Abstract
Objectives
Chronic obstructive pulmonary disease (COPD) is becoming a leading cause of morbidity and mortality in China. In the IMPACT trial, fluticasone furoate[FF]/umeclidinium[UMEC]/vilanterol[VI] single-inhaler triple therapy demonstrated lower rates of moderate/severe exacerbations than dual therapy with FF/VI or UMEC/VI in patients with symptomatic COPD and a history of exacerbations. This analysis investigates the China cohort and its consistency with the overall ITT population.
Methods
10,355 patients were randomized 2:2:1 to once-daily FF/UMEC/VI 100/62.5/25 µg, FF/VI 100/25 µg, or UMEC/VI 62.5/25 µg for 52 weeks. Endpoints included: annual rates of exacerbations, time-to-first on-treatment moderate/severe exacerbation and change from baseline in trough forced expiratory volume in 1 s (FEV1) at Week-52. Clinical trial registration is NCT02164513 (CTT116855).
Results
535 patients (5.2%) were from China. Annual on-treatment moderate/severe exacerbation rate was 0.81 with FF/UMEC/VI versus 0.96 with FF/VI (rate ratio: 0.84; 95% confidence interval [CI]: 0.64, 1.11; p = .227) and 0.80 with UMEC/VI (rate ratio: 1.02; 95% CI: 0.72, 1.44; p = .929). Hazard ratio for time-to-first moderate/severe exacerbation was 0.84 (95% CI: 0.63, 1.11; p = .218) for FF/UMEC/VI versus FF/VI and 0.89 (95% CI: 0.62, 1.27; p = .516) versus UMEC/VI. Significant improvements in mean change from baseline in trough FEV1 were observed for FF/UMEC/VI versus FF/VI (treatment difference 137 mL; 95% CI: 86, 188; p < .001) and UMEC/VI (63 mL; 0, 125; p = .050). Health status was improved with FF/UMEC/VI versus both dual therapies. Results were similar to the overall ITT population. No new safety signals were identified.
Conclusions
Single-inhaler triple therapy with FF/UMEC/VI versus FF/VI or UMEC/VI reduced the rate and risk of exacerbations, and improved lung function and health status in the China cohort similar to the overall ITT population. No new safety signals were identified.
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive respiratory disease characterized by persistent respiratory symptoms, including dyspnea, cough and/or sputum production, as well as airflow limitation, which results in a decrease in lung functionCitation1. The global burden of COPD is high, with the World Health Organization listing the disease as the third leading cause of death worldwide in 2016Citation2. Furthermore, this substantial burden is predicted to continue with COPD projected to remain a leading cause of death by 2030Citation3.
The burden of COPD in China is greater than that seen in developed countries, with the condition rapidly becoming a leading cause of morbidity and mortality among Chinese peopleCitation4,Citation5. The prevalence of COPD in China has recently been estimated to be between 8.6% and 13.6%, with a higher prevalence in men than in womenCitation4,Citation6. Findings from the Global Burden of Disease Study 2010 show that, regardless of gender, China has higher mean years of life lost to COPD when compared with all other G20 members apart from IndiaCitation7. It has also been noted that approximately 70% of all COPD deaths worldwide occur in South and South East AsiaCitation5,Citation8. In a recent cross-sectional survey of a nationally representative sample of patients with COPD from China, the majority (56.4%) had mild disease (Global initiative for chronic Obstructive Lung Disease [GOLD] stage I), 36.3% moderate disease (GOLD stage II), 6.5% severe disease (GOLD stage III), and 0.9% very severe disease (GOLD stage IV)Citation6. Chinese physicians are encouraged to follow COPD therapy strategies recommended in the GOLD report, i.e. treatment escalation based on symptoms and exacerbation riskCitation9. However, many physicians fail to implement these guidelines adequately and, as a result, appropriate use of COPD medication is inconsistentCitation5,Citation9.
The GOLD management strategy for COPD recommends triple therapy with an inhaled corticosteroid (ICS), a long-acting muscarinic antagonist (LAMA), and a long-acting β2-agonist (LABA) in patients with persistent breathlessness or exercise limitation on dual ICS/LABA therapy, or in patients with recurrent exacerbations despite treatment with dual ICS/LABA or LAMA/LABACitation1. The InforMing the PAthway of COPD Treatment (IMPACT) trial, conducted in 37 countries worldwide, compared once-daily single inhaler triple therapy (fluticasone furoate [FF]/umeclidinium [UMEC]/vilanterol [VI]) with two dual therapies (UMEC/VI [LAMA/LABA] and FF/VI [ICS/LABA]) in patients with symptomatic COPD with at least one moderate/severe exacerbation in the previous year. FF/UMEC/VI significantly reduced the rate of moderate/severe COPD exacerbations and improved lung function and health status compared with either dual therapyCitation10.
The pharmacokinetic profile of inhaled COPD therapies can be impacted by a number of factors including lung receptor occupancy and the amount of drug absorbed into the systemic circulation, which could elicit additional pharmacological effects and contribute to the safety and tolerability profile of the drugCitation11. Moreover, responses to certain treatments have been reported to differ in Chinese patients compared to their Caucasian counterparts, thought to be caused in some cases by differences in the frequency of receptor polymorphisms in patients of differing ethnicity and in some cases to differing adherence to treatmentCitation12,Citation13. Differences in the pharmacokinetic profile of inhaled FF have been observed between Chinese and Caucasian populations, for example, higher FF plasma concentrations and higher Cmax and area under the concentration–time curve (AUC) values, therefore it is of interest to evaluate the efficacy and safety of FF/UMEC/VI triple therapy in patients with COPD in ChinaCitation14. The IMPACT trial was conducted in 37 countries and analyses by geographic region were performed to investigate potential differences from the overall intent-to-treat (ITT) population in the efficacy and safety of FF/UMEC/VI triple therapy. In the analysis of IMPACT, patients enrolled in Japan, improvements in moderate/severe exacerbation rates, lung function and health status with FF/UMEC/VI versus FF/VI and UMEC/VI were similar as those seen in the overall ITT population, with no new safety signals identifiedCitation15. Here we present results of a subgroup analysis based on patients from China enrolled in the IMPACT trial. The primary objective was to evaluate the efficacy of FF/UMEC/VI to reduce the annual rate of moderate/severe exacerbations compared with FF/VI or UMEC/VI dual therapy in the China cohort. Secondary objectives included evaluation of the long-term safety and other efficacy assessments of FF/UMEC/VI compared with dual therapies in this population. The purpose of this analysis was to determine if the efficacy and safety data for patients from China enrolled in the IMPACT trial was consistent with the overall ITT population of the IMPACT trial.
Methods
Study design
The IMPACT trial (GSK study CTT116855, NCT02164513) is a Phase III, randomized, double-blind, parallel-group, multicenter trial conducted in 37 countries between June 2014 and July 2017. Here we report a subgroup analysis in patients from China. The trial design has been described in detail previouslyCitation10,Citation16. Briefly, the total trial duration was approximately 55 weeks, consisting of a 2-week run-in period, a 52-week treatment period, and a 1-week safety follow-up period. All patients in the overall ITT population provided written informed consent. The trial was conducted in accordance with Good Clinical Practice guidelines and the provisions of the Declaration of Helsinki and received approval from local institutional review boards or independent ethics committees.
Study population
Eligibility criteria for enrollment in the trial have been reported previouslyCitation10. Eligible patients were ≥40 years of age with a diagnosis of COPD as defined by the American Thoracic Society/European Respiratory Society (ATS/ERS)Citation17, current or former smokers with a smoking history of ≥10 pack-years, had a COPD Assessment Test (CAT) score ≥10 at screening, and had either a post-bronchodilator forced expiratory volume in 1 s (FEV1) <50% of the predicted normal value and a history of ≥1 moderate or severe exacerbation in the previous year, or a post-bronchodilator FEV1 of 50–80% of the predicted normal value and ≥2 moderate exacerbations or ≥1 severe exacerbation in the previous year. Patients had to have been receiving daily maintenance treatment for COPD for ≥3 months prior to screening.
Exclusion criteria have also been reported previouslyCitation10. Of note, patients with pneumonia or a severe COPD exacerbation that had not resolved ≤14 days prior to screening and ≤30 days following the last dose of corticosteroids (if applicable) were excluded, as were those with respiratory tract infection (RTI) that had not resolved within 7 days of screening, abnormal chest X-ray or resting oxygen requirement of >3 L/min at screening. Patients with a current diagnosis of asthma were also excluded.
The population comprised all randomized patients, excluding those who were randomized in error. The China cohort was derived from the overall IMPACT trial ITT population and only included patients enrolled in China across 41 sites.
Study treatments
Patients were randomized (2:2:1) to receive either once-daily triple therapy FF/UMEC/VI 100/62.5/25 µg, or dual therapy FF/VI 100/25 µg, or UMEC/VI 62.5/25 µg administered via the Ellipta inhaler. Patients continued to use their existing COPD medications during the run-in period and were provided with salbutamol on an as-needed basis (rescue medication) throughout the trial.
Study endpoints
The trial endpoints have been described previouslyCitation10,Citation16. This subgroup analysis evaluated the following endpoints in the China cohort: annual rate of on-treatment moderate/severe exacerbations (primary endpoint), and secondary efficacy endpoints of time-to-first on-treatment moderate/severe exacerbation, on-treatment severe exacerbations, and change from baseline in trough FEV1 and change from baseline in St George’s Respiratory Questionnaire (SGRQ) at Week 52. Other efficacy endpoints included the proportion of SGRQ responders (patients with ≥4-point decrease in SGRQ total score from baseline), change from baseline in CAT score, the proportion of CAT score responders (patients with ≥2-point decrease in CAT score from baseline), and rates of other exacerbations (mild/moderate/severe, moderate only, and requiring treatment with systemic/oral corticosteroids or antibiotics).
Mild exacerbations were defined as exacerbations that were self-managed by the patient and did not require treatment with oral/systemic corticosteroids or antibiotics, moderate exacerbations were defined as requiring treatment with oral/systemic corticosteroids and/or antibiotics, and severe exacerbations were defined as requiring hospitalization or resulting in death.
Safety endpoints included the incidence of adverse events (AEs), serious AEs (SAEs), and AEs of special interest (AESI). AESIs are AEs that were prespecified AEs associated with the known profiles of ICS, LAMAs or LABAs and allow for a comprehensive review of safety data that is not limited to a specific Preferred Term. Adjudication of SAEs was performed by an independent adjudication committee, who categorized the primary event in the SAE report.
Statistical analyses
Details of sample size calculations have been described previouslyCitation10. The trial was not powered for subgroup analysis by country. All summaries, analyses or comparisons performed for the China cohort are for descriptive purposes only. No multiplicity adjustment was applied for analyses of these data.
In the overall ITT population, the number of moderate or severe COPD exacerbations was analyzed using a generalized linear model assuming a negative binomial distribution with covariates of treatment group, sex, exacerbation history (≤1, ≥2 moderate/severe), smoking status (screening), geographical region, and post-bronchodilator % predicted FEV1 (screening)Citation10. In the China cohort, the region covariate was defined as China/Not China and an additional covariate of treatment group by region (China/Not China) interaction was also included in the analysis. Time-to-first on-treatment moderate or severe COPD exacerbation was analyzed using Cox proportional hazards model. Least squares (LS) mean change from baseline in trough FEV1 was analyzed using a mixed-model repeated measures (MMRM) analysis. For the overall ITT population, covariates for FEV1 included group, smoking status (screening), geographical region, visit, baseline, baseline-by-visit, and treatment group-by-visit interactionsCitation10. For the China cohort, the analyses included the same covariates, except for geographical region which was replaced with China/Not China, and the addition of treatment group by region, visit by region and treatment group by a visit by region interactions. For the overall ITT population, the proportion of SGRQ and CAT responders was analyzed using a generalized linear mixed model with a logit link function with the following covariates: treatment group, smoking status (screening), geographic region, visit, baseline value, and baseline-by-visit and treatment-by-visit interactionsCitation10. For the China cohort, the analyses included the same covariates in the model as the overall ITT population, except for geographic region which was replaced with China/Not China. The proportion of patients reporting AEs and SAEs was summarized for each treatment group. In the overall ITT population, multiplicity across selected treatment comparisons and key secondary endpoints was controlled using a hierarchical, truncated Hochberg closed testing procedure. Since all treatment comparisons in the testing hierarchy demonstrated adjusted p-values of <.001, both adjusted and unadjusted p-values were the same, and therefore only unadjusted p-values are presented here.
Results
Study population
Results in the overall ITT population have been previously publishedCitation10. A total of 10,355 patients were randomized to treatment in the overall ITT populationCitation10. Of these, 535 were randomized in China (213 to FF/UMEC/VI, 216 to FF/VI, and 106 to UMEC/VI). Patient disposition for each study cohort is shown in Supplementary Figure 1. The percentages of pre-screen or screen failures and reasons for failures were similar across cohorts. The majority of patients completed study treatment and completed the study; of those treated in the China cohort, 463 (87%) completed study treatment and 479 (90%) completed the study (patients who prematurely discontinued study treatment were encouraged to stay in the study to minimize data loss).
Demographics and baseline characteristics were similar across treatment groups. As anticipated, some demographic characteristics in the China cohort differed from the overall ITT population (). These included body mass index (BMI), % predicted post-bronchodilator FEV1, CAT score and the proportion of current smokers, which were notably lower in patients from China compared with the overall ITT population. In the China cohort, the majority of patients were male (95–96% compared with 66–67% in the overall ITT), with mean age ranging from 65.5 to 66.1 years across the treatment groups (compared with 65.2–65.3 in the overall ITT) and mean BMI ranging from 22.1 to 22.6 kg/m2 (compared with 26.6 to 26.7 kg/m2 in the overall ITT).
Table 1. Demographics and baseline characteristics.
Primary efficacy analysis
In the China cohort, the rate of on-treatment moderate/severe exacerbations among patients randomized to FF/UMEC/VI was 0.81 per year, compared with 0.96 per year among those randomized to FF/VI (rate ratio: 0.84; 95% confidence interval [CI]: 0.64, 1.11; p = .227) and 0.80 per year among those randomized to UMEC/VI (rate ratio: 1.02; 95% CI: 0.72, 1.44; p = .929) (). These results were similar to the overall ITT population, with a point estimate in favor of FF/UMEC/VI versus FF/VI for the reduction in the rate of moderate/severe exacerbations. However, unlike in the overall ITT population, there was no difference between FF/UMEC/VI and UMEC/VI for this endpoint ().
Table 2. Rates and risk (time-to-first analysis) of on-treatment moderate/severe exacerbation.
Secondary efficacy analyses
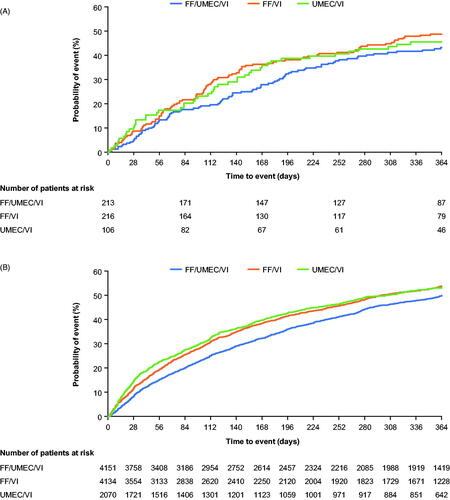
In the analysis of the time-to-first moderate/severe exacerbation in the China cohort, hazard ratios for FF/UMEC/VI versus FF/VI and UMEC/VI were 0.84 (95% CI: 0.63, 1.11; p = .218) and 0.89 (95% CI: 0.62, 1.27; p = .516), respectively ( and ).
Figure 1. Time-to-first on-treatment moderate/severe exacerbation in (A) China; (B) overall ITT*. Abbreviations. FF, fluticasone furoate; ITT, intent-to-treat; UMEC, umeclidinium; VI, vilanterol. *From Lipson et al.Citation10. Copyright © 2018 Massachusetts Medical Society, Reprinted with permission from Massachusetts Medical Society.
The proportion of patients experiencing severe exacerbations while on treatment in China were slightly higher compared to the incidence in the overall ITT population across FF/UMEC/VI, FF/VI, and UMEC/VI treatment groups (18% vs 11%, 17% vs 11%, and 17% vs 13%, respectively; Supplementary Table 1).
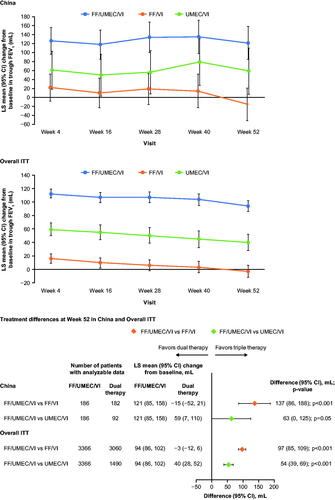
In the China cohort, FF/UMEC/VI significantly improved trough FEV1 at Week 52 versus FF/VI (treatment difference 137 mL; 95% CI: 86, 188; p < .001) and versus UMEC/VI (treatment difference 63 mL; 95% CI: 0, 125; p = .050) (). These improvements were similar to the overall population, although the point estimates for the between-treatment difference were in each case slightly higher in China than in the overall ITT ().
Other efficacy endpoints
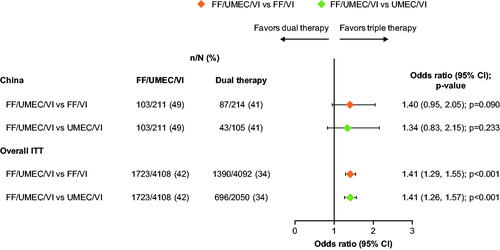
In the China cohort, there was a trend for an increased proportion of SGRQ total score responders at Week 52 with FF/UMEC/VI (49%) compared with FF/VI (41%; odds ratio [OR]: 1.40; 95% CI: 0.95, 2.05; p = .090) and UMEC/VI (41%; OR: 1.34; 95% CI: 0.83, 2.15; p = .233) (). These differences were in line with those observed in the overall ITT population. At Week 52, the LS mean change from baseline data were consistent with the responder data, with significantly greater improvements seen with FF/UMEC/VI versus both dual therapy comparators (Supplementary Figure 2).
Figure 3. Odds of SGRQ response with FF/UMEC/VI versus dual therapy comparators at Week 52. Response is defined as a decrease from baseline in SGRQ total score of ≥4 units. Non-response is defined as a decrease from baseline in SGRQ total score <4 units below baseline, or an increase from baseline in SGRQ total score or a missing SGRQ total score with no subsequent on-treatment scores. Patients did not have a responder status derived if baseline SGRQ total score was missing, or if the SGRQ total score at a particular visit was missing but subsequent on-treatment SGRQ total scores were present. Abbreviations. n, number of responders; N, total number of analyzable patients; CI, confidence interval; FF, fluticasone furoate; ITT, intent-to-treat; SGRQ, St George’s Respiratory Questionnaire; UMEC, umeclidinium; VI, vilanterol.
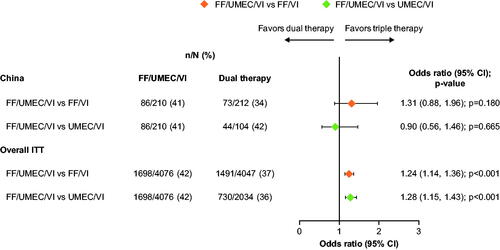
In the China cohort, the proportion of CAT score responders at Week 52 was higher in the FF/UMEC/VI group (41%) than in the FF/VI group (34%; OR: 1.31; 95% CI: 0.88, 1.96; p = .180); the proportion of CAT score responders at Week 52 was similar between FF/UMEC/VI and UMEC/VI (42%; OR: 0.90; 95% CI: 0.56, 1.46; p = .665) (). At Week 52, there was no statistically significant difference in LS mean change from baseline in CAT score between treatment arms (Supplementary Figure 3).
Figure 4. Odds of CAT response with FF/UMEC/VI versus dual therapy comparators at Week 52. Response is defined as a decrease from baseline in CAT score of ≥2 units. Non-response is defined as a decrease from baseline in CAT score <2 units, or an increase from baseline in CAT score, or a missing CAT score with no subsequent non-missing on-treatment scores. Patients did not have a responder status derived if baseline CAT score was missing but subsequent on-treatment CAT scores were present. Abbreviations. CAT; COPD Assessment Test; n, number of responders; N, total number of analyzable patients; CI, confidence interval; FF, fluticasone furoate; ITT, intent-to-treat; UMEC, umeclidinium; VI, vilanterol.
In the China cohort, the rates of mild/moderate/severe COPD exacerbations and COPD exacerbations requiring systemic/oral corticosteroids were lowest in the FF/UMEC/VI group, compared with the FF/VI and UMEC/VI groups, which was also similar to what was observed in the overall ITT (Supplementary Table 2).
Safety endpoints
The incidence of AEs within the China cohort was similar across the three treatment groups, ranging from 75% to 79% (). The most common AESI in the China cohort were cardiovascular effects (15%) and pneumonia (4–13%). These were also among the most frequent AESI in the overall ITT population (10–11% and 5–8%, respectively). There was a higher incidence of pneumonia AESIs in the FF/UMEC/VI and FF/VI groups in the China cohort (13%) compared with the overall ITT population (8% and 7%), while the incidence of pneumonia AESIs in the UMEC/VI group was similar in China and the overall ITT (4% and 5%, respectively). There were no differences across the cohorts for the incidence of adjudicated pneumonia SAEs. The incidence of COPD exacerbation with evidence of pneumonia adjudicated SAEs in the FF/UMEC/VI, FF/VI, and UMEC/VI groups was 6%, 5%, and 5% in China and 3%, 3%, and 3% in the overall ITT, respectively. The incidence of pneumonia/RTI without COPD exacerbation adjudicated SAEs in the FF/UMEC/VI, FF/VI, and UMEC/VI groups was 2%, <1%, and 0% in China and 2%, 2%, and 1% in the overall ITT, respectively ().
Table 3. Summary of on-treatment AESI and adjudicated SAEs.
SAEs occurred in 25–29% of patients in the China cohort, compared with 21–23% in the overall ITT population. In total, there were seven fatal SAEs in the China cohort (two [<1%] in the FF/UMEC/VI group, three [1%] in the FF/VI group, and two [2%] in the UMEC/VI group); these proportions were similar to those seen in the overall ITT population (2% across treatment groups) ().
Discussion
In the China cohort of the IMPACT trial once-daily single-inhaler triple therapy with FF/UMEC/VI reduced the rate of moderate/severe exacerbation versus FF/VI and reduced the risk of these events versus both FF/VI and UMEC/VI in patients with symptomatic COPD and a history of exacerbations, though these reductions were not statistically significant. Furthermore, FF/UMEC/VI significantly improved lung function (as assessed by trough FEV1), and there were trends for improved health-related quality of life (as assessed by SGRQ score response) versus both dual therapy comparators, and improved health-related quality of life (as assessed by CAT score response) versus FF/VI in this cohort. Given the small size of the China cohort and the fact that the trial was not specifically powered for between-treatment comparisons in this cohort, the results of the current analysis should be interpreted based on the similarity of the results relative to the overall ITT population.
As anticipated in a population of patients with a history of exacerbationsCitation18, a high proportion of patients experienced moderate/severe exacerbations during the trial. In the China cohort, FF/UMEC/VI reduced the annual rate of on-treatment moderate/severe exacerbations compared with FF/VI and, while this was not a significant reduction, it was consistent with the pattern in the overall ITT population. This improvement was of a similar direction and magnitude to the results in the overall ITT population. In contrast, there was no clear difference in the annual rate of moderate/severe exacerbations between FF/UMEC/VI and UMEC/VI among patients from China. This may be due to chance variation in the relatively small patient sample receiving UMEC/VI, as patients were randomized to FF/UMEC/VI, FF/VI, and UMEC/VI in a 2:2:1 ratio. Alternatively, it is worth noting that the Chinese population had a slightly more severe disease presentation in terms of lung function and symptoms compared with the overall ITT population, while the exacerbation history profiles were similar, and <1% of the population were receiving LABA/LAMA therapy at baseline compared with 8% of the overall ITT population. Together, these factors may help to explain the slightly better performance of the dual bronchodilator in this population. Importantly, the treatment effects of both FF/UMEC/VI versus FF/VI and FF/UMEC/VI versus UMEC/VI on the time-to-first on-treatment moderate/severe exacerbation in the China cohort were similar to the results seen in the overall ITT population. Of note, rates of mild/moderate/severe COPD exacerbations and COPD exacerbations requiring systemic/oral corticosteroids were lowest in the FF/UMEC/VI group in the China cohort, as compared with FF/VI and UMEC/VI treatment groups. These results illustrate that ICS may have less impact on infective exacerbations treated with antibiotics but could play an important role in the prevention of inflammatory exacerbations that respond to oral corticosteroid use, which is consistent with a previous reportCitation19. Complex patterns of exacerbation data and prevention can occur between the three treatment regimens and this can be impacted by the way exacerbations are treated, including the preference for antibiotic or oral corticosteroid use in various countries. The proportion of patients experiencing severe exacerbations was higher in China than in the overall ITT population across all treatment arms. This difference between the two populations may be due to access to healthcare, as in China many people use hospital care as their primary means of healthcare accessCitation20.
A number of studies have demonstrated the importance of wider measures of clinical efficacy on the patient experience of COPDCitation21–23. As such, other clinically relevant non-exacerbation measures were also analyzed in this China cohort and demonstrated results that were generally consistent with those seen in the overall ITT population. Firstly, statistically significant differences between FF/UMEC/VI and both FF/VI and UMEC/VI were shown for the mean change from baseline in trough FEV1 at Week 52 in the China cohort. Furthermore, the proportion of SGRQ responders at Week 52 was highest in the FF/UMEC/VI group compared with either dual therapy in China, with between-treatment differences similar to those in the overall ITT population. In addition, the proportion of CAT responders at Week 52 in the China cohort was higher in the FF/UMEC/VI group compared with FF/VI; the proportion of CAT responders at Week 52 in the China cohort was similar between FF/UMEC/VI and UMEC/VI.
As previously reported, the safety profile of FF/UMEC/VI in the original IMPACT trial was in line with the profile of each of the individual components and that of the dual combinations FF/VI and UMEC/VICitation24,Citation25. This sub-analysis in the China cohort identified no new safety signals compared with the overall ITT population, with similar incidences of overall AEs, drug-related AEs, and SAEs across the three treatment groups. As expected, based on the class effect for ICS in patients with COPDCitation1, the incidence of pneumonia was highest in ICS-containing treatment groups. The incidences of reported pneumonia AESI were 13%, 13%, and 4% in the FF/UMEC/VI, FF/VI, and UMEC/VI groups in the China cohort compared with 8%, 7%, and 5%, respectively, in the overall ITT population. Due to the higher proportion of males, much lower BMI, and worse lung function, the China cohort had a higher baseline risk of pneumonia compared with the overall ITT populationCitation26,Citation27. It is worth noting that the IMPACT trial used a broad definition of pneumonia to ensure all pneumonia events were captured; serious adverse reports were adjudicated to determine the primary event. Reassuringly, incidences of these reports, primarily adjudicated to be pneumonia with or without evidence of COPD exacerbation, were consistent across these treatment groups in the two cohorts and of much lower magnitude compared with the reported pneumonia events (COPD exacerbation with evidence of pneumonia – China: FF/UMEC/VI 6%, FF/VI 5%, UMEC/VI 5%; overall ITT: FF/UMEC/VI 3%, FF/VI 3%, UMEC/VI 3%; pneumonia/RTI without COPD exacerbation – China: FF/UMEC/VI 2%, FF/VI <1%, UMEC/VI 0%; overall ITT: FF/UMEC/VI 2%, FF/VI 1%, UMEC/VI 1%). The results presented here are similar to those in the KRONOS study, which evaluated patients at low risk of pneumonia or exacerbation, of whom 23% were from ChinaCitation28,Citation29. In the KRONOS study, physician-reported pneumonia rates were higher than adjudicated rates in the overall study population, and the incidence of adjudicated pneumonia in the budesonide/glycopyrrolate/formoterol fumarate treatment arm of the China sub-group was higher than that observed in the overall populationCitation28,Citation29.
This study provides valuable data on the efficacy and safety of FF/UMEC/VI relative to FF/VI and UMEC/VI in a population of patients from China with symptomatic COPD and a history of exacerbations. However, some limitations should be considered when interpreting the results. Firstly, and importantly, the analysis was carried out in only a small sample of patients, representing only 5% of the appropriately statistically powered overall ITT population. In addition, there were clear and distinct demographic differences between the China and overall ITT cohorts which may have affected the magnitude in results seen in drug efficacy and safety. Finally, differences in medical practice between China and the overall ITT population, including lower total use of oral/systemic corticosteroids and higher use of antibiotics, may limit the comparability of the China cohort and overall ITT population. Despite these limitations, our data demonstrate that FF/UMEC/VI is an effective treatment in China for patients with symptomatic COPD and a history of exacerbations. There are no inter-country differences in response to FF/UMEC/VI between patients with COPD in China and the overall ITT population, across multiple efficacy endpoints, providing valuable and relevant information for prescribing physicians in China.
Conclusions
While the IMPACT trial was not powered to demonstrate statistical significance for the primary endpoint of annual rate of on-treatment moderate/severe exacerbations in the China cohort, greater improvements in this endpoint were seen with FF/UMEC/VI versus FF/VI, similar to the results in the overall ITT population, although these improvements did not reach statistical significance. Furthermore, treatment with FF/UMEC/VI resulted in statistically significant improvements in lung function and clinically meaningful improvements in health-related quality of life compared with FF/VI and UMEC/VI. No new safety signals were identified in this cohort. These results highlight the favorable benefit/risk profile of FF/UMEC/VI single-inhaler triple therapy in China for patients with symptomatic COPD and a history of exacerbations.
Transparency
Declaration of funding
Editorial support (in the form of writing assistance, assembling figures, collating author comments, grammatical editing and referencing) was provided by Hayley Mukherjee, PhD, and Eloise Morecroft, at Fishawack Indicia Ltd, UK, and was funded by GSK. This study was funded by GSK (study number CTT116855). The funders of the study had a role in the study design, data analysis, data interpretation, and writing of the report.
Declaration of financial/other relationships
JZ is conducting a cohort study that is supported by GSK. NZ, CW, XDZ, LZ, YDY, BH, BW and LPW do not have any conflicts of interest to report. XD, DAL and JS are GSK employees and hold stock/shares in GSK. Peer reviewers on this manuscript have received an honorarium from CMRO for their review work. One of the reviewers reports grants and personal fees from Boehringer Ingelheim, GSK, Novartis, and AZ and personal fees from Chiesi, Zambon, Guidotti/Malesci, Menarini, Mundipharma, TEVA, and Almiral. The remaining reviewers have no other relevant financial relationships or otherwise to disclose.
Author contributions
The authors meet criteria for authorship as recommended by the International Committee of Medical Journal Editors, take responsibility for the integrity of the work as a whole, contributed to the writing and reviewing of the manuscript, and have given final approval for the version to be published. All authors had full access to the data in this study and take complete responsibility for the integrity of the data and accuracy of the data analysis. NZ and JZ were involved in the acquisition of data and data analysis/interpretation. CW, LPW, XDZ, LZ, YDY, BH, JS, and BW were involved in analysis/interpretation of data. XD was involved in data analysis/interpretation. DAL was involved in the conception/design of the study, acquisition of data and data analysis/interpretation.
Supplemental Material
Download MS Word (294.5 KB)Acknowledgements
Editorial support (in the form of writing assistance, assembling figures, collating author comments, grammatical editing and referencing) was provided by Hayley Mukherjee, PhD, and Eloise Morecroft, at Fishawack Indicia Ltd, UK, and was funded by GSK. The authors thank the investigators, patients and their families, for participation in the IMPACT trial.
Data availability statement
Anonymized individual participant data and study documents can be requested for further research from www.clinicalstudydatarequest.com.
References
- Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease (2019 report); 2019.
- World Health Organization. The top 10 causes of death. 2018. [cited 2019 Jul 30]. Available from: http://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death.
- Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3(11):e442.
- Wang C, Xu J, Yang L, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. 2018;391(10131):1706–1717.
- Kurmi OP, Davis KJ, Hubert Lam KB, et al. Patterns and management of chronic obstructive pulmonary disease in urban and rural China: a community-based survey of 25 000 adults across 10 regions. BMJ Open Resp Res. 2018;5(1):e000267.
- Fang L, Gao P, Bao H, et al. Chronic obstructive pulmonary disease in China: a nationwide prevalence study. Lancet Respir Med. 2018;6(6):421–430.
- Yang G, Wang Y, Zeng Y, et al. Rapid health transition in China, 1990-2010: findings from the Global Burden of Disease Study 2010. Lancet. 2013;381(9882):1987–2015.
- Burney PG, Patel J, Newson R, et al. Global and regional trends in COPD mortality, 1990-2010. Eur Respir J. 2015;45(5):1239–1247.
- Gao J, Prasad N. Chronic obstructive pulmonary disease in China: the potential role of indacaterol. J Thorac Dis. 2013;5(4):549–558.
- Lipson DA, Barnhart F, Brealey N, et al. Once-daily single-inhaler triple versus dual therapy in patients with COPD. N Engl J Med. 2018;378(18):1671–1680.
- Hu C, Jia J, Dong K, et al. Pharmacokinetics and tolerability of inhaled umeclidinium and vilanterol alone and in combination in healthy Chinese subjects: a randomized, open-label, crossover trial. PLoS One. 2015;10(3):e0121264.
- Zheng JP, Yang L, Wu YM, et al. The efficacy and safety of combination salmeterol (50 microg)/fluticasone propionate (500 microg) inhalation twice daily via accuhaler in Chinese patients with COPD. Chest. 2007;132(6):1756–1763.
- Xie HG, Stein CM, Kim RB, et al. Frequency of functionally important beta-2 adrenoceptor polymorphisms varies markedly among African-American, Caucasian and Chinese individuals. Pharmacogenetics. 1999;9(4):511–516.
- Allen A, Bal J, Cheesbrough A, et al. Pharmacokinetics and pharmacodynamics of intravenous and inhaled fluticasone furoate in healthy Caucasian and East Asian subjects. Br J Clin Pharmacol. 2014;77(5):808–820.
- Kato M, Tomii K, Hashimoto K, et al. The IMPACT study – single inhaler triple therapy (FF/UMEC/VI) versus FF/VI and UMEC/VI in patients with COPD: efficacy and safety in a Japanese population. Int J Chron Obstruct Pulmon Dis. 2019;14:2849–2861.
- Pascoe SJ, Lipson DA, Locantore N, et al. A phase III randomised controlled trial of single-dose triple therapy in COPD: the IMPACT protocol. Eur Respir J. 2016;48(2):320–330.
- Celli BR, MacNee W, Force AET. Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J. 2004;23(6):932–946.
- Husebo GR, Bakke PS, Aanerud M, et al. Predictors of exacerbations in chronic obstructive pulmonary disease–results from the Bergen COPD cohort study. PLoS One. 2014;9(10):e109721.
- Wedzicha JA, Calverley PM, Seemungal TA, et al. The prevention of chronic obstructive pulmonary disease exacerbations by salmeterol/fluticasone propionate or tiotropium bromide. Am J Respir Crit Care Med. 2008;177(1):19–26.
- The Commonwealth Fund. The Chinese Health Care System. [cited 2019 Jun 11]. Available from: https://international.commonwealthfund.org/countries/china/.
- Lee SD, Huang MS, Kang J, et al. The COPD assessment test (CAT) assists prediction of COPD exacerbations in high-risk patients. Respir Med. 2014;108(4):600–608.
- Wacker ME, Jorres RA, Karch A, et al. Assessing health-related quality of life in COPD: comparing generic and disease-specific instruments with focus on comorbidities. BMC Pulm Med. 2016;16(1):70.
- Tantucci C, Modina D. Lung function decline in COPD. Int J Chron Obstruct Pulmon Dis. 2012;7:95–99.
- Zheng J, de Guia T, Wang-Jairaj J, et al. Efficacy and safety of fluticasone furoate/vilanterol (50/25 mcg; 100/25 mcg; 200/25 mcg) in Asian patients with chronic obstructive pulmonary disease: a randomized placebo-controlled trial. Curr Med Res Opin. 2015;31(6):1191–1200.
- Donohue JF, Maleki-Yazdi MR, Kilbride S, et al. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25 mcg in COPD. Respir Med. 2013;107(10):1538–1546.
- Crim C, Calverley PM, Anderson JA, et al. Pneumonia risk in COPD patients receiving inhaled corticosteroids alone or in combination: TORCH study results. Eur Respir J. 2009;34(3):641–647.
- Crim C, Dransfield MT, Bourbeau J, et al. Pneumonia risk with inhaled fluticasone furoate and vilanterol compared with vilanterol alone in patients with COPD. Annals ATS. 2015;12(1):27–34.
- Ferguson GT, Rabe KF, Martinez FJ, et al. Triple therapy with budesonide/glycopyrrolate/formoterol fumarate with co-suspension delivery technology versus dual therapies in chronic obstructive pulmonary disease (KRONOS): a double-blind, parallel-group, multicentre, phase 3 randomised controlled trial. Lancet Respir Med. 2018;6(10):747–758.
- Wang C, Yang T, Kang J, et al. Efficacy and safety of inhaled triple therapy budesonide/glycopyrrolate/formoterol fumarate metered dose inhaler (BGF MDI) versus dual therapies in chinese patients with moderate-to-very severe COPD. Am J Respir Crit Care Med. 2019;199:A3333.