Abstract
Objective
Insulin lispro 200 U/mL (IL200) is a treatment choice for people with diabetes who have daily mealtime insulin (MTI) requirements of >20 U/day. We report clinical characteristics of real world IL200 users in Germany to understand clinical settings and the type of patients who would benefit from IL200 treatment.
Methods
This retrospective database analysis used the patient-level data from “IMS Disease Analyzer” in Germany from February 2015 to June 2016. Clinical and demographic information were collected and analyzed for IL200 users alongside that of those who were using more than 20 U a day of 100 U/mL analog MTI.
Results
Of the 17,261 patients using insulin, 811 were identified in IL200 group. The IL200 group had 60% men, mean ± SD age of 63.6 ± 11.9 years, and BMI of 36.2 ± 6.7 kg/m2. Of these, 63.5% (n = 515) were seen by diabetologists, while 36.5% (n = 296) were seen by general practitioners (GPs). In the IL200 group, 77.7% used basal insulin concomitantly, >90% had ≥1 comorbidity, and 52% had ≥4 comorbidities; the most common being hypertension (75.2%), neuropathy (66.0%), and nephropathy (59.6%). Diabetologist-treated IL200 users were more likely to have multiple comorbidities as compared with those treated by GPs (15.0% vs. 12.9% for >5 comorbidities).
Conclusions
IL200 is prescribed to people with diabetes who need more than 20 U/day of mealtime insulin and tend to be more obese, older, and with multiple comorbidities. Future research should explore how concentrated MTI can impact adherence and long-term glycemia.
Introduction
Insulin is the treatment for all people with type 1 diabetes (T1D) and eventually for many people with type 2 diabetes (T2D) due to the progressive nature of the diseaseCitation1. When lifestyle modifications and antihyperglycemic drugs (oral or injectable) are not effective enough to achieve glycemic targets, patients with T2D often begin insulin therapy. The introduction of insulin in most people with T2D starts with long-acting insulin (basal only) and then eventually with the addition of prandial insulin (basal-bolus therapy)Citation1. Alternatively, insulin therapy can also start with prandial-insulin-only therapyCitation2,Citation3. A basal-bolus regimen is always required for people with T1D. In people with diabetes, older age and increased body-mass index (BMI) with central obesity are factors that increase insulin resistance; often calling for, greater doses of insulin to maintain near-euglycemiaCitation4,Citation5.
Higher concentrations of insulin with or without modified pharmacokinetic (PK) and pharmacodynamics (PD) profiles have recently entered the market that fulfill different patient needs like the problems of injecting higher insulin dosages/volumes such as, unpredictable absorption, leakage, and increased pain and discomfortCitation6,Citation7. Concentrated basal insulin analogs are currently available at 200 U/mL and 300 U/mL, whereas mealtime insulin (MTI) analog is available at 200 U/mL. High concentration human insulin is available at 500 U/mL. These high concentration insulins may offer benefits to certain patients because of their diverse pharmacological profiles and clinical characteristicsCitation8,Citation9.
The insulin lispro 200 U/mL (hereafter IL200) (HumalogFootnotei U-200 U/mL KwikPenFootnoteii or LiprologFootnoteiii 200 U/mL KwikPen) was introduced in Germany in 2015. IL200 is available in a disposable insulin pen (KwikPen) that delivers one unit in half the volume as MTI U-100 U/mL and its features allow for dosing in one-unit increments in the range from 1 to 60 U. IL200 is bioequivalent to insulin lispro 100 U/mL (herein IL100) with similar efficacy and safety profileCitation10,Citation11. IL200 pen exhibited significantly lower glide force and glide force variability compared with the IL100 deviceCitation12, indicating that patients may have to exert less effort to self-inject, allowing for a smooth injection experience. In a simulated injection study of people with T1D and T2D, majority of participants preferred the IL200 device compared to the IL100 deviceCitation13. The primary drivers of preference for the IL200 device included reduced glide force, reduced injection volume, and ability to have a total of 600 U of insulin in the pen compared to 300 U in the IL100 pen.
This study was performed to characterize the type of patients and clinical settings that use IL200 to understand the practice patterns in real world setting. This report primarily focuses on patient characteristics of patients who were prescribed IL200 while providing a context for these findings by characterizing the patient population and treatment patterns for patients using >20 U/day of 100 U/mL analog MTI (herein referred to as the “reference group”). A dose greater than 20 U of rapid-acting insulin/day was chosen because IL200 should be reserved for this patient group according to its EU summary of product characteristicsCitation14. It allows the patients to use the entire content of a pen before the insulin expires within 28 days of opening. To ensure adequate sample size of patients using IL200 in the real world, Germany was the chosen country of interest as it was the first European country where the product was available and reimbursed.
Methods
IMS Disease Analyzer database
This study was a retrospective database analysis using patient-level data from IMSFootnoteiv Disease Analyzer (DA) database (IQVIA) from February 2015 to June 2016. DA is a representative database of primary care practices in Germany that compiles demographic, clinical, and pharmaceutical data obtained anonymously from computer systems used in outpatient practicesCitation15. The data collected includes patient information (age, gender, comorbidities, body weight, height, laboratory test results, medical history, diagnosis, risk factors, hospitalizations, and referrals) and treatment information (date of treatment, molecule/brand, form, strength, and dose). The quality and accuracy of the data in DA is regularly monitored by IQVIA. More than 15 million patient records and 100 million prescriptions, with over 20 years of history are available in DA. The Data Protection Commissioner of the data protection agency of the German Federal state Hesse endorses the data transmission, processing, and storage procedures for DA. DA has been used in other studies focusing on different outcomes in diabetes treatmentCitation16–18.
In this study, data from DA were anonymized, truncated, and coded to ensure confidentiality of patient identity. Patient parameters included here are year of birth, gender, height, and body weight. Data were accessed and extracted securely by trained employees of IQVIA. This study followed the regulatory and ethical requirements prevalent in Germany as well as the European Pharmaceutical Marketing Research Association (EphMRA) guidelines. This article is based on retrospective database analysis and does not involve direct participation of humans.
Study design and population
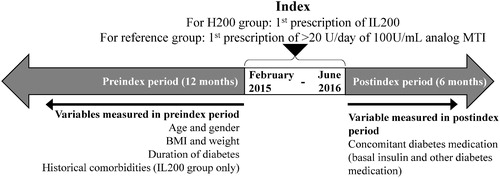
The study index date was between February 2015 and June 2016. We determined a 12-month preindex period and a 6-month postindex period for the assessment of data for different patient characteristics (). The index date for data collection for the IL200 group was the date of the first prescription of Humalog 200 U/mL KwikPen or Liprolog 200 U/mL KwikPen, whereas for the reference group was the date of the first prescription of more than 20 U/day on any 100 U/mL analog MTI. Information on the variables such as demographics (age and gender), BMI, weight, duration of diabetes, and historical comorbidities (observed throughout the entirety of a patient’s history) were collected during the 12-month preindex period, whereas for the concomitant diabetes medication was collected in the 6-month postindex period. This analysis used the date of first recorded diabetes diagnosis as a proxy for duration of diabetes, which is not directly recorded in DA.
Figure 1. Study design. Abbreviations. BMI, body-mass index; IL200, insulin lispro 200 U; MTI, mealtime insulin.
People included in this database real world evidence study were more than 18 years old with T1D or T2D, with more than 12 months of data available preindex, and who were using either IL200 or any other analog MTI (aspart, lispro, or glulisine) more than 20 U/day, with or without basal insulin. For the description of patient characteristics (such as age, gender, weight, BMI, prescriber specialty, concomitant medications, and comorbidities) the patient population excluded those using insulin pumps or human MTI. The insulin regimen data was from a larger population of 63,787 insulin users that included those using basal or prandial insulin within February 2015 to June 2016 period. Patients were stratified by the prescriber specialty, that is, a general practitioner (GP) or a diabetologist and the insulin regimen the patients received. For the description of patient characteristics, patients on a prandial-insulin-only regimen were defined as those included in the study with a prescription of IL200 or >20 U/day of 100 U/mL of analog MTI in the index window without a prescription for a basal insulin between index date and the duration of their prandial use. Prefilled pen users were defined as patients with a prescription of MTI with the form indicated as a pre-filled pen (KwikPen, FlexPenFootnotev, SoloStarFootnotevi), whereas reusable pen users were defined as the 100 U/mL analog MTI users with a prescription of MTI with their form indicated as “cartridge” without prior prescription of an insulin pump.
Statistical analysis
The analyses conducted were primarily descriptive and included summary statistics (mean and SD), proportions, and frequency of outcomes. BMI was analyzed as both a continuous variable and within the categories of <30, 30–34.9, 35–39.9, 40–44.9, and ≥45 kg/m2. Body weight was analyzed as a continuous variable and presented for both the overall group and stratified by gender. Data for concomitant medications and comorbidities were analyzed only for the IL200 group. Analysis of concomitant diabetes medications included all other diabetes medications prescribed to the patient for the duration of their treatment with IL200. Historical comorbidities were analyzed in terms of the number of comorbidities per patient and the proportion of patients with each respective comorbidity. The data for historical comorbidities were considered over the entirety of a patient’s available historical data. Use of cardiovascular risk management concomitant medications was analyzed in terms of the number of classes of concomitant medications in a patient’s record and the proportion of patients with a prescription of each respective class of drug. The results for the IL200 and reference groups were tested by chi-square test for differences in gender, BMI, duration of diabetes, and for age and weight by the Wilcoxon test.
Results
Of the 17,261 people injecting MTI, 811 were in the IL200 group and 16,450 were in the reference group using >20 U/day of 100 U/mL analog MTI (refer Supplemental Figure S1 for patient selection details). The number of patients in the IL200 and reference groups differed across the different patient characteristics evaluated in the preindex period (). About 6.5% of the people in IL200 group and 18.8% of those in the reference group had T1D. The small T1D population limited us from drawing conclusions or comparisons between T1D and T2D populations.
Table 1. Patient characteristics of IL200 and reference groups in the preindex period.
Patient characteristics
The reference group of 16,450 users had an average ± SD age of 62.5 ± 14.9 years and comprised 58% men. Data on BMI were available for only 4107 (25%) patients whose mean ± SD (95% CI) BMI was 31.9 ± 6.7 (31.7, 32.1) kg/m2.
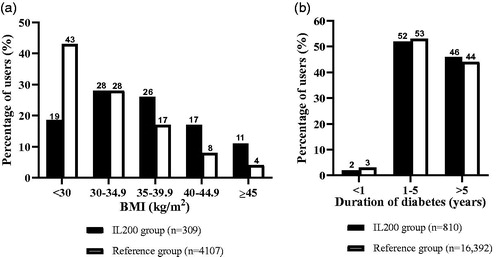
The IL200 group (n = 811) had an average age of 63.6 ± 11.9 years with 60% men (). About 62% of the people had missing data for BMI, but within the available data, the mean ± SD (95% CI) BMI of patients in the IL200 group (n = 309) was 36.2 ± 6.7 (35.4, 36.9) kg/m2. About 18.5% of the patients in IL200 group and 42.9% of those in the reference group had BMI <30 (; ). Within the IL200 group, 63.5% of the patients were prescribed IL200 by diabetologists, while for the rest, it was prescribed by GPs ().
Concomitant medications and comorbidities in the IL200 group
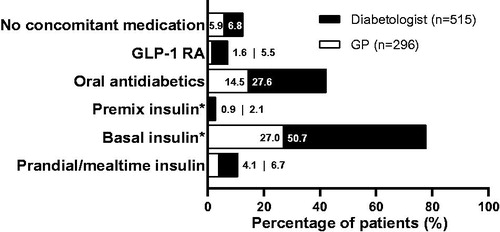
shows the overall usage of concomitant diabetes medications for the IL200 group and prescriber specialty subgroups. With 77.7% of all users in the IL200 group using basal insulin concomitantly, it was the most commonly used concomitant diabetes medication with IL200. Diabetologists were more likely to prescribe insulins, glucagon-like peptide-1 receptor agonists, or oral antidiabetics in combination with IL200 than GPs.
Figure 3. Usage pattern of concomitant diabetes medications in the IL200 group treated either by a general practitioner or a diabetologist. *Basal insulin group includes both intermediate and long-acting insulin, while premix insulin group includes intermediate or long-acting combined with fast-acting insulin. Abbreviations. GLP-1 RA, glucagon-like peptide-1 receptor agonist; GP, general practitioner; IL200, insulin lispro 200 U.
About 52% of patients in the IL200 group had four or more comorbidities, and more than half of which had greater than five comorbidities (). About 7.1% of the patients in the IL200 group did not have any comorbidities. The most common comorbidities in the IL200 group were hypertension, neuropathy, and nephropathy (). When comparing prescriber specialties, more patients treated by diabetologists had more comorbidities recorded than patients treated by GPs (15.0% with >5 comorbidities as compared with 12.9% for GPs; ). Patients treated by GPs had recorded cardiovascular comorbidities more often than patients treated by diabetologists (particularly hypertension, ischemic heart disease, dyslipidemia, and peripheral vascular disease) ().
Figure 4. Proportion of patients by number of historical comorbidities in the IL200 group treated either by a general practitioner or a diabetologist. Abbreviations. GP, general practitioner; IL200, insulin lispro 200 U.
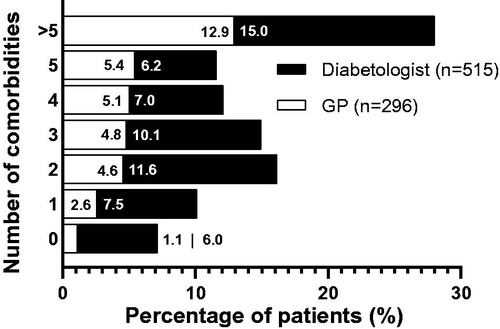
Table 2. Description of common historical comorbidities in more than 25% of patients in IL200 group.
Patient characteristics: Insulin regimen
Of the 63,787 insulin users, 27,683 (43%) were on prandial-insulin-only regimen, 21,052 (33%) were on combined basal + prandial insulin regimen, and the rest were on basal-insulin-only regimen. There were no clinically meaningful differences in the proportion of GPs and diabetologists that prescribed prandial-insulin-only regimen (GP = 16,555 [43%], diabetologist = 11,128 [44%]) and combined basal + prandial insulin regimen (GP = 11,481 [30%], diabetologist = 9571 [37%]).
Patients in both IL200 and reference group were categorized as those on prandial-insulin-only or prandial + basal insulin regimen. Patients in the prandial-insulin-only group and in the combined prandial + basal insulin group were broadly similar in terms of gender, age, body weight, BMI, duration of diabetes and prescriber specialty ().
Table 3. Characteristics of patients using IL200 or U100 > 20 U/mL and on different insulin regimens.
Discussion
The results of this study characterized patients who were prescribed IL200 for diabetes treatment as those with higher BMI (including higher than the reference group using >20 U/day of 100 U/mL analog MTI), multiple comorbidities, and multiple co-medications (both diabetes and cardiovascular risk management medications). These characteristics align with those of the diabetes population who generally score high on disease complexityCitation19. Patients who were prescribed IL200 had a gender distribution in line with the general diabetes population with slightly more males than femalesCitation20,Citation21 and patients in the IL200 and the reference group had similar duration of diabetes. Only 6.5% of patients in the IL200 group had the diagnosis of T1D, in contrast to 18.8% in the reference group. These results show that IL200 has been implemented in Germany as prandial-insulin-only regimen mainly for more obese people with T2D and to a lesser amount for T1D. However, IL200 was also prescribed in 18.5% of the people who were leaner (with BMI <30 kg/m2), which indicated that in real world concentrated insulin may be needed for patients with different characteristics regardless of body weight. This may indicate that the current recommendations for individualization of insulin therapy are being followed in real world.
Germany is a country where prandial-insulin-only regimen is followed relatively frequentlyCitation2 and as IL200 is a prandial insulin, this study analyzed the population who were prescribed IL200 for the insulin regimen they followed. Patients on prandial-insulin-only and combined basal + prandial regimen were similar in terms of gender, age, BMI and its distribution, and duration of diabetes in comparison with 100 U/mL insulin users. A recent study reported a decrease in the number of people with diabetes on prandial-insulin-only regimen from 12% in 2002 to 8.3% in 2014 in GermanyCitation22, although in our data we identified a high percentage of patients (43%) on prandial-insulin-only regime. Another study in 4045 people with T2D initiating insulin therapy in the UK primary care reported that only 2.1% of patients were initiated with prandial-insulin-only regimenCitation23. The reasons for this contrasting observation are unclear. In Germany, the trends in insulin prescription may differ owing to lack of financial restrictions and wide availability of insulin analogs.
There were no clear differences in the characteristics of patients treated either by a GP or a diabetologist regarding the MTI regimen recommendation. Higher proportion of patients with more comorbidities were seen by diabetologists in comparison with GPs. This may be expected as diabetologists may be consulted by those patients with advanced and more complicated condition with longer duration of diabetes. However, higher proportion of patients visiting GPs reported specific common comorbidities such as hypertension, dyslipidemia and other cardiovascular diseases as listed in .
As is expected in all electronic medical records, information is limited by the level of detail and quality of information recorded by the physician and systematic biases may be introduced by the nature of the recording physician. An important limitation is the amount of missing data in the source database, which limits the strength of interpretation from the observed data differences and similarities. Another caveat is that the date of first recorded diabetes diagnosis was used for quantifying the duration of diabetes. If patients switched physicians at this date, this may underestimate the true duration of diabetes. Data does not capture the diagnostics or therapies prescribed during hospital visits or by specialists the patient is referred to. Prescription refills through in-person practitioner visits or over telephonic calls cannot be distinguished in the data. Patient histories are not carried over in the electronic medical records if a patient shifts from one GP or diabetologist to another. We acknowledge that the conclusions of this report in German population may not be generalizable.
Conclusion
In conclusion, this analysis of patient data from IMS Disease Analyzer during 2015–2016 in Germany, shows that the characteristics of people with diabetes using IL200 are in line with what is expected for people with diabetes in need of a high dose of MTI. Patients who were prescribed IL200 demonstrated to have higher body weight than the patients on 100 U/mL analog MTI. Their higher BMI and weight than the reference group may justify their need for more units of insulin. Further studies are required to make any conclusion about how IL200 could impact adherence and glucose control in this patient population.
Transparency
Declaration of funding
This study was supported by Eli Lilly and Company, Indianapolis, Indiana, USA.
Declaration of financial/other relationships
NCS, MPN, CPdO, HS and TO are employees and stockholders of Eli Lilly and Company and/or one of its subsidiaries. NCS is a guest scientist at the German Diabetes Center, Institute for Clinical Diabetology, Düsseldorf, Germany. SMC is a former employee of Eli Lilly and Company and has no other conflicts of interest to declare. SK is an employee of IQVIA and has no other conflicts of interest to declare. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article and have given their approval for this version to be published. NCS, MPN, SK, TO, and SMC were involved in conception and design of the study. CPdO was involved in interpretation of data. HS and SK were involved in data analysis. All authors interpreted the data, critically revised the article, and agreed to be accountable for all aspects of this work.
H200_Germany_RWEmanuscript_CMRO_R2R_Supplemental_Submitted.docx
Download MS Word (121.8 KB)Acknowledgements
Writing and editorial assistance in the preparation of this article was provided by Dr. Shirin Ghodke, a full-time employee of Eli Lilly Services India Pvt. Ltd.
Data availability statement
The datasets analysed during the current study are not publicly available but will be made available on reasonable request.
Notes
i Humalog is a registered trademark of Eli Lilly and Company, Indianapolis, IN, USA.
ii Kwikpen is a trademark of Eli Lilly and Company, Indianapolis, IN, USA.
iii Liprolog is a registered trademark of Eli Lilly and Company, Indianapolis, IN, USA.
iv IMS is a registered trademark of IQVIA, Frankfurt, Germany.
v FlexPen is a registered trademark of Novo Nordisk, Denmark.
vi SoloStar is a registered trademark of Sanofi-Aventis, Deutschland GmBH.
References
- Standards of medical care in diabetes – 2018. Diabetes Care. 2018;41(Suppl 1):S73–S85.
- Liebl A, Jones S, Benroubi M, et al. Clinical outcomes after insulin initiation in patients with type 2 diabetes: 6-month data from the INSTIGATE observational study in five European countries. Curr Med Res Opin. 2011;27(5):887–895.
- Holman RR, Farmer AJ, Davies MJ, et al. Three-year efficacy of complex insulin regimens in type 2 diabetes. N Engl J Med. 2009;361(18):1736–1747.
- Papaetis GS, Papakyriakou P, Panagiotou TN. Central obesity, type 2 diabetes and insulin: exploring a pathway full of thorns. Arch Med Sci. 2015;11(3):463–482.
- Stout MB, Justice JN, Nicklas BJ, et al. Physiological aging: links among adipose tissue dysfunction, diabetes, and frailty. Physiology. 2017;32(1):9–19.
- Brusko CSA, Taylor AD, Jackson JA, et al. Clinical challenges with concentrated insulins: setting the record straight. Diabetes Spectr. 2017;30(4):229–232.
- Lamos EM, Younk LM, Davis SN. Concentrated insulins: the new basal insulins. Ther Clin Risk Manag. 2016;12:389–400.
- Heinemann L, Beals JM, Malone J, et al. Concentrated insulins: history and critical reappraisal. J Diabetes. 2019;11(4):292–300.
- Schloot NC, Hood RC, Corrigan SM, et al. Concentrated insulins in current clinical practice. Diabetes Res Clin Pract. 2019;148:93–101.
- de la Pena A, Seger M, Soon D, et al. Bioequivalence and comparative pharmacodynamics of insulin lispro 200 U/mL relative to insulin lispro (Humalog®) 100 U/mL. Clin Pharmacol Drug Dev. 2016;5(1):69–75.
- Ovalle F, Segal AR, Anderson JE, et al. Understanding concentrated insulins: a systematic review of randomized controlled trials. Curr Med Res Opin. 2017;34(6):1029–1043.
- Rees TM, Lennartz AH, Ignaut DA. A comparison of glide force characteristics between 2 prefilled insulin lispro pens. J Diabetes Sci Technol. 2015;9(2):316–319.
- Wang T, Conrad KA, van Brunt K, et al. Attributes influencing insulin pen preference among caregivers and patients with diabetes who require greater than 20 units of mealtime insulin. J Diabetes Sci Technol. 2016;10(4):923–931.
- Summary of product characteristics. Humalog 200 units/ml KwikPen, solution for injection in prefilled pen [updated 2018 Jun 29. cited 2019 Mar 1]. Available from: https://www.medicines.org.uk/emc/product/3725/smpc.
- Rathmann W, Bongaerts B, Carius HJ, et al. Basic characteristics and representativeness of the German Disease Analyzer database. Int J Clin Pharmacol Ther. 2018;56(10):459–466.
- Basson M, Ntais D, Ayyub R, et al. The cost-effectiveness of dulaglutide 1.5mg versus exenatide QW for the treatment of patients with type 2 diabetes mellitus in France. Diabetes Ther. 2018;9(1):13–25.
- Kostev K, Jacob L. Association between depression and persistence with oral antihyperglycemic drugs in type 2 diabetes mellitus patients in Germany. Psychiatry Res. 2018;261:90–93.
- Kostev K, Rockel T, Jacob L. Prescription patterns and disease control in type 2 diabetes mellitus patients in nursing home and home care settings: a retrospective analysis in Germany. J Diabetes Sci Technol. 2018;12(1):136–139.
- Grant RW, Ashburner JM, Hong CS, et al. Defining patient complexity from the primary care physician's perspective: a cohort study. Ann Intern Med. 2011;155(12):797–804.
- Gale EA, Gillespie KM. Diabetes and gender. Diabetologia. 2001;44(1):3–15.
- International Diabetes Federation Diabetes Atlas [Internet]. International Diabetes Federation; 2017. [cited 2018 Jan 2]. Available from: http://www.diabetesatlas.org/resources/2017-atlas.html.
- Bohn B, Kerner W, Seufert J, et al. Trend of antihyperglycaemic therapy and glycaemic control in 184,864 adults with type 1 or 2 diabetes between 2002 and 2014: analysis of real-life data from the DPV registry from Germany and Austria. Diabetes Res Clin Pract. 2016;115:31–38.
- Blak BT, Smith HT, Hards M, et al. A retrospective database study of insulin initiation in patients with Type 2 diabetes in UK primary care. Diabet Med. 2012;29(8):e191-198–e198.