Abstract
Objectives
To evaluate patient engagement and usage of a prescription digital therapeutic (PDT) and associated outcomes of opioid use and treatment retention in a large real-world dataset of patients with opioid use disorder (OUD) treated with buprenorphine medication for opioid use disorder (MOUD). PDTs are software-based disease treatments evaluated for safety and effectiveness in randomized clinical trials (RCTs), and authorized by the U.S. Food and Drug Administration (FDA) to treat disease with approved directions for use (label).
Methods
A real-world observational evaluation of an all-comer population of patients who redeemed a 12-week prescription for the reSET-O PDT. Engagement and therapeutic use data were collected and analysed on a population level. Substance use was evaluated as a composite of self-reports recorded with reSET-O and urine drug screens (UDS).
Results
Data from 3144 individuals with OUD were evaluated. 45.5% were between ages 30 and 39 years. 80% completed at least 8 of the 67 possible therapeutic modules, 66% completed half of all modules, and 49% completed all modules. Abstinence during the last 4 weeks of treatment was calculated with two imputation methodologies: 66% abstinent using “missing data excluded (patients with no data as positive)”, and 91% abstinent with “missing data removed (patients with no data excluded)”. 91% of patients met the responder definition of ≥80% of self-report or UDS negative. 74.2% of patients were retained through the last 4 weeks of treatment. Subgroup analysis of patients using reSET-O appropriately (4 or more modules per week for the first 4 weeks) showed 88.1% abstinence using “missing data excluded (patients with no data as positive)”, and retention at weeks 9–12 of 85.8%.
Conclusions
Results demonstrate that reSET-O is readily and broadly used by patients with OUD and that high real-world engagement with the therapeutic is positively associated with abstinence and retention in treatment. ReSET-O is a potentially valuable adjunct to buprenorphine MOUD therapy for patients with OUD.
Introduction
In 2011, the Centers for Disease Control and Prevention (CDC) described the level of opioid use and overdose in the United States as an “epidemic”Citation1. Nearly a decade later, the epidemic has dramatically worsened. In the 12-month period ending February 2020, 51,574 individuals in the United States died from an opioid-related overdose (licit or illicit opioids of any kind), which is approximately 141 overdose deaths each dayCitation2. The rate of overdose deaths related to fentanyl, in particular, has risen exponentially since 2011Citation3.
In addition, an estimated 2.1 million people in the U.S. aged ≥12 years in 2017 were diagnosed with Opioid Use Disorder (OUD), but only about 1 in 5 received treatmentCitation4. As severe as the toll of opioid use and overdose is on those grappling with OUD, the psychological, societal, and economic impacts that ripple out from each individual with OUD greatly multiply the devastating impact of the opioid epidemic.
These alarming statistics persist despite strong evidence that medical treatment delivered by primary care physicians, or in specialized clinics, can significantly reduce rates of death, overdose, and relapse in persons with OUDCitation5,Citation6. These evidence-based treatments, however, remain vastly under-used, and the number of providers authorized to provide these treatments remains inadequateCitation6.
Addiction treatment systems are struggling to address the multiple needs of individuals with OUD. Medications for opioid use disorder (MOUD) along with behavioral therapy, are standard of care for OUDCitation7. But between 80 and 90% of individuals who need MOUD and/or behavioral therapy do not receive recommended careCitation8. Common reasons for this gap in care include refusal to seek treatment, cost of care, stigma, and lack of, or limited access to, treatmentCitation6. Only 25% of outpatient treatment facilities in the US offer MOUDCitation9, and those relatively few providers authorized to provide MOUD prescribe these medications far below their patient limitsCitation10. These difficulties are magnified in rural communities, where substance use treatment centers or addiction specialists are often difficult to access.
Evidence-based behavioral approaches for OUD are resource-intensive and challenging to implement as they require intensive training and ongoing supervision to ensure correct and consistent deliveryCitation11,Citation12. Furthermore, in-person behavioral therapy may not be readily available to support patients in a moment of crisis. In a 2017 survey, of 8.5 million adults with co-occurring substance use disorder and any mental health issue only an estimated 8.3% received behavioral therapyCitation4. MOUD is critical to reduce cravings via partial-agonism or antagonism of opioid receptors, but behavioral therapy is essential for reinforcing behaviors that help patients progress towards a sustainable long-term recovery. Behavioral therapy, in addition to helping patients better manage a range of life challenges, can also exert therapeutic benefits via changes in the physical structure and neurofunctional responses of the brain (i.e. neuroplasticity)Citation13.
Attrition from OUD treatment (pharmacological, behavioral, or both) is another major barrier to successful outcomes with an estimated 30% attrition rate within the first month and attrition rates of 50% or higher within the first 3 monthsCitation14–18. Studies have shown an association between actively engaging patients in their treatment and improved retention and successful recoveryCitation19,Citation20.
Prescription digital therapeutics (PDTs) are software-based disease treatments that deliver evidence-based treatment that is evaluated for safety and effectiveness in randomized clinical trials (RCTs), and are authorized by the U.S. Food and Drug Administration (FDA) to treat disease with approved directions for use (label). Prescribed and initiated by treating physicians, and delivered on mobile devices, PDTs have the potential to safely expand access to evidence-based therapies, which is highly relevant in the context of limited access to clinicians, either because of geographical variations or as a result of restrictions due to the COVID-19 pandemic. PDTs can also enhance the therapeutic relationship by giving clinicians real-time information about their patients and their progress in treatment.
ReSET-O received FDA market authorization in late 2018 and is an 84-day PDT intended as a behavioral treatment for patients with OUD at any stage of buprenorphine MOUDCitation21. It delivers a form of evidence-based neurobehavioral therapy founded on the community reinforcement approach (CRA), an intensive addiction-specific form of cognitive behavioral therapy (CBT) validated in a 2014 clinical trial for OUDCitation22. The CRA approach is based on the idea that drugs compete with more delayed prosocial reinforcers, hence treatment teaches patients to increase satisfaction with drug-free sources of reinforcmentCitation23. ReSET-O content consists of a series of 67 interactive, on-demand audio, text, and video CRA modules (also called therapy lessons) which are sequentially unlocked as patients progress through the therapeutic. Modules are designed to deliver approximately 30 min of treatment. It is recommended that patients complete 4 modules per week. Participants can revisit already-completed modules, but are required to complete the sequence of modules in the order prescribed.
Comprehension of, and proficiency with, therapeutic content is supported by a neurobehavioral approach known as “fluency training” that uses a quiz format to reinforce understanding of positive adaptive behaviors immediately following each lesson. Contingency management, a highly-effective evidence-based approach for OUD treatment, follows each lesson to immediately recognize and reward patients’ engagement with treatment.
In the pivotal randomized controlled trial (RCT) on which FDA authorization was based, 82% of 170 adult OUD patients at a single addiction treatment center who received treatment with the PDTFootnotei stayed in treatment versus 68% of those who only received treatment as usual (TAU), and the likelihood of abstinence during weeks 9–12 was 77.3 vs. 62.1%, respectively (p < .05 for both comparisons)Citation24,Citation25.
RCTs are the standard for evaluating safety and efficacy of novel therapeutics, but RCTs are conducted using Good Clinical Practice (GCP) and design conditions optimized to evaluate internal validity and causality using gold-standard outcome measures to ensure maximum scientific validity. Real-world evidence complements RCTs by evaluating the generalizability of therapeutic use in the context of real-world clinical careCitation26,Citation27. Real-world data collected in the course of day-to-day health care delivery and in the absence of stringent research constraints provides a complementary evaluation of therapeutic performance, including measures of patient engagement and assessments of clinically relevant outcomes, which provide data to evaluate the external validity of RCT resultsCitation26,Citation27.
This real-world analysis is based on a large dataset of patients prescribed and using reSET-O on mobile devices (i.e. smartphones or tablets) in the routine course of their treatment in clinics across the US and focuses on patient engagement/product usage data and the clinical outcomes of opioid use and retention, including associations with other relevant variables. Although PDTs have great potential to address the unmet needs of OUD treatment, it has been questioned if patients with OUD will engage with and use a PDT and whether such use is associated with improvements in relevant clinical outcomes in real-world settings.
Methods
This is a real-world observational evaluation of an all-comer population of patients who accessed an initial prescription for reSET-O under routine care by their clinician. Patients were under the care of clinicians in 30 US states in a range of outpatient treatment settings and organizations. Engagement and other therapeutic use data (de-identified and patient-consented [via terms of the service agreement]) were collected and analysed on a population level. An “active day” was defined as a day in which a patient used any feature of reSET-O. The dose, or unit of treatment, is a 30-minute module, with patients instructed to complete four per week.
Substance use was evaluated as a composite of patient self-reports recorded via the reSET-O therapeutic as well as with urine drug screens (UDS) performed in clinics and recorded by clinicians. UDS schedules varied widely by sites as different organizations follow different UDS protocols and UDS frequency also varies by the phase of treatment individual patients are in.
Two endpoints of opioid use were analyzed. The primary endpoint was a conservative measure of achieving abstinence for the last 4 weeks (weeks 9–12) of the PDT 12-week prescription. This was the same endpoint evaluated in the pivotal RCTs for both reSET and reSET-OCitation25,Citation28. To be deemed abstinent the patient must not have any positive UDS or self-reports during the last 4 weeks.
An “abstinent week” was defined as a week with no positive self-reported use or UDS. The entire 4-week period was then evaluated for any positive self-report or UDS. Consistent with prior real-world and observational studiesCitation29, missing abstinence data for any given week was imputed in two different ways. The first approach was “missing data excluded (patients with no data as positive),” where weeks with no outcomes were excluded and participants were considered positive if any self report or urine drug screen was positive in the last 4 weeks or if all results were missing. The second approach was “missing data removed (patients with no data excluded),” where patients without any self-reports or urine drug screen results during the last 4 weeks were dropped from the analysis population.
The secondary endpoint of substance use was a responder analysis of patients with ≥80% negative UDS or self-reports for the duration of their 12-week prescription, a standard that has been used in other studies of OUD treatment and that is consistent with FDA guidanceCitation30–32.
Results
Demographics
De-identified data from 3,144 individuals with OUD across the US who received their first prescription for reSET-O by their clinician, consented to use, redeemed their prescription, and completed at least one module define the sample evaluated in this real-world analysis. 15.4% of individuals were between ages 19 and 29, 45.5% were between 30 and 39, 25% were between 40 and 49, 11.2% were between 50 and 59, and 2.9% were age 60 or older. Information on gender was incomplete: 33.7% of the population provided no data on gender, 39% reported female gender, and 27.3% reported male gender. Patients are not asked to provide other demographic variables (e.g. race, ethnicity, socioeconomic status) as part of their use of reSET-O, hence, these data are not available at this time.
Therapeutic use/patient engagement
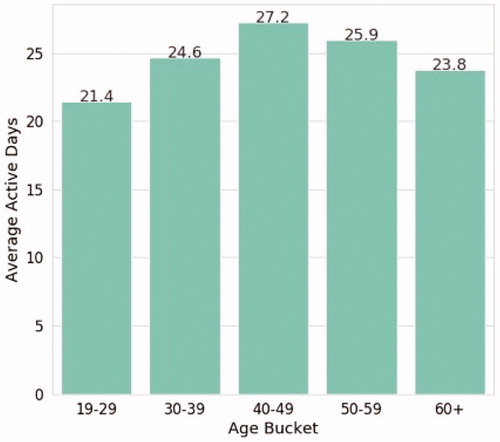
Engagement with reSET-O, defined as any use of the therapeutic during a 24-hour period, as well as completion of therapy lessons (modules), was well-distributed across age categories (). Patients are recommended to complete 4 therapy lessons per week as a standard dose, starting with the 31 core modules and then, when the core modules are completed, an additional 36 supplemental modules.
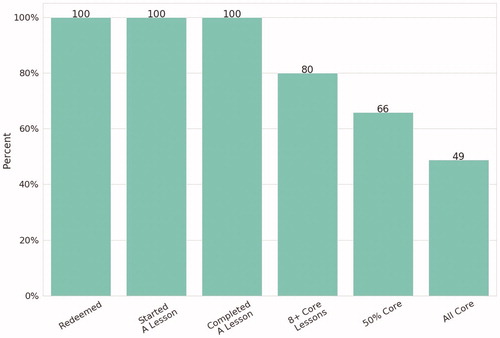
In a pattern similar to that seen in earlier RCTs, not all individuals completed all of the therapeutic core modules (), but in this population of individuals coping with the challenges of OUD and other potential comorbidities 80% completed 8 or more core modules, 66% completed half of all core modules, and 49% completed all core modules. As context, 8 core modules correspond roughly to a minimal amount of behavioral support, half of all core modules represents robust use, and completing all core modules is approaching full-dose.
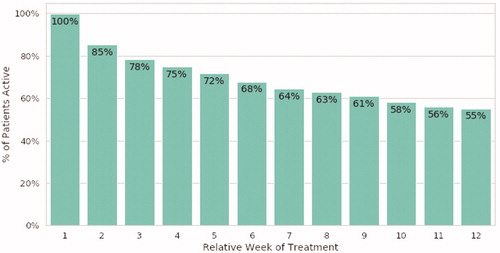
These data demonstrate a real-world engagement and product use pattern where there is a gradual reduction in the use of the therapeutic with time, from 100% in the first week, to 55% of individuals in week 12 continuing to engage and use the therapeutic ().
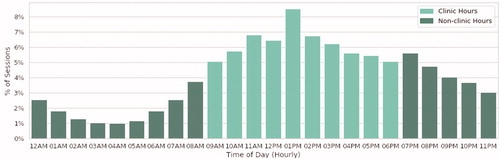
An analysis of program uses by time of day showed substantial evening use (i.e. from 7 p.m. to midnight) ().
Clinical outcomes
Substance use/abstinence
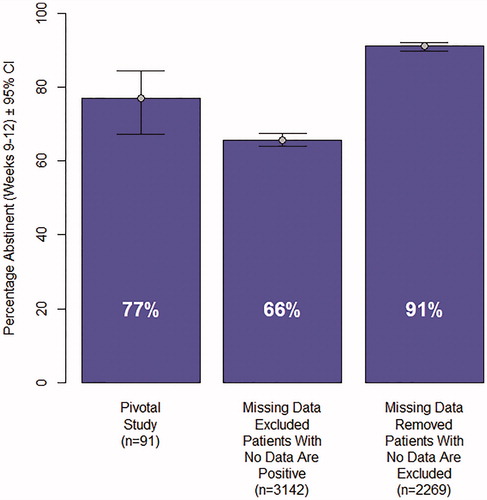
compares abstinence in the last 4 weeks of treatment in the pivotal study (n = 91, 77% abstinent) with the abstinence rates observed in the real-world data using two methods (described above) for imputing missing data. In the “missing data excluded (patients with no data as positive)”, approach the abstinence rate of patients was observed to be 66%. 873 individuals in the real-world dataset had no UDS or self-report data in the last 4 weeks of treatment. In the “missing data removed (patients with no data excluded)”, imputation analysis, these 873 patients were removed from the analysis. The abstinence rate in the remaining sample of 2269 patients was 91%.
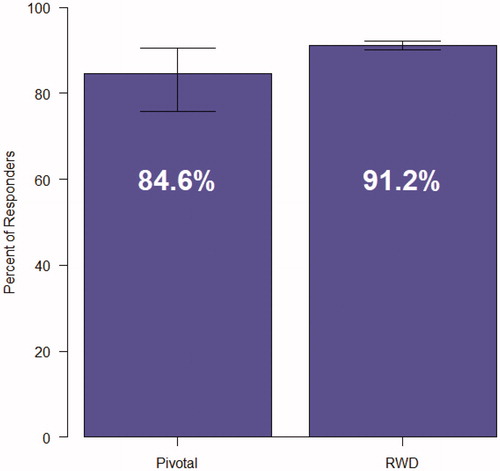
A secondary endpoint evaluating opioid use was a responder analysis of participants who had ≥80% negative UDS or self-reports (). 91.2% of patients in this analysis met the criteria for responder vs. 84.6% of patients in the pivotal trial.
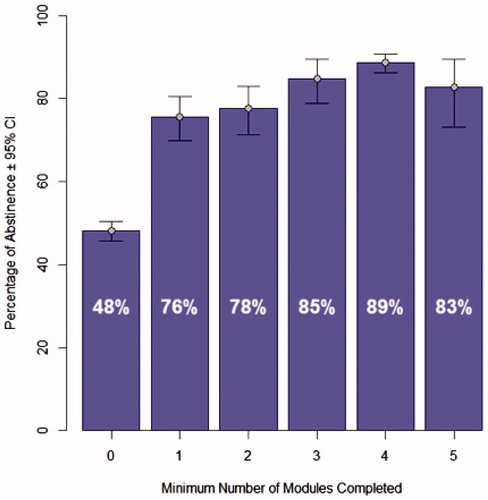
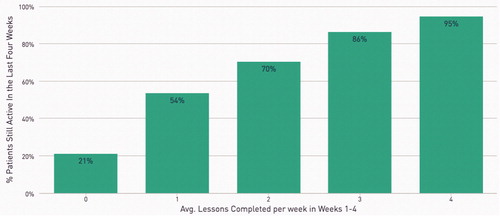
Previous analyses have found that the use of the therapeutic in the first four weeks is predictive of long-term outcomes. Abstinence in the last 4 weeks of treatment was positively associated with engagement/therapeutic use of reSET-O in the first 4 weeks of treatment (). A sizeable increase in abstinence in the final 4 weeks was observed in patients who went from a minimum of 0 modules per week to patients who completed at least 1 module in the first 4 weeks (48% abstinent to 76% abstinent). (Note: a minimal number of modules means that in at least 1 week no modules were completed, but modules could have been completed in other weeks). Abstinence rates generally rose with progressively more modules completed in the first four weeks up to four modules per week, in which patients achieved an 89% abstinence rate.
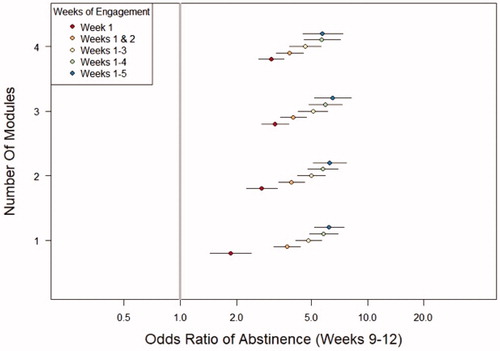
As shown in , a consistent pattern of association between abstinence in the last 4 weeks of treatment and duration of engagement with reSET-O was observed, a pattern that varied when analysed by how many modules individuals completed. 89.4% of the variation in the odds ratio for abstinence was due to duration of engagement, whereas 7.7% of the variation was attributable to how many modules an individual completed each week. Both consistency as well as therapeutic use/dose with the therapeutic, were important for achieving improved outcomes of abstinence.
Retention
Retention is a measure of patients continuing to participate in treatment. In the pivotal clinical trial, where appointment logs where available, 82% of participants were still active in the last week of treatment, defined as the last face-to-face visit. In this analysis of real-world data, patient appointment logs were not available. Retention at the end of prescription was thus defined using a surrogate of patient activity in the software treatment during the last 4 weeks of the 12-week prescription. Using this surrogate definition 74.2% of patients with OUD were continuing to participate at the end of treatment.
Retention in the last 4 weeks of treatment was positively associated with increasing numbers of modules completed in the first 4 weeks of treatment (). Only 22% of individuals completing no modules in the first 4 weeks remained in treatment in the last 4 weeks, whereas 94% of individuals completing an average of 4 modules per week in the first 4 weeks of treatment met the definition of retention.
A subgroup analysis was performed of the 29% of patients who used reSET-O appropriately and consistently for the first 4 weeks, i.e. completing 4 or more modules per week. In this group, median number of lessons completed in the first 4 weeks was 18, median number of lessons per entire prescription duration was 48, abstinence (“missing data excluded [patients with no data as positive]”) was observed to be 88.1%, and retention (defined as activity in weeks 9–12) was 94.6% ().
Table 1. Subgroup analysis of patients using reSET-O appropriately in first 4 weeks of treatment.
Discussion
With real-world data from 3144 individuals with OUD, this cohort represents one of the largest datasets of community use of OUD treatment analyzed to date. It is more than 30 times larger than the patient population in the pivotal study on which FDA authorization of reSET-O was based and is drawn from a geographically diverse and clinically heterogeneous population.
The data from these analyses robustly demonstrate that individuals with OUD across the age span will use and readily engage with a prescription digital therapeutic across the entire prescription duration, and that a majority will access high levels of the recommended content dose. The engagement and retention data seen in these analyses of reSET-O are consistent with levels of adherence seen in large observational studies of retention in buprenorphine treatment, i.e. 37% adherence after 12 monthsCitation33 and 47.5% adherence at 6 monthsCitation34. Exponential declines in use, as reported in other real-world data of health and wellness apps, were not observed, and these adherence and engagement data were superior to adherence rates of buprenorphine treatment in prior observational studiesCitation33–35.
The data also revealed positive associations (e.g. real-world dose-response) between reSET-O and the key outcomes of abstinence in the last 4 weeks of treatment and retention in treatment. These results may be relevant to clinicians, public health experts, and health care policy makers who are challenged to provide effective, widely-available treatments to patients with OUD.
The use in this analysis of a composite self-report and/or UDS measure of abstinence/substance use is consistent with other RWE and observational studies of patients with OUD. Studies by Ling et al.Citation36 and Soeffing et al.Citation37 used self-report/UDS data; studies by ZhuCitation38 and DarkeCitation39 used self-reported abstinence data only, and a study by Leonardi et al.Citation40 used UDS data only. Unlike a clinical trial where UDS and/or self-report are collected for evaluating effectiveness, decisions to collect UDS in real-world clinical practice settings are determined by the care model of a particular addiction facility, by clinicians based on the specific needs and status of individual patients, or by attempts to minimize unnecessary testing.
It is worth reiterating that evaluation of real-world data is not a replacement, but rather an augmentation of RCT-derived data, which can inform complementary dimensions of care, such as patterns of treatment, patient needs, treatment adherence, and patterns of patient use of a therapeuticCitation41. Real-world data are the foundation for analyses that result in real-world evidence (RWE), the clinical evidence about the use and potential benefits or risks of a medical productCitation41. RWE is part of the continuum of evidence that includes data from RCTs and health economics outcomes research (HEOR) and must be interpreted with an awareness of its limitations.
Limitations
While real-world data and evidence can facilitate evaluation of generalizability such data have intrinsic limitations compared to clinical trials. First, all data come from the use of reSET-O. Unlike in a clinical trial, there is no source document verification by monitors in a GCP-conducted study. The data come from direct inputs entered by the patient, the clinician, or that are captured automatically by the software and the mobile device log. Further limitations include the absence of comparison groups, a mixed population of patients at different stages of treatment, potentially sub-optimal use of the therapeutic, variability in use of the therapeutic, and a lack of demographic data beyond age and gender. This analysis shows, however, that a majority of patients engaged in a meaningful way with the therapeutic despite this variability of use under real-world conditions.
Second, results are highly sensitive to methods of imputation for missing data and frequency of outcomes reports, both self-report and UDS (as observed in ). Clinical trials of treatments for addiction typically involve frequent and standardized UDS and standardized assessments in order to evaluate efficacy. In real-world practice, collection of UDS and other drug use assessments are highly variable and depend on policies at provider organizations and individual clinician behaviors. Infrequent UDS collection, particularly in patients who are progressing well in their recovery, is often the rule rather than the exception. How to impute missing data in addiction clinical trials as well as in observational and real-world settings is an ongoing research challenge.
Patterns of UDS ordering and collection (e.g. low use among those on long-term buprenorphine therapy) may introduce confounders that are difficult to control for in real-world analyses. The two imputation methods used in these analyses are commonly used and standard, particularly in observational and cohort studies. While some RCTs use regularly-scheduled weekly, twice-weekly, or three-times weekly UDS and self-report collection, this resource-intensive testing approach is not widely used in a resource-constrained healthcare system. In the “missing data excluded (patients with no data as positive)” analysis, patients who had no UDS or self-report during the final four weeks, were coded as positive, which is a conservative imputation methodology for real-world data and may under-estimate rates of abstinence.
These analyses did not look at a number of additional questions such as approaches for clinicians and provider organizations to engage more patients in treatment or how to increase levels of consent/redemption of their prescription for reSET-O.
Conducting research in a real-world patient population coping with the difficult disease of OUD has its challenges, and such data must be interpreted in the context of pivotal trial data, other RCTs, and real-world utilization research.
Conclusions
The results of this evaluation of a large, real-world addiction interventional dataset are consistent with similar positive findings of safety and effectiveness of the PDT reSET-O in RCTs and health economic analysesCitation22,Citation25,Citation42–44.
In summary, the findings of this study include: first, patients across a broad range of ages and sex used the therapeutic. In fact, patients in the age cohort of 40–49 years had the highest rates of use and engagement, demonstrating that in the real world as well as in clinical trials use is not limited to young adults. Second, for a patient population with a disease characterized by poor seeking of and participation in treatment, therapeutic use and engagement were robust and were much higher than levels seen in many other therapeutic categories as well as health and wellness software applications in mental health, supporting the relevance of the therapeutic content for this underserved patient populationCitation33–35. Third, positive outcomes in terms of abstinence, retention, and responders with ≥80% negative UDS or self-report were observed and consistent with prior findings in clinical trialsCitation22,Citation25,Citation43,Citation44. Lastly, the observed positive association between patient use of the therapeutic and key clinical outcomes shows a “dose-response” relationship also seen in prior clinical trials. Together, the data and analyses presented here suggest the generalizability and real-world impact of a PDT integrated into standard of care for patients with OUD.
Transparency
Declaration of funding
This evaluation was funded entirely by Pear Therapeutics Inc.
Declaration of financial/other relationships
All authors are employees of, or contractors for, Pear Therapeutics Inc. Peer reviewers on this manuscript have received an honorarium from CMRO for their review work but have no other relevant financial relationships to disclose.
Author contributions
YAM led and contributed to the design and conduct of the real-world analysis, and assisted with conceptualization of the data analysis, review, and revision of the manuscript. RG contributed to the design and conduct of the real-world analysis, and assisted with conceptualization of the data analysis, review, and revision of the manuscript. FV contributed to the design and conduct of the real-world analysis, and assisted with conceptualization of the data analysis, review, and revision of the manuscript. BI contributed to the review and revision of the manuscript. KB contributed to the review and revision of the manuscript. AK contributed to the design and conduct of the real-world analysis and assisted with conceptualization of the data analysis, review and revision of the manuscript. HFL contributed to the review and revision of the manuscript. KW contributed to the review and revision of the manuscript. SB wrote the initial draft of the manuscript and contributed to revision of the manuscript. XX contributed to the design and conduct of the real-world analysis and assisted with conceptualization of the data analysis, review and revision of the manuscript. All authors agree to be accountable for all aspects of the work.
Acknowledgements
No assistance in the preparation of this article beyond that listed in Author Contributions is declared.
Notes
i In the pivotal clinical trial, patients accessed the clinical content, at the time called TES, via a browser whereas reSET-O delivers the equivalent clinical content and treatment logic via a mobile application downloaded from an app store.
References
- Centers for Disease Control and Prevention. Vital signs: overdoses of prescription opioid pain relievers—United States, 1999–2008. MMWR Morb Mortal Wkly Rep. 2011;60(43):1487–1492.
- Centers for Disease Control and Prevention. 12 month-ending provisional number of drug overdose deaths by drug or drug class. [cited 2020 Sep 28]. Available from: https://www.cdc.gov/nchs/nvss/vsrr/drug-overdose-data.htm.
- Spencer MR, Warner M, Bastian BA, et al. Drug overdose deaths involving fentanyl, 2011–2016. National Vital Statistics Reports. 2019;68(3):108.
- Substance Abuse Mental Health Services Administration. Key substance use and mental health indicators in the United States: results from the 2018 National Survey on Drug Use and Health. Rockville (MD): Center for Behavioral Health Statistics and Quality, Substance Abuse and Mental Health Services Administration; 2019.
- Wakeman SE, Barnett ML. Primary care and the opioid-overdose crisis – buprenorphine myths and realities. N Engl J Med. 2018;379(1):1–4.
- National Academies of Sciences Engineering and Medicine. Medications for opioid use disorder save lives. Washington (DC): The National Academies Press; 2019.
- American Society of Addiction Medicine. National practice guideline for the use of medications in the treatment of addiction involving opioid use. Chevy Chase (MD): American Society of Addiction Medicine, Inc.; 2015.
- Center for Behavioral Health Statistics and Quality. 2017 National survey on drug use and health: detailed tables. Rockville (MD): Substance Abuse and Mental Health Services Administration; 2018.
- Substance Abuse and Mental Health Services Administration. National survey of substance abuse treatment services (N-SSATS): 2017. Data on substance abuse treatment facilities. Rockville (MD): Substance Abuse and Mental Health Services Administration; 2018.
- Duncan A, Anderman J, Deseran T, et al. Monthly patient volumes of buprenorphine-waivered clinicians in the US. JAMA Netw Open. 2020;3(8):e2014045.
- Marsch LA, Dallery J. Advances in the psychosocial treatment of addiction: the role of technology in the delivery of evidence-based psychosocial treatment. Psychiatr Clin North Am. 2012;35(2):481–493.
- Pincus HA, England MJ. Improving the quality of psychosocial interventions for mental and substance use disorders: a report from the IOM. JAMA. 2015;314(12):1227–1228.
- Mansson KN, Salami A, Frick A, et al. Neuroplasticity in response to cognitive behavior therapy for social anxiety disorder. Transl Psychiatry. 2016;6:e727.
- Stark MJ. Dropping out of substance abuse treatment: a clinically oriented review. Clin Psychol Rev. 1992;12(1):93–116.
- Simpson DD. Treatment for drug abuse. Follow-up outcomes and length of time spent. Arch Gen Psychiatry. 1981;38(8):875–880.
- Kang SY, Kleinman PH, Woody GE, et al. Outcomes for cocaine abusers after once-a-week psychosocial therapy. Am J Psychiatry. 1991;148(5):630–635.
- Harris PM. Attrition revisited. Am J Eval. 1998;19(3):293–305.
- Simpson DD, Joe GW, Brown BS. Treatment retention and follow-up outcomes in the Drug Abuse Treatment Outcome Study (DATOS). Psychol Addict Behav. 1997;11(4):294–307.
- Hser YI, Evans E, Huang D, et al. Relationship between drug treatment services, retention, and outcomes. Psychiatr Serv. 2004;55(7):767–774.
- Marchand KI, Oviedo-Joekes E, Guh D, et al. Client satisfaction among participants in a randomized trial comparing oral methadone and injectable diacetylmorphine for long-term opioid-dependency. BMC Health Serv Res. 2011;11:174.
- De Novo classification request for reSET (DEN160018). In Health CFD, radiological. Washington (DC): U.S. Food & Drug Administration; 2017.
- Marsch LA, Guarino H, Acosta M, et al. Web-based behavioral treatment for substance use disorders as a partial replacement of standard methadone maintenance treatment. J Subst Abuse Treat. 2014;46(1):43–51.
- Budney AJ, Higgins ST. Therapy manuals for drug addiction, a community reinforcement plus vouchers approach: treating cocaine addiction. Rockville, MD: National Institute on Drug Abuse; 1998.
- Maricich YA, Bickel WK, Marsch LA, et al. Safety and efficacy of a prescription digital therapeutic as an adjunct to buprenorphine for treatment of opioid use disorder. Curr Med Res Opin. 2020. DOI:10.1080/03007995.2020.1846022
- Christensen DR, Landes RD, Jackson L, et al. Adding an internet-delivered treatment to an efficacious treatment package for opioid dependence. J Consult Clin Psychol. 2014;82(6):964–972.
- Blonde L, Khunti K, Harris SB, et al. Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther. 2018;35(11):1763–1774.
- Dreyer NA. Advancing a framework for regulatory use of real-world evidence: when real is reliable. Ther Innov Regul Sci. 2018;52(3):362–368.
- Campbell AN, Nunes EV, Matthews AG, et al. Internet-delivered treatment for substance abuse: a multisite randomized controlled trial. Am J Psychiatry. 2014;171(6):683–690.
- National Institute on Drug A. Principles of drug addiction treatment. A research based guide. Bethesda (MD): National Institutes of Health, U.S. Department of Health and Human Services; 2012. p. 1–75.
- Haight BR, Learned SM, Laffont CM, et al. Efficacy and safety of a monthly buprenorphine depot injection for opioid use disorder: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2019;393(10173):778–790.
- Lofwall MR, Walsh SL, Nunes EV, et al. Weekly and monthly subcutaneous buprenorphine depot formulations vs daily sublingual buprenorphine with naloxone for treatment of opioid use disorder: a randomized clinical trial. JAMA Intern Med. 2018;178(6):764–773.
- Food & Drug Administration. Opioid use disorder: endpoints for demonstrating effectiveness of drugs for medication‐assisted treatment guidance for industry [cited 2020 Sep 29]. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/opioid-use-disorder-endpoints-demonstrating-effectiveness-drugs-medication-assisted-treatment.
- Ronquest NA, Willson TM, Montejano LB, et al. Relationship between buprenorphine adherence and relapse, health care utilization and costs in privately and publicly insured patients with opioid use disorder. SAR. 2018;9:59–78.
- Mark TL, Hinde JM, Zarkin GA, et al. Adherence to buprenorphine treatment guidelines among individuals with an opioid use disorder who have private insurance. J Subst Abuse Treat. 2020;116:108062.
- Baumel A, Muench F, Edan S, et al. Objective user engagement with mental health apps: systematic search and panel-based usage analysis. J Med Internet Res. 2019;21(9):e14567.
- Ling W, Nadipelli VR, Aldridge AP, et al. Recovery from opioid use disorder (OUD) after monthly long-acting buprenorphine treatment: 12-month longitudinal outcomes from recover, an observational study. J Addict Med. 2020;14(5):e233–e240.
- Soeffing JM, Martin LD, Fingerhood MI, et al. Buprenorphine maintenance treatment in a primary care setting: outcomes at 1 year. J Subst Abuse Treat. 2009;37(4):426–430.
- Zhu Y, Evans EA, Mooney LJ, et al. Correlates of long-term opioid abstinence after randomization to methadone versus buprenorphine/naloxone in a multi-site trial. J Neuroimmune Pharmacol. 2018;13(4):488–497.
- Darke S, Ross J, Mills KL, et al. Patterns of sustained heroin abstinence amongst long-term, dependent heroin users: 36 months findings from the Australian Treatment Outcome Study (ATOS). Addict Behav. 2007;32(9):1897–1906.
- Leonardi C, Hanna N, Laurenzi P, Group IDAC, et al. Multi-centre observational study of buprenorphine use in 32 Italian drug addiction centres. Drug Alcohol Depend. 2008;94(1–3):125–132.
- Food & Drug Administration. Framework for FDA's real-world evidence program [cited 2020 Aug 25]. Available from: http://www.fda.gov/media/120060/download.
- Velez FF, Colman S, Kauffman L, et al. Real-world reduction in healthcare resource utilization following treatment of opioid use disorder with reSET-O, a novel prescription digital therapeutic. Expert Rev Pharmacoecon Outcomes Res. 2020. DOI:10.1080/14737167.2021.1840357
- Bickel WK, Marsch LA, Buchhalter AR, et al. Computerized behavior therapy for opioid-dependent outpatients: a randomized controlled trial. Exp Clin Psychopharmacol. 2008;16(2):132–143.
- American Academy of Addiction Psychiatry. Poster presentation abstracts. Am J Addict. 2018;27:285–328.