Abstract
Objective
To assess the relative safety of oral tapentadol PR and other opioid analgesics for moderate or severe chronic pain in adults, we conducted a systematic review and network meta-analysis (NMA).
Methods
A systematic review was conducted to identify randomized controlled trials (RCTs) and randomized withdrawal trials of tapentadol with other WHO stage II and III opioid analgesics in patients with moderate or severe chronic pain. Searches were conducted in MEDLINE, EMBASE, PubMed, Cochrane databases and trial registries. Feasibility assessment evaluated the trials’ suitability for NMA. Outcomes assessed were overall AEs, overall serious adverse events, constipation, nausea, dizziness, somnolence, headache, and discontinuation due to AEs. Randomized withdrawal trials were analyzed separately to other RCTs.
Results
Searches conducted in April 2019 identified 16,604 records. Following screening and feasibility assessment, 29 RCTs and 19 randomized withdrawal trials were identified and included in the NMA. Consistent with existing research, evidence from RCTs suggested that tapentadol is associated with relatively lower odds of adverse events occurring than most active comparators. The withdrawal trial data were less clear, with higher uncertainty around the results, and results that appear to contradict the RCT evidence. There are a number of trial design factors that may be affecting these results.
Conclusions
RCT evidence suggests that tapentadol can be a useful treatment option for patients suffering from chronic pain and in need of an opioid analgesic. Opioids should be prescribed by a qualified physician only after other analgesics have been considered, taking side effects and misuse risk into account.
Introduction
A position paper by the European Pain FederationCitation1 suggests that chronic pain affects 20% of European citizens. This figure is similar to that reported by a recent review of the use of prescription opioids in EuropeCitation2, which found that while 19% of adult Europeans suffer chronic pain of moderate to severe intensity, around half are receiving inadequate pain management. In the US, the National Institutes of Health (NIH) state that chronic pain affects an estimated 100 million Americans (one-third of the US population), 25 million of whom experience significant pain-related activity limitations and diminished quality of lifeCitation3.
Management of pain is difficult, and analgesics, including nonsteroidal anti-inflammatories (NSAIDs), opioids, and others, are an important part of a multimodal approach to the treatment of chronic pain, supporting other medical and nonmedical treatment options. It is important that patients receive appropriate pain treatment with careful consideration of the benefits and risks of the available optionsCitation4. Physicians should take an individualized approach to therapy, carefully weighing the clinical benefits against the potential risks posed by each treatment option on a case-by-case basis.
This manuscript describes the conduct and results of a systematic review (SR) and subsequent network meta-analysis (NMA) of randomized controlled trials (RCTs) and randomized withdrawal trials comparing the safety of an oral formulation of tapentadol PR with other WHO stage II and III opioid analgesics for moderate or severe chronic pain in adult patients.
Methods
This systematic review was undertaken according to the principles of systematic reviewing embodied in the first edition of the Cochrane handbookCitation5 and guidance published by the Centre for Reviews and Dissemination (CRD)Citation6. The protocol has been registered on the PROSPERO database (CRD42018088044)Citation7. details the eligibility criteria for the review.
Table 1. Eligibility criteria.
The search was designed to identify RCTs on the interventions of interest (buprenorphine, codeine, fentanyl, hydrocodone, hydromorphone, morphine, oxycodone, oxymorphone, propoxyphene, pentazocine or tramadol) in patients with chronic pain. Supplementary Appendix A provides details of the MEDLINE search strategy and details of the databases and resources searched.
Records were screened by two reviewers independently, with a third reviewer to adjudicate disagreements. Studies excluded after full-text assessment were listed with reasons for exclusion.
Data extraction and risk of bias assessment was conducted in Excel by a single reviewer, with a second reviewer checking the extracted data points. For each trial, we extracted data on the trial characteristics and design, patient baseline characteristics, details of the intervention and details of the outcomes and results. A full list of data extraction elements can be found within the PROSPERO registration. The risk of bias of each included study was assessed using the Cochrane risk of bias toolCitation8. The risk of bias assessment is summarised in Supplementary Appendix C.
A feasibility assessment (FA) was conducted to identify whether it was appropriate to combine data from the identified trials for the purpose of network meta-analysis. We used guidance produced by the Australian Pharmaceutical Benefits Advisory Committee (PBAC)Citation9 on best practice for the conduct of NMA to help guide this assessment.
Trials in cancer pain patients were analyzed separately to trials of patients with non-cancer-related pain.
It was assumed that event frequencies were not related to study duration because the majority of treatment-related adverse events could be expected to occur towards the beginning of treatment with the study drug. This assumption was assessed by plotting, for each study, the number of events per person against the length of follow-up.
Most studies only reported results for a range of titrated doses, rather than individual doses. Therefore in order to create connected evidence networks, doses were pooled within treatments. This required the assumption that different doses of a particular treatment are equally safe.
To analyse the combined data from the identified studies, Bucher style analysis and Bayesian NMA were used. For the cancer pain networks, the Bucher type analysisCitation10 was used as these networks were small and simple. For all other networks, the analysis was performed within a Bayesian framework using the proportion of patients that experience the event of interest in each arm of each study. A regression model with a binomial likelihood and a logit link function was usedCitation11. Both fixed and random effects models were investigated. With a fixed-effect model, we assume that there is a true treatment effect that is the same across studies, and the differences between studies are due to chance alone. With random-effects however, we assume that the effects being estimated are not the same, but that they are related and follow a distribution. The mean of this distribution is the estimate of the treatment effect. Due to the size of the evidence base, an informative prior was used for the between-study heterogeneity parameter. The log-normal (−2.10, 1.582) prior was chosen; this was based on the work by Turner et al.Citation12 Uninformative priors were used for all other parameters. In the analyses reported here, both fixed and random effects models were fitted where possible. The two model types were then compared through the use of the deviance information criterion (DIC) which gives an indication of model fit, that is, how well the model explains observed patterns in the data.
Relative treatment effects were estimated as log odds ratios and transformed into odds ratios for presentation.
Between-study heterogeneity was assessed using pairwise meta-analyses. A visual assessment was made of the resulting forest plots, and a quantitative assessment was made using the I2 statistics.
Inconsistency is unexplained heterogeneity between direct and indirect evidence made possible by loops in the networks. This was assessed using the node-splitting methodCitation13 which allows comparison of results based on direct evidence against those based on indirect evidence.
Results
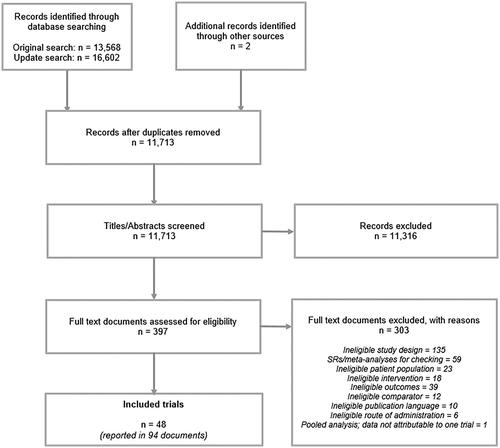
The searches were conducted in December 2017 and identified 13,570 records. An update search, conducted in April 2019 identified 16,602 records. Following deduplication of all the records, 11,713 records were assessed for eligibility ().
29 RCTs, published in 58 documents, were identified that met the eligibility criteria and are presented in Supplementary Appendix B.
An additional 19 randomized withdrawal trials (also presented in Supplementary Appendix B), published in 36 documents, were identified that met the eligibility criteria. Randomized withdrawal trials are a subset of the randomized controlled trial study designCitation14. Randomized withdrawal trials feature a titration phase and a maintenance phase. During the titration phase, the studies are effectively single-arm; all patients receive the investigational treatment and those patients that do not respond to the treatment or that have unacceptable adverse effects do not proceed to the randomized maintenance phase. This difference in trial design means that patient populations in RCTs and randomized withdrawal trials are not considered to be comparable, and they were therefore analyzed in separate networks. For the withdrawal trials, only the comparative maintenance phase results were used in the analysis.
Randomized withdrawal trials are not usually considered for NMA as the patients remaining for randomization to the second phase of the trial no longer represent the population enrolled at the start of the trial. Placebo arms are therefore no longer comparable across trials. Despite these limitations, we analyzed the results of the withdrawal trial maintenance phases in order to investigate the consistency of their results with the results of the RCT analyses.
Cancer pain trials usually include opioid rescue medication for ethical reasons, while non-cancer pain trials often do not. Therefore, cancer and non-cancer trials differ in the adverse event (AE) profiles reported because all arms of a cancer pain trial are influenced by the rescue opioid (which is usually not the investigated molecule but is instead a molecule that has proven efficacy in that indication already). Tolerability advantages of intervention can therefore be masked in cancer pain trials, while they are more obvious in trials that either use no opioid as rescue or allow the investigated intervention as a rescue.
In addition, treatments for cancer are known to cause adverse events, and patients would also be expected to experience events related to cancer as an underlying disease. Patients with cancer pain were expected to experience more adverse events than patients with non-cancer-related pain, and the two populations were considered not to be comparable.
For these reasons, we analyzed trials in cancer pain patients separately to trials in patients with non-cancer-related pain.
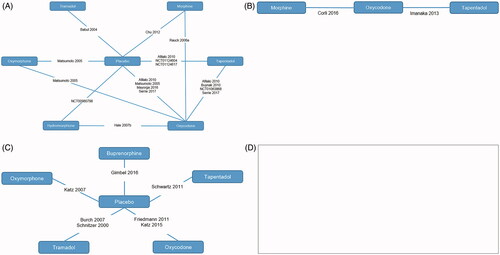
Four sets of networks were explored (); that is, the included trials were broken down into the following broadly homogenous groups in order to permit analysis of the extracted data.
RCTs in patients with non-cancer pain
RCTs in patients with cancer pain
Randomized withdrawal trials in patients with non-cancer pain
Randomized withdrawal trials in patients with cancer pain
Following the feasibility assessment, no networks were possible for withdrawal trials in cancer patients as no relevant trials were identified.
Feasibility assessment
Randomized controlled trials
10 of the 29 RCTs identified in the review were excluded from further consideration in the networks due to differences in study design, opioid status, washout period and interventions.
Five trials had no washout period prior to randomization or had a washout of fewer than two days, which was not considered long enough to wash out the effects of any previous opioid treatmentsCitation15–19. Three further trials reported insufficient details of washout to make a decision as to whether the washout period was adequateCitation20–22. One trial was not genuinely randomizedCitation23 and the final trial lacked a comparator following the decision to collapse all doses for the purposes of the NMACitation24.
19 RCTsCitation25–43 were eligible for inclusion in the networks. presents the 19 RCTs and the interventions assessed by each study.
Table 2. Site of pain and interventions assessed in the RCTs.
Three of the RCTs were conducted in cancer patientsCitation26,Citation30,Citation43, of which twoCitation30,Citation43 shared a common comparator and reported data for two outcomes.
Randomized withdrawal trials
Of the 19 randomized withdrawal trials considered in the feasibility assessment, 8 trials were removed from further consideration in the networks due to differences in opioid status and washout period.
Six trials had no washout period or a washout of less than two daysCitation44–49, and two trials reported insufficient details of washout to make a decision as to whether the washout period was adequateCitation50,Citation51.
presents the 11 randomized withdrawal trialsCitation52–62 and the interventions assessed by each study. All the randomized withdrawal trials were in non-cancer patients and fit together in a single network.
Table 3. Site of pain and interventions assessed in the withdrawal trials.
Following an assessment of data availability, eight outcomes from those detailed in the protocol were selected for an assessment of network feasibility: Overall AEs, overall serious adverse events (SAEs), constipation, nausea, dizziness, somnolence, headache, discontinuation due to AEs. These were the most frequently reported specific AEs from the GI, respiratory and CNS system organ classes, for which networks were feasible. As opioid side effects usually start early in the treatment and either subside (nausea and vomiting) or remain (constipation), the different length of studies was not seen as a relevant confounder when comparing the safety profile, and it was deemed appropriate to compare data across the range of timepoints reported.
Results of the analyses
Randomized controlled trials
Results were all estimated from random-effects models. While for discontinuation due to AE there was a smaller (i.e. better) deviance information criterion (DIC) value for the random-effects model, in general, there was little difference between fixed and random effects versions of the model for each outcome. To ensure consistency, the random effects assumption has therefore been used throughout.
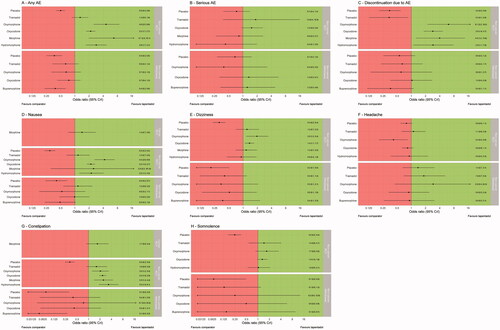
All treatments are compared to tapentadol, with forest plots for all outcomes analyzed presented in . The plots represent the point estimate (black dots) and 95% credible interval of the odds ratio. The dashed vertical line represents the point at which there is no difference between tapentadol and a comparator treatment. Positive estimates favour tapentadol, that is, lower estimated odds of a safety event relative to a comparator.
Figure 2. (A) RCTs in non-cancer patients: all trials contributing to networks; (B) RCTs in cancer patients: all trials contributing to networks; (C) withdrawal trials in non-cancer patients: all trials contributing to networks; (d) withdrawal trials in non-cancer patients: no network was possible.
For the outcome “patients experiencing any AE”, there is evidence that patients treated with tapentadol have lower odds of an AE than those patients treated with one of the active comparators. For the outcome “patients experiencing any serious AE”, low event counts were recorded across trials, with high uncertainty and the analyses showing no strong evidence of a difference in favour of any particular treatment.
For the outcome “discontinuation due to AE”, there is evidence that all active treatments aside from tramadol are associated with higher odds of discontinuation due to AE than tapentadol. This is an outcome of particular interest, as the AE profile for any given group of patients does not only reflect the safety and tolerability profile of the investigated substances but also reflect events occurring due to underlying disease or concomitant medications. However, it is difficult to determine what proportion of AEs were related to the study drug, and this information is not always reported in publications. The exception to this may be “discontinuation due to AE”, as patients are usually discontinued because trial investigators suspect a causal relationship to the study drug. Comparing the results of the analyses of discontinuation due to AE is therefore of particular interest, as a link to the study drug was at least suspected, although it cannot be confirmed because the trial was blinded at the time of investigator’s report and assessment.
For the nausea outcome, the network results are broadly in favour of tapentadol (i.e. tapentadol is less likely to cause nausea), particularly against oxycodone, oxymorphone and hydromorphone, where it even presents a statistically significant difference. The results also favour tapentadol over tramadol and morphine, although no statistical significance could be shown.
For the constipation outcome, tapentadol presents a statistically significantly better profile than all treatments other than tramadol, against which it appears to be numerically better, but could not present a significant difference. In cancer patients, the results are in favour of tapentadol, but the difference is not statistically significant.
For the headache outcome, the only statistically significant result observed was a difference in favour of oxymorphone over tapentadol; all other comparators showed a comparable profile than tapentadol.
For the dizziness and somnolence outcomes, there is evidence in favour of tapentadol when compared with oxycodone and oxymorphone, although for somnolence the differences did not reach statistical significance.
Randomized withdrawal trials
Results of the analyses of randomized withdrawal trials (in non-cancer populations only) showed weak evidence and less conclusive effects; however, any effects shown were generally in the opposite direction to those shown by the RCT evidence. The “any serious AE” outcome showed particularly high uncertainty in the treatment effect estimates, and the 95% credible intervals for all estimates crossed the line of “no difference”. For the “discontinuation due to AE” outcome, there was weak evidence marginally in favour of the comparators for all comparisons apart from that versus oxycodone (where there was no evidence of a difference). Three outcomes (any AE, constipation, and nausea) showed evidence in favour of buprenorphine. The dizziness and somnolence outcomes showed weak evidence in favour of the most active comparators. Finally, there was no evidence of a difference in the odds of experiencing a headache for any of the comparators when compared to tapentadol.
Due to the limitations around the designs of the randomized withdrawal trials, including heterogeneity across trials in the population and duration of the maintenance phases, no meaningful comparative conclusions could be drawn. Most of the outcomes showed high uncertainty in the treatment effect estimates, with wide 95% credible intervals.
Discussion
Discussion of results of randomized controlled trials analyses
In a key 2011 network meta-analysis assessing the efficacy of tapentadol, Riemsma et al.Citation63, concluded that the benefit–risk of tapentadol in patients with chronic severe or moderate pain appears to be improved in comparison with other opioids defined as “step 3” by the World Health OrganizationCitation64. More recent studies have found that tapentadol is equally efficacious as conventional opioidsCitation65,Citation66 in reducing pain following joint surgery.
Riemsma et al.Citation63 found that the superior efficacy of tapentadol over oxycodone shown in their head to head comparison could in part be related to tapentadol’s improved tolerability (with regards to constipation, nausea and vomiting). Tapentadol also presented a favourable tolerability profile against buprenorphine, fentanyl, oxymorphone, oxycodone, hydromorphone and morphine, leading to a reduced number of patients discontinuing treatment. This allowed the full therapeutic effect to be established more often under treatment with tapentadol.
Based on the findings of this review and NMA, evidence from RCTs suggests that tapentadol is associated with relatively lower odds of an adverse event occurring than with most active comparators. This is consistent with an existing network meta-analysisCitation67 and with the current understanding of the mechanism of action of tapentadol. Tapentadol does not rely on (µ-)opioid activity only but also on noradrenaline reuptake inhibition. The two mechanisms and the synergy between them allows for an analgesic activity least comparable with classic strong opioids while requiring less opioid activity (lower µ-load)Citation68. This reduced µ-load leads to lower frequency and magnitude of opioid side effects when comparing tapentadol to pure opioid agonistsCitation68.
Discussion of results of randomized withdrawal trial analyses
Data from randomized withdrawal trials are not usually used to inform NMA due to the significant methodological limitations of the trial design. We also identified heterogeneity across trials in the patient populations and the duration of the titration phases, which ranged from two weeks to eight weeks.
The results of the current analyses of withdrawal trial data are less clear than the results of the RCT analyses; we see higher uncertainty around the results and some results that appear to contradict the RCT evidence. An existing meta-analysis of withdrawal trial dataCitation69 assessed the efficacy rather than safety profile of opioids for chronic pain, and as such could not be directly compared with the results of this NMA. However, they do note that a key limitation when assessing safety in these trials is that patients are deliberately excluded if they experience tolerability issues in the initial titration phase and so the adverse event rates do not represent rates that would occur in RCTs.
Analyses of the randomized withdrawal trials produced inconsistent results. Although none of the factors considered by the authors appears to fully explain this inconsistency, there are a number of methodological factors relating to randomized withdrawal trials that may have contributed.
Only data from the maintenance phase of the randomized withdrawal trials were used in the analyses, as in the titration phase, all patients were treated as a single “arm” and adverse event data were not reported separately for the patients subsequently randomized into the treatment groups. However, it would be expected that patients experience most AEs immediately after receiving an opioid treatment for the first time, that is, at the start of the titration phase. This raises the possibility that analyses of the maintenance phase of randomized withdrawal trials may be consistently underestimating adverse events of the opioid treatments studied as patients are already adapted to opioid intake and their preliminary side effects.
Following the titration phase, the randomized withdrawal trial design allows for discontinuation of those patients who experienced intolerable AEs prior to randomization to the maintenance phase. This means that patients experiencing the most severe and/or frequent AEs would not be represented in data from the maintenance phase. It is, therefore, possible that if one treatment was producing a greater number of adverse events than other treatments, this effect would not be seen in the maintenance phase of the trial as the patients experiencing these events would already have discontinued.
In addition, different criteria between trials for the discontinuation of patients prior to the maintenance phase results in groups of placebo randomized patients which are not necessarily comparable between trials.
The 11 randomized withdrawal trials employed a variety of criteria for selecting which patients would be eligible for randomization to the double-blind phase of the trial. All but one of the studiesCitation53 conducted an efficacy assessment prior to randomization, that is, only patients fulfilling set efficacy criteria would be eligible to continue the trial. Of the ten trials applying efficacy criteria, one of the trialsCitation58 used patient defined criteria, asking patients “has this treatment helped your pain enough so that you would continue to take this medication?” Six of the 11 trials also assessed patients for safetyCitation53–55,Citation60–62. Each of these trials only put patients forward for randomization if they experienced no, or tolerable adverse events. The different criteria used in each of the studies may have contributed to the formation of a slightly different patient population for the double-blind phase of each of the studies.
Further disadvantages of the randomized withdrawal design include potential carryover effects. Due to the unique profile of tapentadolCitation70,Citation71, it is possible that reduced central sensitization could be maintained in tapentadol patients even after they have been re-randomized to placebo, resulting in a lower rate of adverse events than might be expected in patients who were not previously treated with tapentadol. Whether such a potential carryover effect of tapentadol has any impact on side effects while tapering down the drug (i.e. more severe withdrawal syndrome) has not been investigated to date.
Limitations of the analyses
While the analyses of data from randomized withdrawal trials included limitations specific to this study design, additional limitations applied to the analyses of both the RCT and randomized withdrawal trials
In order to make the analyses possible, it was necessary to make a number of assumptions. The majority of the studies treated patients within a dose range for each intervention, rather than at a fixed-dose. Dose ranges overlapped across studies and for this reason, it was not possible to categorize intervention arms into range groups. Due to reporting methods in the included studies, it was not possible to see whether patients at the top of any given dose range experienced more or fewer adverse events than patients at the bottom of the range. For these reasons, it was, therefore, necessary to collapse all doses for each intervention. This represents a major limitation of the review, as frequency/severity of AEs cannot be related to the dose of any particular intervention received. However, it was necessary to take this step to facilitate a network.
Reporting of adverse events by the included studies was inconsistent. Some trials reported only AEs that occurred in at least 5% or 10%Citation32 of patients. Some trials reported only treatment-emergent adverse events (TEAEs), while others reported all AEs. Some trials reported SAEs separately to AEs, while others included SAEs in the figure given for overall AEs. Following clinical input, it was agreed that for the specific adverse events (i.e. all those other than the “any AE” and “any SAE”), only AE data (i.e. not SAE) would be considered in the networks. Where reported, TE-AE data were used in the networks; where TEAE data were not reported, but AE data were reported, the AE data were used.
Use of rescue medication was reported by just under half of the studies used to create the networks. Of the 18 RCTs used in the networks, eightCitation26,Citation29,Citation31,Citation39–42 reported some information on whether patients were allowed rescue medication, whether they were taking it, and if so, what they were taking. Not all of these points were reported by all trials. One RCTCitation33 reported that “Patients had a…washout period when all analgesic medications were stopped”, that is, no rescue medication should have been taken, but data on whether this was actually the case were not given. One RCTCitation30 reported that patients received “ongoing cancer therapy” but did not define this further. Of the 11 randomized withdrawal trials used in the networks, fourCitation54–57 reported details of the use of concomitant medication. One further randomized withdrawal studyCitation58 reported that no rescue medication was permitted, but did not state whether patients adhered to this. The incomplete and inconsistent nature of the reporting of use of rescue medication in both RCT and randomized withdrawal trials meant that it was not possible to make use of concomitant or rescue medications into account in the analyses.
Limitations of the available data
Some (although not all) opioids have been implicated in the development of serotonin syndrome, on the basis that they raise intra-synaptic serotoninCitation72–74. This effect is seen especially when opioids are combined with other serotonergic agents. Although tapentadol has very low serotonergic activity, single cases of serotonin syndrome (SS) have been reported after concomitant use of tapentadol and serotonergic agentsCitation75. As there is no evidence so far on the causal relationshipCitation76, cases of SS were of particular interest to this review. However, none of the included trials reported any instances of serotonin syndrome with tapentadol. It was unclear in some cases whether this was because no cases had occurred, or because the study had not set out to capture cases of SS as a specific named outcome. Larger scale studies and/or analyses incorporating more patients would be necessary to draw any conclusions about the causal relationship and frequency of SS following the use of tapentadol compared to other opioids.
For both the RCT and the randomized withdrawal trial networks, only a small number of studies contributed data for some interventions. Insufficient data were available to investigate all the hypothesized prognostic factors and limitations; interpreting the outcomes observed would ideally require a larger dataset. This conclusion was also reached by the authors of a 2018 NMACitation67 comparing individual opioids.
The available data were also insufficient to determine whether comparator opioids already showed a worse safety profile as compared to tapentadol in the first phase of the randomized withdrawal trials. If this was the case the blinded switch to the intervention arm (same drug, no changes) would lead to tolerance to the side effects of the comparator drug over time, whereas a switch to placebo would lead to reduced side effects from early on in the maintenance phase. If tapentadol had shown the overall better safety profile already in the first phase of the randomized withdrawal trials compared to the comparator drugs, the blinded switch to the intervention arm (same drug, no changes) would theoretically lead almost no changes, and the switch to placebo would lead to fewer changes over time. This could lead to a much more pronounced positive rating when the more incompatible comparator drugs are tapered down and switched to placebo.
Conclusions
The findings of our analyses of RCT evidence are consistent with existing research and suggest that tapentadol’s safety profile may involve less nausea and constipation than that of other opioids and less dizziness and somnolence than oxycodone and oxymorphone. Patients treated with tapentadol have lower odds of an AE than patients treated with other opioids, as well as lower odds of discontinuing therapy due to an AE (except for patients treated with tramadol). Altogether, this suggests that tapentadol can be a useful treatment option for patients suffering from chronic pain and in need of an opioid analgesic.
The finding of our analyses of randomized withdrawal trials was inconsistent and likely not appropriate for assessing safety. This is consistent with the conclusions drawn by existing analyses of data from randomized withdrawal trials, which found weak patterns of evidence with little difference shown between treatment and placeboCitation69, and a less strong association between treatment and outcome than was found in the same outcome from RCT trialsCitation77.
Despite their potential for misuse, opioid analgesics remain an important part of the management of chronic cancer and non-cancer pain. Current guidelines and expert recommendations point out that they should be prescribed by a qualified physician, always after considering alternatives such as non-opioid analgesics, primary disease management, cognitive-behavioral therapy, physical therapy or exercise. Opioid analgesics should only be considered for carefully evaluated, closely monitored patients, and only if they show clear benefits for pain and function. Careful prescribing, with vigilance and caution, is needed to limit potential harmsCitation1.
It is imperative to take an individualized approach to therapy. Important factors include pharmacology of the drug, mode of action of the drug, and pain mechanism present in the patient, and careful weighing of the clinical benefits against the potential risks posed by opioid analgesics must be undertaken on a case-by-case basis. Indication, efficacy, side effects and therapeutic objectives should be regularly assessed in order to adapt or discontinue treatment as necessary.
Transparency
Declaration of funding
YHEC was commissioned by Grünenthal Group to carry out this review.
Declaration of financial/other relationships
CE, TR, MS, and HL are employees of Grünenthal Group. RMC and ME are employees of YHEC; DJ was contracted by YHEC to provide statistical support for the NMA. RF was a consultant to Grünenthal for data analysis and clinical input for the project.
Author contributions
RMC, ME, CE, TR, MS, and HL designed the review protocol. RMC and ME conducted the review with contributions from other YHEC employees. Statistical analyses were carried out by DJ. ME and RMC drafted the manuscript, RF provided clinical input, and all authors contributed to critical revisions and development of the final manuscript. All authors have approved the version to be published, and all authors agree to be accountable for all aspects of the work.
Supplemental Material
Download MS Word (113 KB)Acknowledgements
Mick Arber and Jaana Isojärvi developed the search strategies and conducted the searches for this systematic review. Maria Cikalo worked on record selection and data extraction. MA, JI and MC were employed by YHEC at the time of their work on this review.
Notes
i As there is no established clinical definition of moderate or severe chronic pain, any study in which the authors define the population as experiencing moderate or severe chronic pain was eligible for inclusion. The authors’ definitions or diagnostic criteria were extracted from the texts, where reported, to facilitate comparison.
References
- O’Brien T, Christrup L, Drewes A, et al. European Pain Federation position paper on appropriate opioid use in chronic pain management. Eur J Pain. 2017;21(1):3–19.
- Van Amsterdam J, van den Brink W. The misuse of prescription opioids: a threat for Europe? Curr Drug Abuse Rev. 2015;8(1):3–14.
- National Institutes of Health. Pathways to prevention workshop: the role of opioids in the treatment of chronic pain. Rockville (MD): National Institutes of Health; 2014.
- Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain-United States, 2016. J Am Med Assoc. 2016;315(15):1624–1645.
- Higgins JPT, Green S, editors. Cochrane handbook for systematic reviews of interventions. Version 5.1.0. Oxford (UK): The Cochrane Collaboration; 2011.
- Khan KS, Ter Riet G, Glanville J, et al. Undertaking systematic reviews of research on effectiveness: CRD’s guidance for those carrying out or commissioning reviews. 2nd ed. York (UK): Centre for Reviews and Dissemination (CRD); 2001. (CRD Report 4).
- Centre for Reviews and Dissemination [Internet]. PROSPERO. York (UK): CRD; 2011. [Cited 2015 June 29]. Available from: http://www.crd.york.ac.uk/PROSPERO/
- Higgins JPT, Altman DG, Sterne JAC. Assessing risk of bias in included studies. In: Cochrane handbook for systematic reviews of interventions. Version 510. Oxford (UK): The Cochrane Collaboration; 2011.
- Australian Government Department of Health and Aging. Guidelines for preparing submissions to the Pharmaceutical Benefits Advisory Committee. Version 4.3. Canberra (Australia): Pharmaceutical Benefits Advisory Committee (PBAC); 2008.
- Bucher HC, Guyatt GH, Griffith LE, et al. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50(6):683–691.
- Dias S, Welton NJ, Sutton AJ, et al. NICE DSU technical support document 2: a generalised linear modelling framework for pairwise and network meta-analysis of randomized controlled trials. Sheffield (UK): Decision Support Unit, ScHARR, 2011.
- Rhodes KM, Turner RM, Higgins JP. Predictive distributions were developed for the extent of heterogeneity in meta-analyses of continuous outcome data. J Clin Epidemiol. 2015;68(1):52–60.
- Dias S, Welton NJ, Sutton AJ, et al. NICE DSU technical support document 4: Inconsistency in networks of evidence based on randomized controlled trials. Sheffield (UK): Decision Support Unit, ScHARR, 2011.
- European Patients’ Academy (EUPATI) [Internte]. Clinical trial designs. Brussels (Belgium): European Patients’ Forum (EPF); 2015 [updated 2015 November 23; cited 2020 January]. Available from: https://www.eupati.eu/clinical-development-and-trials/clinical-trial-designs/#Withdrawal_trials
- Binsfeld H, Szczepanski L, Waechter S, et al. A randomized study to demonstrate noninferiority of once-daily OROS(®) hydromorphone with twice-daily sustained-release oxycodone for moderate to severe chronic noncancer pain. Pain Pract. 2010;10(5):404–415.
- Imanaka K, Tominaga Y, Etropolski M, et al. Ready conversion of patients with well-controlled, moderate to severe, chronic malignant tumor-related pain on other opioids to tapentadol extended release. Clin Drug Investig. 2014;34(7):501–511.
- Yu S, Shen W, Yu L, et al. Safety and efficacy of once-daily hydromorphone extended-release versus twice-daily oxycodone hydrochloride controlled-release in Chinese patients with cancer pain: a phase 3, randomized, double-blind, multicenter study. J Pain. 2014;15(8):835–844.
- Markenson JA, Croft J, Zhang PG, et al. Treatment of persistent pain associated with osteoarthritis with controlled-release oxycodone tablets in a randomized controlled clinical trial. Clin J Pain. 2005;21(6):524–535.
- Niesters M, Proto PL, Aarts L, et al. Tapentadol potentiates descending pain inhibition in chronic pain patients with diabetic polyneuropathy. Br J Anaesth. 2014;113(1):148–156.
- Fishman RL, Kistler CJ, Ellerbusch MT, et al. Efficacy and safety of 12 weeks of osteoarthritic pain therapy with once-daily tramadol (Tramadol Contramid OAD). J Opioid Manag. 2007;3(5):273–280.
- Peloso PM, Bellamy N, Bensen W, et al. Double blind randomized placebo control trial of controlled release codeine in the treatment of osteoarthritis of the hip or knee. J Rheumatol. 2000;27(3):764–771.
- Rauck R, Rapoport R, Thipphawong J. Results of a double-blind, placebo-controlled, fixed-dose assessment of once-daily OROS® hydromorphone ER in patients with moderate to severe pain associated with chronic osteoarthritis. Pain Pract. 2013;13(1):18–29.
- Ueberall MA, Mueller-Schwefe GH. Safety and efficacy of oxycodone/naloxone vs. oxycodone vs. morphine for the treatment of chronic low back pain: results of a 12 week prospective, randomized, open-label blinded endpoint streamlined study with prolonged-release preparations. Curr Med Res Opin. 2015;31(7):1413–1429.
- Caldwell JR, Rapoport RJ, Davis JC, et al. Efficacy and safety of a once-daily morphine formulation in chronic, moderate-to-severe osteoarthritis pain: results from a randomized, placebo-controlled, double-blind trial and an open-label extension trial. J Pain Symptom Manage. 2002;23(4):278–291.
- Afilalo M, Etropolski MS, Kuperwasser B, et al. Efficacy and safety of tapentadol extended release compared with oxycodone controlled release for the management of moderate to severe chronic pain related to osteoarthritis of the knee: a randomized, double-blind, placebo- and active-controlled phase III study. Clin Drug Investig. 2010;30(8):489–505.
- Arbaiza D, Vidal O. Tramadol in the treatment of neuropathic cancer pain: a double-blind, placebo-controlled study. Clin Drug Investig. 2007;27(1):75–83.
- Babul N, Noveck R, Chipman H, et al. Efficacy and safety of extended-release, once-daily tramadol in chronic pain: a randomized 12-week clinical trial in osteoarthritis of the knee. J Pain Symptom Manage. 2004;28(1):59–71.
- Buynak R, Shapiro DY, Okamoto A, et al. Efficacy and safety of tapentadol extended release for the management of chronic low back pain: results of a prospective, randomized, double-blind, placebo- and active-controlled phase III study. [Erratum appears in Expert Opin Pharmacother. 2010 Nov;11(16):2773]. Expert Opin Pharmacother. 2010;11(11):1787–1804.
- Chu LF, D’Arcy N, Brady C, et al. Analgesic tolerance without demonstrable opioid-induced hyperalgesia: a double-blinded, randomized, placebo-controlled trial of sustained-release morphine for treatment of chronic nonradicular low-back pain. Pain. 2012;153(8):1583–1592.
- Corli O, Floriani I, Roberto A, et al. Are strong opioids equally effective and safe in the treatment of chronic cancer pain? A multicenter randomized phase IV ‘real life’ trial on the variability of response to opioids. Ann Oncol. 2016;27(6):1107–1115.
- Gana TJ, Pascual MLG, Fleming RRB, et al. Extended-release tramadol in the treatment of osteoarthritis: a multicenter, randomized, double-blind, placebo-controlled clinical trial. Curr Med Res Opin. 2006;22(7):1391–1401.
- Hale M, Tudor IC, Khanna S, et al. Efficacy and tolerability of once-daily OROS hydromorphone and twice-daily extended-release oxycodone in patients with chronic, moderate to severe osteoarthritis pain: results of a 6-week, randomized, open-label, noninferiority analysis. Clin Ther. 2007;29(5):874–888.
- Matsumoto AK, Babul N, Ahdieh H. Oxymorphone extended-release tablets relieve moderate to severe pain and improve physical function in osteoarthritis: results of a randomized, double-blind, placebo- and active-controlled phase III trial. Pain Med. 2005;6(5):357–366.
- Mayorga AJ, Wang S, Kelly KM, et al. Efficacy and safety of fulranumab as monotherapy in patients with moderate to severe, chronic knee pain of primary osteoarthritis: a randomized, placebo- and active-controlled trial. Int J Clin Pract. 2016;70(6):493–505.
- Janssen-Cilag International NV. Placebo-controlled trial with OROS hydromorphone hydrochloride to treat patients with moderate to severe pain induced by osteoarthritis of the hip or the knee. Bethesda (MD): US National Library of Medicine; 2008.
- Johnson & Johnson Pharmaceutical Research & Development LLC. A long-term safety study with tapentadol ER and oxycodone CR in patients with moderate to severe pain due to chronic, painful diabetic peripheral neuropathy (DPN). Bethesda (MD): US National Library of Medicine; 2010.
- Janssen Pharmaceutical. An efficacy and safety study for tapentadol extended release (JNS024ER) in chronic pain participants. Bethesda (MD): US National Library of Medicine; 2010.
- Janssen Pharmaceutical. A phase 2 study of tapentadol extended-release (JNS024ER)) in Japanese participants with chronic pain due to diabetic neuropathic pain or postherpetic neuralgia. Bethesda (MD): US National Library of Medicine; 2010.
- Rauck RL, Bookbinder SA, Bunker TR, et al. The ACTION study: a randomized, open-label, multicenter trial comparing once-a-day extended-release morphine sulfate capsules (AVINZA) to twice-a-day controlled-release oxycodone hydrochloride tablets (OxyContin) for the treatment of chronic, moderate to severe low back pain. J Opioid Manag. 2006;2(3):155–166.
- Serrie A, Lange B, Steup A. Tapentadol prolonged-release for moderate-to-severe chronic osteoarthritis knee pain: a double-blind, randomized, placebo- and oxycodone controlled release-controlled study. Curr Med Res Opin. 2017;33(8):1423–1432.
- Spierings EL, Fidelholtz J, Wolfram G, et al. A phase III placebo- and oxycodone-controlled study of tanezumab in adults with osteoarthritis pain of the hip or knee. Pain. 2013;154(9):1603–1612.
- Wild JE, Grond S, Kuperwasser B, et al. Long-term safety and tolerability of tapentadol extended release for the management of chronic low back pain or osteoarthritis pain. Pain Pract. 2010;10(5):416–427.
- Imanaka K, Tominaga Y, Etropolski M, et al. Efficacy and safety of oral tapentadol extended release in Japanese and Korean patients with moderate to severe, chronic malignant tumor-related pain. Curr Med Res Opin. 2013;29(10):1399–1409.
- Hale ME, Ahdieh H, Ma T, et al. Efficacy and safety of OPANA ER (oxymorphone extended release) for relief of moderate to severe chronic low back pain in opioid-experienced patients: a 12-week, randomized, double-blind, placebo-controlled study. J Pain. 2007;8(2):175–184.
- Rauck RL, Nalamachu S, Wild JE, et al. Single-entity hydrocodone extended-release capsules in opioid-tolerant subjects with moderate-to-severe chronic low back pain: a randomized double-blind, placebo-controlled study. Pain Med. 2014;15(6):975–985.
- Hale ME, Zimmerman TR, Eyal E, et al. Efficacy and safety of a hydrocodone extended-release tablet formulated with abuse-deterrence technology in patients with moderate-to-severe chronic low back pain. J Opioid Manag. 2015;11(6):507–518.
- Hale ME, Laudadio C, Yang R, et al. Efficacy and tolerability of a hydrocodone extended-release tablet formulated with abuse-deterrence technology for the treatment of moderate-to-severe chronic pain in patients with osteoarthritis or low back pain. J Pain Res. 2015;8:623–636.
- Kress HG, Koch ED, Kosturski H, et al. Tapentadol prolonged release for managing moderate to severe, chronic malignant tumor-related pain. Pain Physician. 2014;17(4):329–343.
- Purdue Pharma LP. Efficacy and safety of hydrocodone bitartrate (HYD) in subjects with moderate to severe chronic low back pain. Bethesda (MD): US National Library of Medicine; 2011.
- Bartoli A, Michna E, He E, et al. Efficacy and safety of once-daily, extended-release hydrocodone in individuals previously receiving hydrocodone/acetaminophen combination therapy for chronic pain. Postgrad Med. 2015;127(1):5–12.
- Hale M, Khan A, Kutch M, et al. Once-daily OROS hydromorphone ER compared with placebo in opioid-tolerant patients with chronic low back pain.[Erratum appears in Curr Med Res Opin. 2010 Aug;26(8):1904]. Curr Med Res Opin. 2010;26(6):1505–1518.
- Burch F, Fishman R, Messina N, et al. A comparison of the analgesic efficacy of tramadol contramid OAD versus placebo in patients with pain due to osteoarthritis. J Pain Symptom Manage. 2007;34(3):328–338.
- Friedmann N, Klutzaritz V, Webster L. Efficacy and safety of an extended-release oxycodone (remoxy) formulation in patients with moderate to severe osteoarthritic pain. J Opioid Manag. 2011;7(3):193–202.
- Gimbel J, Spierings EL, Katz N, et al. Efficacy and tolerability of buccal buprenorphine in opioid-experienced patients with moderate to severe chronic low back pain: results of a phase 3, enriched enrollment, randomized withdrawal study.[Erratum appears in Pain. 2017 Feb;158(2):366; PMID: 28092653]. Pain. 2016;157(11):2517–2526.
- Katz N, Rauck R, Ahdieh H, et al. A 12-week, randomized, placebo-controlled trial assessing the safety and efficacy of oxymorphone extended release for opioid-naive patients with chronic low back pain. Curr Med Res Opin. 2007;23(1):117–128.
- Katz N, Kopecky EA, O’Connor M, et al. A phase 3, multicenter, randomized, double-blind, placebo-controlled, safety, tolerability, and efficacy study of Xtampza ER in patients with moderate-to-severe chronic low back pain. Pain. 2015;156(12):2458–2467.
- Rauck RL, Potts J, Xiang Q, et al. Efficacy and tolerability of buccal buprenorphine in opioid-naive patients with moderate to severe chronic low back pain. Postgrad Med. 2016;128(1):1–11.
- Schnitzer TJ, Gray WL, Paster RZ, et al. Efficacy of tramadol in treatment of chronic low back pain. J Rheumatol. 2000;27(3):772–778.
- Schwartz S, Etropolski M, Shapiro DY, et al. Safety and efficacy of tapentadol ER in patients with painful diabetic peripheral neuropathy: results of a randomized-withdrawal, placebo-controlled trial. Curr Med Res Opin. 2011;27(1):151–162.
- Vinik AI, Shapiro DY, Rauschkolb C, et al. A randomized withdrawal, placebo-controlled study evaluating the efficacy and tolerability of tapentadol extended release in patients with chronic painful diabetic peripheral neuropathy. Dia Care. 2014;37(8):2302–2309.
- Vorsanger GJ, Xiang J, Gana TJ, et al. Extended-release tramadol (tramadol ER) in the treatment of chronic low back pain. J Opioid Manag. 2008;4(2):87–97.
- Kawamata M, Iseki M, Kawakami M, et al. Efficacy and safety of controlled-release oxycodone for the management of moderate-to-severe chronic low back pain in Japan: results of an enriched enrollment randomized withdrawal study followed by an open-label extension study. J Pain Res. 2019;12:363–375.
- Riemsma R, Forbes C, Harker J, et al. Systematic review of tapentadol in chronic severe pain. Curr Med Res Opin. 2011;27(10):1907–1930.
- World Health Organization. Impact of impaired access to controlled medications. Geneva (Switzerland): WHO; 2020 [Cited 2020 August 21]. Available from: https://www.who.int/medicines/areas/quality_safety/Impaired_Access/en/
- D’Amato T, Martorelli F, Fenocchio G, et al. Tapentadol vs oxycodone/naloxone in the management of pain after total hip arthroplasty in the fast track setting: an observational study. J Exp Orthop. 2019;6(1):36.
- Rian T, Skogvoll E, Hofstad J, et al. Tapentadol versus oxycodone for postoperative pain treatment the first 7 days after total knee arthroplasty: a randomized clinical trial. Pain. 2020. DOI:https://doi.org/10.1097/j.pain.0000000000002026
- Meng Z, Yu J, Acuff M, et al. Tolerability of opioid analgesia for chronic pain: a network meta-analysis. Sci Rep. 2017;7(1):1995.
- Raff RB, Elling C, Tzschentke TM. Does ‘strong analgesic’ equal ‘strong opioid’? Tapentadol and the concept of ‘µ-load’. Adv Ther. 2018;35(10):1471–1484.
- Meske DS, Lawal OD, Elder H, et al. Efficacy of opioids versus placebo in chronic pain: a systematic review and meta-analysis of enriched enrollment randomized withdrawal trials. J Pain Res. 2018;11:923–934.
- Coluzzi F, Fornasari D, Pergolizzi J, et al. From acute to chronic pain: tapentadol in the progressive stages of this disease entity. Eur Rev Med Pharmacol Sci. 2017;21(7):1672–1683.
- Langford RM, Knaggs R, Farquhar-Smith P, et al. Is tapentadol different from classical opioids? A review of the evidence. Br J Pain. 2016;10(4):217–221.
- Buckley NA, Dawson AH, Isbister GK. Serotonin syndrome. BMJ. 2014;348:g1626.
- Sun-Edelstein C, Tepper SJ, Shapiro RE. Drug-induced serotonin syndrome: a review. Expert Opin Drug Saf. 2008;7(5):587–596.
- Gillman PK. Monoamine oxidase inhibitors, opioid analgesics and serotonin toxicity. Br J Anaesth. 2005;95(4):434–441.
- Gov.uk [Internet]. Tapentadol (Palexia): risk of seizures and reports of serotonin syndrome when co-administered with other medicines. London (UK): Government Digital Service; 2019 [updated 2019 January 09; cited 2020 January 03]. Available from: https://www.gov.uk/drug-safety-update/tapentadol-palexia-risk-of-seizures-and-reports-of-serotonin-syndrome-when-co-administered-with-other-medicines
- Stollenwerk A, Sohns M, Heisig F, et al. Review of post-marketing safety data on tapentadol, a centrally acting analgesic. Adv Ther. 2018;35(1):12–30.
- Busse JW, Wang L, Kamaleldin M, et al. Opioids for chronic noncancer pain: a systematic review and meta-analysis. JAMA. 2018;320(23):2448–2460.