and other trials, albeit in a population without CNS metastasis. After start of study treatment, LCSS data showed no difference between treatment arms in time to deterioration in the LCSS Total Score (HR = 0.962, 95% CI = 0.690–1.343) and the ASBI (HR = 1.012, 95% CI = 0.732–1.400). Additional LCSS data analyses (MMRM analysis) focusing on mean change from baseline, yielded results consistent with the TtD results. These findings suggest that overall quality of life and average symptom burden did not differ with the addition of ramucirumab to erlotinib compared to placebo plus erlotinib.
Further analysis examining LCSS Total Scores for both arms at each time point confirmed that overall quality of life did not differ by study arm throughout the duration of treatment with ramucirumab added to erlotinib. Likewise, the mean EQ-5D Index and VAS scores across treatment cycles did not vary from baseline and were similar in both treatment arms. These results reveal that there was consistent health status (summary score of patient-assessed mobility, self-care, usual activities, pain or discomfort, and anxiety or depression) throughout the trial and no overall difference in health status between treatment arms. These consistent scores occurred throughout the relatively long treatment duration: on the ramucirumab/erlotinib arm, the median treatment duration was 11 months for ramucirumab and 14 months for erlotinib. Only at the 30 day post-discontinuation assessment was there a decline in mean EQ-5D-5L scores for both treatment arms, and this might reflect the deterioration associated with disease progression.
In parallel with the consistent patient-reported outcome summary scores, the ECOG PS observed for most patients during treatment remained stable. Most patients on both treatment arms (94%) maintained a PS of 0 or 1. Thus, the addition of ramucirumab to erlotinib did not diminish patients’ suitability for subsequent treatments upon progression.
The similar patient-reported outcome scores for the two treatment arms were as expected. As observed in our study and in others, low baseline symptom scores are frequently observed in patients receiving first-line treatmentCitation25. Without substantial symptom burden or impaired quality of life, there is limited room for symptom improvement in either arm. Furthermore, although the ramucirumab/erlotinib treatment improved median PFS by 7 months over placebo (19.4 versus 12.4 months) and significantly lengthened the duration of response (18.0 versus 11.1 months), both treatment arms had a similar overall response rate (ramucirumab/erlotinib 76% versus placebo/erlotinib 75%) and a similar disease control rate (ramucirumab/erlotinib 95% versus placebo/erlotinib 96%); thus, a difference in symptom improvement was not expected.
Blood in sputum was the LCSS item with the lowest number of events (n = 47 ramucirumab/erlotinib arm; n = 23 placebo/erlotinib arm) with median times to deterioration not reached, and the only one that was significantly different between treatment arms. Hemoptysis or “blood in sputum” is a symptom of concern among patients and known to cause substantial distress. The blood-in-sputum HR was 1.987 (95% CI = 1.206–3.275), indicating a significant deterioration in this symptom for the ramucirumab/erlotinib arm. This item is summarized as “hemoptysis” when reporting results from the LCSSCitation11. When completing the LCSS questionnaire, however, patients responded to a question about blood in sputum, but were not provided with any additional context, nor were they expected to identify the source of blood. Thus, the source could either be pulmonary (clinician-reported hemoptysis) or nasopharyngeal (clinician-reported epistaxis). As an adverse event, treatment-emergent hemoptysis (blood in sputum) occurred at a rate of 5% in the ramucirumab/erlotinib arm and <1% in the placebo/erlotinib arm; all events were Grade 1 or 2, with no patient discontinuing ramucirumab due to hemoptysis. Treatment-emergent epistaxis occurred at a rate of 33.5% in the ramucirumab/erlotinib arm and 12.0% in the placebo/erlotinib arm; all events were Grade 1 or 2Citation9. Patient-reported blood in sputum occurring more frequently among ramucirumab-treated patients over placebo was consistent with expected symptoms in NSCLC and the known bleeding risk for hemoptysis and epistaxis with VEGF inhibitors.
Use of different PRO instruments across studies limits comparisons of the completed analyses. For example, the FLAURA trialCitation25 used EORTC QLQ-LC13 and QLQ-C30, two other well established questionnaires, rather than the LCSS and EQ-5D-5L as used in RELAY. Another limitation of the study is that the PRO data focuses on patient symptoms and overall health status; therefore, no conclusions about the patient experience of possible toxicities related to treatment, such as diarrhea or skin rash, can be made. A further limitation, one often inherent in trial-based PRO assessments, is an informative censoring bias that occurs when patients drop out at progression when symptoms are expected to worsen. Finally, the RELAY trial was not powered for specific hypotheses in the PRO secondary endpoints, thus conclusions should be interpreted accordingly.
Patients in RELAY experienced a PFS benefit, extended duration of response and some additional toxicity, while most of the lung cancer symptoms, symptom burden, overall quality of life and health status did not significantly differ between study arms with the addition of ramucirumab to erlotinib, with the one exception of blood in sputum. Overall, these patient-reported outcomes results support the clinical benefit of the RELAY regimen in the first-line setting of EGFR-mutated metastatic NSCLC.
Transparency
Declaration of funding
The study sponsor provided the study drug and worked with academic investigators to design the study and to collect, analyze and interpret the trial data, and write the report.
Declaration of financial/other relationships
K.Y. has disclosed that he has received during the conduct of this study grants from Eli Lilly and, outside the submitted work, grants and personal fees from Chugai Pharma, AstraZeneca, Eli Lilly, Ono Pharmaceutical, Novartis, MSD, Bristol-Myers Squibb, Taiho Pharmaceutical and Daiichi Sankyo; grants from Bayer, Pfizer, and Takeda; and personal fees from Boehringer Ingelheim and Kyowa Kirin. S.A. has disclosed that he has received personal fees from AstraZeneca, Chugai, MSD, Ono, Taiho, Boehringer Ingelheim, Pfizer, Bristol-Myers Squibb and Eli Lilly; and institutional support from AstraZeneca, Chugai, MSD, Ono, Taiho, Boehringer Ingelheim, Pfizer, Bristol-Myers Squibb, Eli Lilly, and F. Hoffmann-La Roche. M.R. has disclosed that he has received outside the submitted work personal fees from Abbvie, Amgen, AstraZeneca, BMS, Boehringer-Ingelheim, Celgene, Eli Lilly, Merck, MSD, Novartis, Pfizer and Roche. E.B.G. has disclosed that he has received during the conduct of the study institutional grants from Eli Lilly and, outside the submitted work, institutional grants from AstraZeneca, BMS, Eli Lilly, Genentech, Merck, Novartis, Iovance, Neon, Dynavax, EMD Serono and Mirati; and personal fees from Dracen, Novartis, GSK and EMD Serono. S.P.A. has disclosed that he has received personal fees from Eli Lilly outside the submitted work. D.M.S. has disclosed that he has received during the conduct of the study personal fees from Eli Lilly and, outside the submitted work, personal fees from Roche, BMS, MSD, Takeda, Abbvie, Pfizer, Novartis, AstraZeneca, Eli Lilly and Boehringer Ingelheim. K.B.W., B.F.M., A.Z. and C.V.G. have disclosed that they are employees and stockholders of Eli Lilly. K.N. has disclosed that he has received during the conduct of the study grants and personal fees from AstraZeneca, Astellas Pharma, MSD, Nippon Boehringer Ingelheim, Novartis Pharma, Bristol Myers Squibb, Chugai Pharmaceutical, Daiichi Sankyo and Merck Serono/Merck Biopharma; and grants, personal fees and a consulting role with Ono Pharmaceutical, Pfizer Japan and Eli Lilly Japan; he has also received, outside the submitted work, personal fees from Clinical Trial Co., Medicus Shuppan Publishers, Care Net, Reno Medical, Medical Review Co, Roche Diagnostics, Bayer Yakuhin, Medical Mobile Communications Co, 3H Clinical Trial, Nichi-Iko Pharmaceutical, Nanzando Co, Yodosha Co, Nikkei Business Publications, Thermo Fisher Scientific, Yomiuri Telecasting Corp, Nippon Kayaku Co; personal fees and an advisory role with Kyorin Pharmaceutical and Takeda Pharmaceutical; grants and personal fees from Taiho Pharmaceutical, SymBio Pharmaceuticals, and AbbVie; and grants from inVentiv Health Japan, ICON Japan, Gritsone Oncology, Parexel International Corp, Kissei Pharmaceutical Co, EPS Corp, Syneos Health, Pfizer R&D Japan, A2 Healthcare Corp., Quintiles/IQVIA Services Japan, EP-CRSU Co, Linical Co, Eisai Co, CMIC Shift Zero, Kyowa Hakko Kirin Co, Bayer Yakuhin, EPS International Co and Otsuka Pharmaceutical Co. CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
In addition to the named authors of the paper, the following RELAY investigators are all non-author contributors: Quincy Chu, Alexis Cortot, Jean-Louis Pujol, Elizabeth Fabre, Corinne Lamour, Helge Bischoff, Jens Kollmeier, Martin Kimmich, Walburga Engel-Riedel, Stefan Hammerschmidt, Wolfgang Schütte, Konstantinos Syrigos, James Chung Man Ho, Kwok-Hung Au, Silvia Novello, Andrea Ardizzoni, Giulia Pasello, Vanessa Gregorc, Alessandro Del Conte, Domenico Galetta, Toshiaki Takahashi, Makoto Nishio, Takashi Seto, Fumio Imamura, Toru Kumagai, Katsuyuki Hotta, Yasushi Goto, Yukio Hosomi, Hiroshi Sakai, Yuichi Takiguchi, Young Hak Kim, Takayasu Kurata, Hiroyuki Yamaguchi, Haruko Daga, Isamu Okamoto, Miyako Satouchi, Satoshi Ikeda, Kazuo Kasahara, Koichi Azuma, Toru Kumagai, Keisuke Aoe, Yoshitsugu Horio, Nobuyuki Yamamoto, Hiroshi Tanaka, Satoshi Watanabe, Naoyuki Nogami, Tomohiro Ozaki, Ryo Koyama, Tomonori Hirashima, Hiroyasu Kaneda, Keisuke Tomii, Yuka Fujita, Masahiro Seike, Naoki Nishimura, Terufumi Kato, Masao Ichiki, Hideo Saka, Katsuya Hirano, Yasuharu Nakahara, Shunichi Sugawara, Keunchil Park, Sang-We Kim, Young Joo Min, Hyun Woo Lee, Jin-Hyoung Kang, Ho Jung An, Ki Hyeong Lee, Jin-Soo Kim, Gyeong-Won Lee, Sung Yong Lee, Aurelia Alexandru, Anghel Adrian Udrea, Óscar Juan-Vidal, Ernest Nadal-Alforja, Ignacio Gil-Bazo, Luis Paz-Ares, Belén Rubio-Viqueira, Miriam Alonso Garcia, Enriqueta Felip Font, Jose Fuentes Pradera, Juan Coves Sarto, Meng-Chih Lin, Wu-Chou Su, Te-Chun Hsia, Gee-Chen Chang, Yu-Feng Wei, Chao-Hua Chiu, Jin-Yuan Shih, Jian Su, Irfan Cicin, Tuncay Goksel, Hakan Harputluoglu, Ozgur Ozyilkan, Ivo Henning, Sanjay Popat, Olivia Hatcher, Kathryn Mileham, Jared Acoba, Gabriel Jung, Moses Raj, William Martin and Shaker Dakhil.
Author contributions
Conception and design: K.N., A.Z.; acquisition of data for the work: K.Y., S.A., M.R., E.B.G., S.P.A., D.M.S., K.N.; analysis of data for the work: A.Z., C.V.G., M.R.; interpretation of data for the work: all authors; drafting of the paper or critical revision for intellectual content: all authors; final approval for publication: all authors.
Acknowledgements
The authors thank the patients and their caregivers, and the investigators and their support staff for their invaluable participation in the RELAY trial. Medical writing assistance was provided by Mary Dugan Wood, funded by Eli Lilly and Company.
Data availability statement
Eli Lilly provides access to all individual participant data collected during the trial, after anonymization, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the USA and EU and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once they are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, and blank or annotated case report forms will be provided in a secure data sharing environment for up to 2 years per proposal. For details on submitting a request, see the Vivli website: www.vivli.org.
References
- National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: non-small cell lung cancer, Version 2 [Internet]. 2020 [cited 2020 Feb 21]. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf
- Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29(Suppl 4):iv192–iv237.
- Planchard D, Popat S, Kerr K, et al. Correction to: “metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up”. Ann Oncol. 2019;30(5):863–870.
- Hsu WH, Yang JC, Mok TS, et al. Overview of current systemic management of EGFR-mutant NSCLC. Ann Oncol. 2018;29 (Suppl 1):i3–i9.
- Park K, Tan E-H, O’Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17(5):577–589.
- Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(11):1454–1466.
- Mok T, Wu YL, Ahn MJ, et al. Osimertinib or platinum–pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376(7):629–640.
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113–125.
- Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20(12):1655–1669.
- Kovic B, Jin X, Kennedy SA, et al. Evaluating progression-free survival as a surrogate outcome for health-related quality of life in oncology: a systematic review and quantitative analysis. JAMA Intern Med. 2018;178(12):1586–1596.
- Hollen PJ, Gralla RJ, Kris MG, et al. Quality of life assessment in individuals with lung cancer: testing the Lung Cancer Symptom Scale (LCSS). Eur J Cancer. 1993;29(Suppl 1):S51–S58.
- Janssen MF, Birnie E, Haagsma JA, et al. Comparing the standard EQ-5D three-level system with a five-level version. Value Health. 2008;11(2):275–284.
- Pérol M, Ciuleanu TE, Arrieta O, et al. Quality of life results from the phase 3 REVEL randomized clinical trial of ramucirumab-plus-docetaxel versus placebo-plus-docetaxel in advanced/metastatic non-small cell lung cancer patients with progression after platinum-based chemotherapy. Lung Cancer. 2016;93:95–103.
- Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet. 2009;374(9699):1432–1440.
- Bonnetain F, Fiteni F, Efficace F, et al. Statistical challenges in the analysis of health-related quality of life in cancer clinical trials. J Clin Oncol. 2016;34(16):1953–1956.
- Hollen PJ, Gralla RJ, Kris MG, et al. Measurement of quality of life in patients with lung cancer in multicenter trials of new therapies. Psychometric assessment of the Lung Cancer Symptom Scale. Cancer. 1994;73(8):2087–2098.
- Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727–1736.
- EQ-5D-5L [Internet]. EQ-5D, EuroQol Research Foundation; 2020 [cited 2020 Jan 20]. Available from: www.EuroQoL.org
- Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655.
- Eisenhauer EA, Therasse P, Bogaert J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247.
- van Hout B, Janssen MF, Feng YS, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708–715.
- Kaplan EL, Meier P. Nonparametric estimation of incomplete observations. J Am Stat Assoc. 1958;53(282):457–481.
- Cox DR. Regression models and life-tables. J Royal Stat Soc Ser B. 1972;34(2):187–220.
- Villaruz LC, Socinski MA. The clinical viewpoint: definitions, limitations of RECIST, practical considerations of measurement. Clin Cancer Res. 2013;19(10):2629–2636.
- Leighl NB, Karaseva N, Nakagawa K, et al. Patient-reported outcomes from FLAURA: osimertinib versus erlotinib or gefitinib in patients with EGFR-mutated advanced non-small-cell lung cancer. Eur J Cancer. 2020;125:49–57.
