Abstract
Objective
To assess the level of glycemic control among type 2 diabetes patients on sodium-glucose cotransporter 2 inhibitor (SGLT2i) and metformin dual therapy.
Methods
Observational, retrospective database study in adult type 2 diabetes mellitus patients from the IQVIA Electronic Medical Record (EMR) database was conducted. The observation period was June 2015 to June 2018. Patient’s earliest encounter in the observation period while on SGLT2i and metformin dual therapy served as the index date. Patients were required to have at least one HbA1c measure in the 12 months prior to the index date and be on SGLT2i and metformin dual therapy and no other antihyperglycemic treatment as of the HbA1c measurement date or any time during the 90 days prior. The associations between sociodemographic factors and clinical burden on achievement of HbA1c <8% were assessed using multivariable logistic regression with backward stepwise selection.
Results
Of 3491 patients, 2176 (62.3%) achieved HbA1c <8%, with a median distance to goal of 1.1% (IQR 0.5–2.3%) for those not at glycemic target. Mean age was 56.5 years and 52.6% were male. At baseline, 28.3% of patients had established cardiovascular disease/chronic kidney disease, and of those 63.8% had HbA1c <8%. African American patients had lower odds of attaining HbA1c <8% when compared with white patients [OR 0.69], while older patients had marginally higher odds [OR 1.01].
Conclusion
Approximately 3 out of 5 patients on metformin and SGLT2i dual therapy achieved HbA1c <8%, with African Americans having a lower likelihood of achieving this glycemic goal.
Introduction
Diabetes mellitus (DM) is characterized by chronic hyperglycemia, with type 2 DM (T2DM) being the most frequent subtype. Approximately 30 million people live with diabetes in the United States (US)Citation1, with a resulting cost of around $237 billion per yearCitation2. Cardiovascular disease (CVD) and chronic kidney disease (CKD) affect approximately 22% and 24% of T2DM patients in the US, respectivelyCitation3. Diabetes patients with CVD/CKD experience substantial burden of disease and are at risk for downstream health outcomes, such as atrial fibrillation, kidney failure, major adverse cardiovascular events (MACE), and cardiovascular deathCitation4.
The treatment of T2DM includes both pharmacological and lifestyle changes. The current consensus report of the American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) recommends metformin as the general first-line pharmacotherapy to manage hyperglycemia and SGLT2i inpatients with manifest cardiovascular disease, such as those with heart failure or chronic kidney diseaseCitation5. This is further reflected in the current ADA standards of care and the 2019 European Society of Cardiology (ESC) guidelinesCitation6,Citation7.
Cardiovascular outcomes trials (CVOTs) with SGLT2 inhibitor therapy were performed on a background of metformin (Supplemental Appendix for Table 1: Proportion of Metformin patients in CVOTs) and other antihyperglycemic agents (AHAs): Empagliflozin was tested in EMPA-REG OUTCOME and was the only SGLT2 inhibitor to demonstrate a 38% reduction in cardiovascular (CV) mortality in T2DM patients with established CVDCitation8. Results from this study, as well as the Canagliflozin Cardiovascular Assessment Study (CANVAS), the Dapagliflozin Effect on Cardiovascular Events (DECLARE), and the Evaluation of Ertugliflozin Efficacy and Safety Cardiovascular outcomes trial (VERTIS-CV) demonstrated a reduction of hospitalization for heart failure in T2DM patients: HR 0.65 (95% CI 0.50–0.85) in EMPA-REG-OUTCOME, HR 0.67 (95% CI 0.52–0.87) in CANVAS, HR 0.73 (95% CI, 0.61–0.88) in DECLARE, and HR 0.70 (95% CI 0.54–0.90) in VERTIS-CVCitation8–12. Previously, SGLT2i have been shown to reduce the risk of major adverse CV events (MACE) in patients with T2D and established CV disease or high CV risk in 2 CVOTs: EMPA-REG OUTCOME testing empagliflozin (HR 0.86 (95% CI 0.74–0.99)), CANVAS testing canagliflozin (HR 0.86 (95% CI 0.75–0.97). The superiority of SGLT2i versus placebo in reducing the risk of renal events in patients with T2DM was elucidated in several trialsCitation8,Citation9,Citation11,Citation12. These results established SGLT2i as a class of cardioprotective antihyperglycemic agents. However, many patients on SGLT2i may require additional glycemic control.
The current study aimed to assess the level of glycemic control and distance to glycemic thresholds among T2DM patients who were treated with SGLT2i and metformin dual therapy in the real world. Further, the study evaluated patient profiles, assessed potential differences in goal attainment by established Cardiovascular Disease (eCVD) and CKD status, and determined factors associated with HbA1c ≥8%.
Methods
Data source
Using the IQVIA EMR database previously known as the Quintiles Electronic Medical Record research database, a retrospective database study was conducted in adult (> =18 years) T2DM patients who were treated with dual therapy with SGLT2i and metformin. The IQVIA EMR database covers 30 million active patients, treated by over 30,000 healthcare providers across the United States. This database contains medical and pharmacy service data files, with information on demographics, diagnoses, prescription medications, lab assessments, and comorbidities, which were required for the current study. The study was done on a retrospective, proprietary, de-identified data and as such is not considered human subject research hence no Institutional Review Board review/approval was required.
Study population
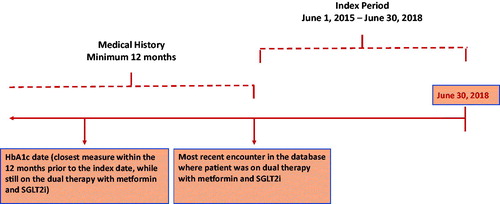
Patients with dual therapy of SGLT2i and metformin between 01 June 2015 and 30 June 2018 were identified using generic drug names (Supplemental Appendix for Table 2: Other Medications). The patient’s earliest encounter (either physician visit, prescription, lab measure, etc.) during the study period while on SGLT2i and metformin dual therapy was defined as the index date. The patients were required to have at least 12 months of medical enrollment history prior to the index date, at least one HbA1c measurement in the 12 months prior to the index date and needed to be on SGLT2i and metformin dual therapy and no other antihyperglycemic treatment as of the measurement date or any time during the 90 days prior to the HbA1c measurement. Additionally, patients were excluded if they had a diagnosis for type 1 diabetes mellitus, gestational diabetes or dialysis in the 12 months prior to index date. The patient’s demographic and clinical characteristics (including comorbidities and laboratory values) were captured in the 12 month period prior to index dateCitation13 ().
eCVD and CKD status
eCVD patients were defined as having International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) or International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes for coronary heart disease, cerebrovascular disease or peripheral vascular disease during the 12 months period prior to index date. Similarly, CKD patients had ICD-9-CM or ICD-10-CM diagnosis code for chronic kidney disease or an estimated glomerular filtration rate (eGFR) < 60 using the most recent eGFR measurement in the 12 months prior to the index dateCitation13.
Outcomes
The primary outcome of the study was the level of glycemic control defined as the proportion of patients with HbA1c ≥8% on the most recent lab test. HbA1c ≥8% was used as a threshold in the absence of knowledge about the individual glycemic level goals. At the very least an HbA1c ≥8% is considered to be a minimum conservative glycemic level goal applicable to most patientsCitation14. However, to address issues surrounding the assumption, sensitivity analyses were also conducted using HbA1c glycemic goals of HbA1c ≥7% and ≥7.5%. For patients not at glycemic goal, the distance to target in % (7%. 7.5% and 8%) HbA1c was reported.
Comorbidities and other risk factors
The analyses also included information on several socio-demographic factors, comorbidities, and lab measurements as covariates. The sociodemographic factors included age, sex, and ethnicity (Asian, African American, Native American, Native Hawaiian Pacific Islander, White, Other/Unknown). The ICD-9-CM/ICD-10-CM diagnosis information during the 12 month history period prior to index date was used to include clinical characteristics such as nicotine dependency and alcohol use disorder, retinopathy, neuropathy, hypoglycemia, liver disease, hypertension, and obesity (Supplemental Appendix for Table 3: Diagnosis codes). Relevant lab measurements, BMI and blood pressure information were analyzed if they were entered during the 12 months prior to index date.
Statistical analyses
Continuous variables were analyzed utilizing t-tests. Chi-square test for categorical variables was utilized when comparing the sample characteristics by eCVD or CKD status. For patients not at glycemic control of HbA1c < 8%, the distance to target (i.e. the distance of the existing HbA1c levels from the optimal glycemic levels) was calculated. The impact of sociodemographic factors and clinical characteristics on achievement of glycemic control was tested using multivariable logistic regression with backward stepwise selection. All analyses were conducted using SAS 9.4.Footnote1
Results
We identified 3491 patients on SGLT2i and metformin dual therapy which represents 9% of all SGLT2i and metformin combination users in the database. 62.3% of patients had HbA1c <8%, while the median distance from target was 1.1% (IQR 0.5, 2.3) for those not at glycemic target. 48.4% achieved HbA1c <7.5% with distance to target of 1.2% (IQR 0.5, 2.2), and 31.5% achieved HbA1c <7% with distance to target 1.1% (IQR 0.5, 2.2). Baseline demographic characteristics are reported in . Mean age was 56.5 (SD 10.9) years, with marginally lower proportion of females (47.4%). The sample was predominantly white (74.5%), with a mean Body Mass Index (BMI) of 34.8 kg/m2 (SD 7.5), and mean HbA1c levels of 7.9% (SD 1.6). Obesity (94.4%), hyperlipidemia (36.4%), hypertension (29.9%), CKD (16.8%), and CVD (15.2%) were the most common conditions recorded for the overall population.
Table 1. Demographic and clinical characteristics.
The prevalence of eCVD and CKD was significantly higher in older, white men, .
The trends in this subgroup were similar to the trends observed for the overall study population. Specifically, patients with eCVD/CKD were older (37.0% vs 14.5% in 65 and above category), male (56.9% vs 51.3%), and white (75.9% vs 69.9%) as compared to patients without eCVD/CKD (Supplemental Appendix for Table 4: Baseline characteristics). The median distance to HbA1c threshold of 8% was similar for patients with eCVD/CKD versus patients without eCVD/CKD (1.0% vs 1.1%, ). Likewise, the distance to threshold of <7% and <7.5% was median 1.2% and 1.1%, respectively, with no differences between those with or without eCVD/CKD.
Table 2. Distance to optimal glycemic control.
Factors associated with achieving HbA1c <8% were assessed (). There was a nominal increased likelihood of goal attainment for older patients (OR 1.01, 95% CI 1.01–1.02), while African American ethnicity was associated with lower likelihood of goal attainment (OR 0.69, 95% CI 0.57–0.85).
Table 3. Factors associated with Goal Attainment among T2DM population.
Sensitivity analyses described the demographic characteristics of patients with and without eCVD; as well as patients with and without CKD. Patients with eCVD and CKD were likely to be at or above 65 years (40.4% vs 20.4% and 43.0% vs 20.3% for with and without CKD and eCVD, respectively), male (49.6% vs 47.0% and 69.6% vs 49.5% for with and without CKD and eCVD, respectively). The subgroup with only eCVD had higher proportion of whites (83.1% vs 73.0%) and diabetic retinopathy (20.7% vs 0.8%) than the subgroup without eCVD (Supplemental Appendix for Table 4: Baseline characteristics).
Discussion
This study assessed the level of glycemic control among T2DM patients who were treated with SGLT2i and metformin dual therapy and captured in a representative EMR database in the US: 31.5%, 48.4% and 62.3% achieved HbA1c <7%, <7.5% and <8% respectively. This is the first time such an examination has been conducted and is particularly relevant given the current ADA/EASD consensus 2019 recommendation for this combination as first-line therapy in a select group of established CVD patientsCitation5.
28.3% of the patients had a history of eCVD or CKD. These patients were more likely to be older, white men, and had higher proportion of patients with hypoglycemic events and comorbidities such as neuropathy and hypertension. Patients with eCVD or CKD had a higher prevalence of other comorbidities, yet cardiovascular disease had no significant impact on attaining HbA1c <8% or distance to this goal. Although the current study used 8% as a minimum standard for glycemic control, the study also included guideline recommended glycemic targets of 7.5% and 7%Citation6. The corresponding sensitivity analyses found that the clinical and sociodemographic characteristics of patients attaining these thresholds were very similar to those of the patients the main analysis (Supplemental Appendix for Table 4: Baseline characteristics).
The leading international societies ADA and EASD, in their consensus statement, recommend SGLT2i agents for treating patients with eCVD/CKD. The consensus statement and the individual guidelines are based on the proven efficacy in clinical trials, such as CANVAS and CREDENCECitation12,Citation13. There were considerable differences between the patient characteristics reported in this real world population vis a vis the CANVAS clinical trial cohort, namely higher proportion of patients with history of CVD (15.2% vs. 65.6%), retinopathy (0.8% vs. 21.0%), and neuropathy (7.0% vs. 30.7%). Patients in both CREDENCE and EMPA-REG OUTCOME populations had an even higher cardiovascular baseline riskCitation8,Citation13. A recently published observational study in the real world likewise described the characteristics of incident diabetic patients using a large integrated healthcare delivery system in the US and identified a similar patient profile, as well a comparable rate of attaining HbA1c ≥8% (37.7% vs 31.0%)Citation16. The population in the current study resembles the sociodemographic characteristics identified by Pantalone et al. in an analysis of T2DM patients in an integrated health systemCitation17. Importantly, we for the first time analyzed the upfront combination treatment of metformin and SGLT2 inhibitors and would have expected a higher proportion of patients at high cardiovascular risk in alignment with international guidelines.
We identified a nominal increased likelihood of goal attainment in patients >65 years of age. Older patients are an increasingly important subgroup as their prevalence increases. Giugliano et al. showed cardiovascular protection with SGLT2 inhibitors was more pronounced in patients 65 years and older. However, there is no significant difference in subgroups and this result could further be driven by a different overall distribution of risk factors in these patients indicating comparable efficacy independent of ageCitation18.
A reduced likelihood of glycemic control was seen in African American versus white patients. Prior research has demonstrated ethnical disparities in access to healthcare for diabetes patients and the impact on glycemic controlCitation19,Citation20. Specifically, a study by Assari et al. reported that greater perceived discrimination was associated with higher HbA1c levelCitation19. A recent meta-analysis by Mishriky et al. indicated that potential differences in efficacy of SGLT2 inhibitors in black versus white patients could not be identified and that based in the low sample size of African American patients in the cardiovascular outcomes trials, it remains unclear whether such a difference would existCitation21. Our study further identified that African Americans were significantly less likely to meet the glycemic target of 8% which may contribute to worse outcomes over time.
Previous literature has highlighted that the primary reasons for suboptimal glycemic control among T2DM patients is clinical inertia, which is defined as defined as failure to intensify or switch therapies on time when necessaryCitation19,Citation20. A study by Ajmera et al. reported that among patients prescribed 2 oral antihyperglycemic drug classes with HbA1c >8%, median time to treatment intensification was 1.5 yearsCitation22. In the current study, 37.7% of patients were above the glycemic threshold of ≥8%. If target HbA1c levels are not achieved after 3 months of follow-up, these patients may require immediate treatment intensification with other antihyperglycemic therapy such as DPP-4i, GLP-1RA, sulfonylurea, or insulinCitation15. A nationwide study in patients without a history of cardiovascular events in Denmark indicated, DPP-4i, GLP-1RA, and SGLT2i had a similar effect in preventing mortality, HF hospitalizations, and MACE outcomes, while SU and insulin had lower effectivenessCitation23. In contrast, early findings from EMPRISE showed that compared with DPP4i, empagliflozin was associated with a decreased risk of HHF, yet a similar risk of myocardial infarction (MI) and stroke in routine clinical care. Empagliflozin was associated with a decreased risk of all-cause mortality, as well as acute kidney injury compared with DPP4i. A subgroup analysis from EMPRISE in T2DM patients >65 years showed that empagliflozin was associated with a decreased risk of MI, stroke, and all-cause mortalityCitation24,Citation25. In this context it is important to note that patients not at glycemic goal under any initial antihyperglycemic combination therapy may require additional agents to reach their individual therapeutic target.
The current study finds that a substantial proportion of patients initiating SGLT2i metformin dual therapy are not at glycemic goal, which is consistent with data from the National Health and Nutrition Examination Surveys (NHANES), which reported that 22% of T2DM patients did not meet glycemic targets(ref) of HbA1C <8%Citation26. Prolonged loss of glycemic control increases the risk of hospitalization, mortality, MACECitation27,Citation28 As patient’s comorbidity burden increases it can lead to increased healthcare utilization and cost and worsen patient’s quality of lifeCitation29–32. Furthermore, patients should be made aware that adherence to prescribed medication is an important factor for improved outcomes and general wellbeing.
Our study has several important limitations. Utilizing EMR data, care obtained outside the hospital or physician network is not recorded, including prescriptions of SGLT2i and metformin as well as eCVD/CKD diagnosis information. Patients were required to have HbA1c measurement along with SGLT2i metformin dual therapy prescription for meaningful analysis of the data. Additionally, patients with hypertension may be under reported solely based on diagnosis information. Moreover, no measure was undertaken to ensure their adherence towards the therapies. Since lab results are not available for all patients, this may result in a sample of patients that might deviate from the entire group of SGLT2i metformin dual therapy users in the US. The IQVIA-EMR database, formerly known as Quintiles Electronic Medical Record database (Q-EMR), is representative of the US population, however current results may not be generalizable to all SGLT2i users more broadly. Additionally, since this is a cross-sectional retrospective observational study, only associations with the baseline characteristics were assessed. Further, the study only considers the earliest prescription for SGLT2i metformin dual therapy. Dose titration after the initiation of the SGLT2i metformin dual therapy may increase glycemic goal attainment, but is not assessed in the current study.
Conclusion
In this real-world study of adult T2DM patients, more than half of the patients who were treated with metformin and SGLT2i dual therapy were below the glycemic target of HbA1c <8%. The vast majority of patients did not have underlying cardiovascular disease. African American patients had significantly lower odds of attaining HbA1c <8%.
Transparency
Declaration of funding
Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
Declaration of financial/other relationships
DL, LY and SR are employees of Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. KI was an employee of Merck Sharp and Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA during the design phase of this study. NB, RM and NK are employees of CHEORS, North Wales, PA, USA, which has done contract work from Merck & Co., Inc. Peer reviewers on this manuscript have received an honorarium from CMRO for their review work but have no other relevant financial relationships to disclose.
Author contributions
KI, LY and SR were involved in the conception and design of the study. NB, NK, RM were involved in the analysis and interpretation of the data. KI, YL and DL designed the statistical analysis plan. DL and NK wrote this manuscript. All authors were involved in drafting of the paper and revising it critically for intellectual content. All authors approved of the final version of this manuscript and agree to be accountable for all aspects of the work.
CMRO-2020-FT-0543_Supplementary_material_CLEAN.docx
Download MS Word (103.5 KB)Acknowledgements
The authors thank Tania Gulati for her suggestions and critical revisions of the manuscript.
Notes
1 SAS Institute Inc., Cary, NC, USA.
References
- National diabetes statistics report, 2017. Atlanta, GA Centers Dis. Control Prev. US Dept Heal. Hum. Serv. 2017;41:917–928.
- Yang W, Dall TM, Beronjia K, et al. Economic costs of diabetes in the U.S. in 2017. Diabetes Care. 2018;41:917–928.
- Iglay K, Hannachi H, Howie PJ, et al. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr Med Res Opin. 2016;32(7):1243–1252.
- Bashier A, Bin Hussain A, Abdelgadir E, et al. Consensus recommendations for management of patients with type 2 diabetes mellitus and cardiovascular diseases. Diabetol Metab Syndr. 2019;11:80. Available from:/pmc/articles/PMC6761728/?report = abstract.
- Buse JB, Wexler DJ, Tsapas A, et al. 2019 update to: management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2020;63(2):221–228. Available from: https://pubmed.ncbi.nlm.nih.gov/31853556/.
- Association AD. Pharmacologic approaches to glycemic treatment: standards of medical care in diabetes-2020. Diabetes Care. 2020;43:S98–S110.
- Cosentino F, Grant PJ, Aboyans V, et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force for diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and the European Associ. Eur Heart J. 2020;41:255–323. Available from: www.escardio.org/guidelines.
- Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373(22):2117–2128.
- Wiviott SD, Raz I, Bonaca MP, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2019;380(4):347–357.
- McMurray JJV, Solomon SD, Inzucchi SE, et al. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med. 2019;381:1995–2008. Available from: http://www.nejm.org/doi/10.1056/NEJMoa1911303.
- Merck and Pfizer’s SGLT2 Inhibitor STEGLATRO™ (ertugliflozin) Meets Primary Endpoint in VERTIS CV Trial for Patients with Type 2 Diabetes and Atherosclerotic Cardiovascular Disease. [Internet]. 2020. https://www.merck.com/news/merck-and-pfizers-sglt2-inhibitor-steglatro-ertugliflozin-meets-primary-endpoint-in-vertis-cv-trial-for-patients-with-type-2-diabetes-and-atherosclerotic-cardiovascular-disease/.
- Neal B, Perkovic V, Mahaffey KW, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377(7):644–657.
- Perkovic V, Jardine MJ, Neal B, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–2306.
- Levey AS, Eckardt KU, Tsukamoto Y, et al. Definition and classification of chronic kidney disease: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO)z. Kidney Int. 2005;67(6):2089–2100.
- American Diabetes Association. Glycemic targets: standards of medical care in Diabetesd2018. Diabetes Care 2018;41:S55–S64.
- Pantalone KM, Misra-Hebert AD, Hobbs TM, et al. Effect of glycemic control on the Diabetes Complications Severity Index score and development of complications in people with newly diagnosed type 2 diabetes. J Diabetes. 2018;10(3):192–199.
- Pantalone KM, Hobbs TM, Wells BJ, et al. Clinical characteristics, complications, comorbidities and treatment patterns among patients with type 2 diabetes mellitus in a large integrated health system. BMJ Open Diabetes Res Care. 2015;3(1):e000093. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4513350/.
- Giugliano D, Longo M, Maiorino MI, et al. Efficacy of SGLT-2 inhibitors in older adults with diabetes: systematic review with meta-analysis of cardiovascular outcome trials. Diabetes Res Clin Pract. 2020;162:108114. Available from: https://pubmed.ncbi.nlm.nih.gov/32165164/.
- Assari S, Lee DB, Nicklett EJ, et al. Racial Discrimination in health care is associated with worse glycemic control among black men but not black women with type 2 diabetes. Front Public Health. 2017;5:235
- Fan T, Koro CE, Fedder DO, et al. Ethnic disparities and trends in glycemic control among adults with type 2 diabetes in the U.S. from 1988 to 2002. Diabetes Care. 2006;29(8):1924–1925.
- Mishriky BM, Powell JR, Wittwer JA, et al. Do GLP-1RAs and SGLT-2is reduce cardiovascular events in black patients with type 2 diabetes? A systematic review and meta-analysis. Diabetes Obes Metab. 2019;21(10):2274–2283. [cited 2020 Aug 6]. Available from: https://pubmed.ncbi.nlm.nih.gov/31168889/.
- Ajmera M, Raval A, Zhou S, et al. A real-world observational study of time to treatment intensification among elderly patients with inadequately controlled type 2 diabetes mellitus. J Manag Care Spec Pharm. 2015;21(12):1184–1193. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4760631/.
- Thein D, Christiansen MN, Mogensen UM, et al. Add-on therapy in metformin-treated patients with type 2 diabetes at moderate cardiovascular risk: a nationwide study. Cardiovasc Diabetol. 2020;19:107. Available from: https://pubmed.ncbi.nlm.nih.gov/32631337/.
- Patorno E, Pawar A, Bessette LG, et al. Effectiveness and safety of Empagliflozin in routine care patients: interim results from the EMPagliflozin compaRative effectIveness and SafEty (EMPRISE) study. Diabetes. 2020; 69(Supplement 1):134-LB.
- Patorno E, Pawar A, Bessette LG, et al. Cardiovascular outcomes in older adults initiating Empagliflozin vs. DPP-4 inhibitors and GLP-1 receptor agonists: a subgroup analysis from the EMPRISE study. Diabetes. 2020; 69(Supplement 1):133-LB.
- Casagrande SS, Fradkin JE, Saydah SH, et al. The prevalence of meeting A1C, blood pressure, and LDL goals among people with diabetes, 1988–2010. Diabetes Care. 2013;36(8):2271–2279. Available from: https://care.diabetesjournals.org/content/36/8/2271.
- Nathan DM, McGee P, Steffes MW, et al. Relationship of glycated albumin to blood glucose and HbA1c values and to retinopathy, nephropathy, and cardiovascular outcomes in the DCCT/EDIC study. Diabetes. 2014;63(1):282–290. Available from: https://pubmed.ncbi.nlm.nih.gov/23990364/.
- Svensson E, Baggesen LM, Johnsen SP, et al. Early glycemic control and magnitude of HbA1c reduction predict cardiovascular events and mortality: Population-based cohort study of 24,752 metformin initiators. Dia Care. 2017;40(6):800–807. Available from: https://pubmed.ncbi.nlm.nih.gov/28404659/.
- France EF, Wyke S, Gunn JM, et al. Multimorbidity in primary care: a systematic review of prospective cohort studies. Br J Gen Pract. 2012;62(597):e297–e307. Available from: https://bjgp.org/content/62/597/e297.
- Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med. 2002;162(20):2269–2276. Available from: https://jamanetwork.com/.
- Lin PJ, Kent DM, Winn A, et al. Multiple chronic conditions in type 2 diabetes mellitus: prevalence and consequences. Am J Manag Care. 2015;21(1):e23–e34.
- Kathe N, Hayes CJ, Bhandari NR, et al. Assessment of reliability and validity of SF-12v2 among a diabetic population. Value Health. 2018;21(4):432–440.