Abstract
Objective
To evaluate outcomes, healthcare resource utilization, and costs associated with mucormycosis in inpatient settings in Japan.
Methods
We performed a systematic literature review, followed by a retrospective database study using hospital health claims for patients in Japan hospitalized with a diagnosis of mucormycosis between January 2010 and January 2019. Outcomes assessed included duration of index hospitalization; index stay mortality; hospital readmission within 30, 60, and 90 days after index hospitalization discharge; drug/treatment utilization and patterns; number of patients examined for mucormycosis during the index hospitalization; and index stay inpatient costs.
Results
From our systematic literature review of articles describing 133 patients with mucormycosis, mortality in the index hospitalization was 55.6%. From our database study of 126 patients hospitalized for mucormycosis, mortality during the index hospitalization was 35.7% and mean index stay duration was 94 days. Hematologic malignancies were the most common risk factor in the literature review and the most common comorbidity in the database study. During the index stay, 39 patients (31.0%) received liposomal amphotericin B (L-AMB) treatment and 74 patients (58.7%) received other antifungal treatments. Median total inpatient costs for the index hospitalization were equivalent to approximately US$60,945, including US$29,283 in drug costs.
Conclusions
This study investigated the healthcare resource utilization and cost of medical resources caused by mucormycosis in Japan. The drug costs for antifungal treatments comprised about half of total inpatient costs. Mucormycosis leads to high mortality, high healthcare resource utilization, and high costs.
Introduction
Mucormycosis is an uncommon but life-threatening infection that occurs in immunocompromised patients, such as organ transplant recipients, and those with hematologic malignancies or diabetes mellitusCitation1–6. Estimates of mortality from mucormycosis vary by population and range from as low as 22%Citation7 to as high as 91% for high-risk patients (i.e. immunocompromised patients with hematologic diseases)Citation8. Antifungal chemotherapy, control of the underlying predisposing condition, and surgery are the cornerstones of mucormycosis managementCitation5,Citation9,Citation10. However, obstacles to appropriate treatment include late diagnosis or even no diagnosis, as well as non-specific clinical presentation of mucormycosis and the limitations of currently implemented diagnostic means (e.g. microbiological culture, molecular and serological techniques)Citation11.
In addition to the clinical burden of mucormycosis, recent studies have demonstrated the economic burden of the disease, which includes healthcare resource utilization (HCRU) and treatment costs. A 2016 US-based study using the Premier Healthcare Database reported that the estimated prevalence of mucormycosis was 0.12 (range 0.09–0.17) per 10,000 discharges and that the average cost associated with a mucormycosis-related hospitalization was US$112,419 which indicates the substantial economic burden attributable to mucormycosisCitation12.
Similarly, a separate, retrospective, US-based study using data from the Healthcare Cost and Utilization Project’s 2004 Nationwide Inpatient Sample (HCUP-NIS) database reported that the mean cost for patients with invasive mucormycosis infections was US$48,673 considerably higher than that for other invasive fungal infections, including aspergillosis, histoplasmosis, and cryptococcosisCitation13. However, the authors of that study comment that differences from other studies in estimates of costs may be related to differences in case selection, differences in costing methodology, or changes in practice patterns over time.
A related, retrospective, US-based study using data from the 2003–10 HCUP-NIS reported that among hospitalized patients with mucormycosis, mortality was 22.1%, mean length of stay was 24.5 days, and mean total costs were US$90,272; all considerably higher than results for high-risk hospitalized patients without mucormycosisCitation7.
A retrospective cost analysis of 46 patients with mucormycosis hospitalized in Germany between 2003 and 2016 reported that mean overall direct treatment costs were €53,261, with antifungal treatment being the primary cost-driver in mucormycosis management (mean €22,819 per patient). Compared with matched patients, those with mucormycosis were treated in hospital for a mean of 26.5 additional days, resulting in mean additional costs of €32,991Citation8.
Information describing the clinical and economic burdens of mucormycosis in Japan is limited. A prevalence study in Japan reported that mucormycosis was detected in 4.3% of autopsy patients infected by visceral mycoses, suggesting that, compared with other invasive fungal infections in Japan, such as aspergillosis and candidiasis, mucormycosis is rare but associated with high mortality and morbidityCitation14. However, most of the reports describing mucormycosis in Japan are case reports or studies with small sample sizes and limited information on clinical outcomes, HCRU, and economic burdenCitation15,Citation16.
In order to gain a better understanding of the disease burden of mucormycosis in Japan, including the HCRU associated with the disease, we performed a systematic literature review using the PubMed and Ichushi-Web databases to identify reports of patients diagnosed with mucormycosis in Japan published up to the end of October 2018. Findings from this literature review then informed the design of a large-scale, retrospective, database study that used hospital health claims and administrative data to evaluate clinical outcomes, HCRU, and the costs associated with mucormycosis in inpatient settings in Japan.
Methods
Systematic literature review
A systematic literature review using the PubMed and Ichushi-Web electronic databases was conducted to identify observational studies of patients diagnosed with mucormycosis in Japan from peer-reviewed journals. The English search terms used to select qualifying studies from the PubMed database published on October 2018 are (“deep mycosis” OR “deep-seated mycosis” OR “invasive fungal” OR “invasive mycose” OR “invasive mycosis” OR “Invasive Fungal Infections”[Mesh] OR mucomycosis OR mucorales OR mucormycoses OR zygomycosis OR mucoromycotina) AND (Japan[tiab] OR Japanese[tiab]) AND Humans[Mesh]. Similar search terms were used for the Ichushi-database (Supplemental Table 1).
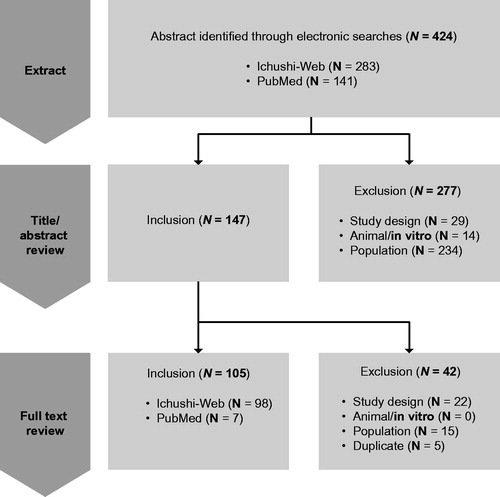
This literature review was limited to studies in individuals in Japan diagnosed with mucormycosis. Articles were independently screened for inclusion criteria in two stages: first, titles and abstracts, followed by the retrieval and screening of full-text articles. All the titles, abstracts, and full-text articles were screened independently by two reviewers. Disagreements were resolved through discussion and consensus or by consulting a third reviewer. Data extracted from the included publications included publication year, study setting, population, sample size, baseline characteristics, medication utilization, examination, HCRU, and clinical outcomes. Extracted data were combined across studies (without statistical weighting) to generate estimates of sex, age, mortality, site of diagnosis, and risk factors from the published reports.
Database study design and study population
We conducted a descriptive, retrospective study using patient data from the Medical Data Vision (MDV) database, a commercially available database of health claims and administrative data from more than 300 Japanese acute hospitals. All patient data were anonymized and included no identifying information, so informed consent was not necessary. We included all patients who were hospitalized with a diagnosis of mucormycosis (ICD-10 code [B46.x]) between 1 January 2010, and 31 January 2019 (the main group). The index date was defined as the date of the first hospital admission that included a diagnosis of mucormycosis.
In addition to this main group, analyses were conducted on a sub-group of patients who received amphotericin B (AMPH-B) or liposomal amphotericin B (L-AMB) as treatment during the index hospitalization. Patient baseline characteristics and clinical information collected in this database study included age, sex, medical history, and site of mucormycosis.
Database study outcomes
Outcomes assessed in this database study included: the duration of the index hospitalization stay; index stay mortality (defined as inpatient death due to any cause, as indicated by the inpatient claim discharge status); hospital readmission within 30, 60, and 90 days after index hospitalization discharge; drug/treatment utilization, including the number of patients who received antifungals (AMPH-B, L-AMB, and other antifungals) as first, second, and third antifungal treatments; the time from the index date to the first antifungal treatment (note that the anti-Mucorales azole drugs posaconazole and isavuconazole were not approved when the study was conducted and were not included in this analysis); index doses and duration of L-AMB and AMPH-B; the number of patients who received examinations for mucormycosis (e.g. microbiologic examinations, imaging studies) during the index hospitalization; and inpatient costs from the index hospitalization, defined as the sum of daily costs incurred in inpatient settings (e.g. hospital bed, intensive care unit [ICU] stay, medication, laboratory tests, imaging). All costs were measured in Japanese yen and multiplied by a factor of 0.009 based on the exchange rate in January 2019 to estimate US dollar equivalents. Claims for mucormycosis-related surgical procedures were not captured in this database analysis. The study comprised a series of descriptive analyses.
Results
Systematic literature review
The literature review identified 424 potential articles: 147 of these articles were selected for full-text review and 105 were selected for inclusion (), describing a total of 133 patients. A table summarizing characteristics of the 105 studies is available as Supplemental Table 1. Among the 133 patients identified, 64.7% were male and 27.8% were aged 65 or older (). Mortality in the index hospitalization was 55.6% (). A total of 38.4% of the identified patients underwent surgery during the index hospitalization. Pulmonary mucormycosis was the most frequent diagnosis site (47.4% of patients; ). The most common risk factors identified for mucormycosis included hematologic malignancies, chemotherapy, steroid use, diabetes, and respiratory disease ().
Table 1. Summary of literature review findings: patient sex, age, and mortality.
Database study: patient characteristics and clinical information
During the study period, 151 patients were identified with at least one mucormycosis diagnosis, 126 of whom (83.4%) had a record of hospitalization in the same month as that diagnosis. Of these 126 patients (the main group), 105 were included in the sub-group that received L-AMB or AMPH-B treatment during the index hospitalization. Baseline characteristics and clinical information are summarized in . Approximately half the patients (52.4%) were aged 65 years or older and 68.3% were male. Common medical histories included hematologic malignancies (61.9%) and diabetes (46.0%). Approximately half the patients (50.8%) had pulmonary mucormycosis at diagnosis; fewer patients had rhinocerebral, gastrointestinal, cutaneous, or disseminated mucormycosis (). Overall, patient characteristics were similar between the main group and the sub-group.
Table 2. Baseline characteristics and clinical information from the database study.
Database study: index hospitalization
The mean duration of the index stay was 94 days (median, 67 days) for the main group and 106 days (median, 82 days) for the sub-group. The mean duration of ICU stay was 8.63 days (median, 9 days) in both groups. Mortality during the index stay was 35.7% in the main group and 41.9% in the sub-group.
Clinical examinations performed during the index stay are summarized in . In both the main group and the sub-group, more than 90% of patients received examination by beta-D-glucan immunologic assay and computed tomography scan. Cultures were obtained for the majority of patients; however, biopsy was only conducted in approximately 30% of patients. More than half (54.8%) of the patients received Aspergillus antigen testing.
Table 3. Clinical examinations performed during the index stay from the database study.
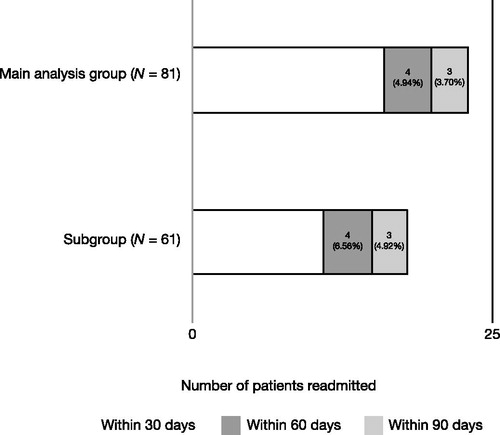
Hospital readmission within 30, 60, and 90 days post-index stay discharge is summarized in . Among the 81 patients in the main group evaluable for hospital readmission (126 patients minus the 45 who died during the index stay), 16 (19.8%) were readmitted within 30 days and a total of 23 (28.4%) had been readmitted by 90 days. Similar readmission results were observed in both the main group and the sub-group ().
Database study: treatment patterns
Treatment patterns for mucormycosis received by the main group during the index hospitalization and subsequent lines of therapy are summarized in . For the first treatment, L-AMB was prescribed to 39 patients (31.0%), and other antifungals, exclusive of L-AMB and AMPH-B, were prescribed to 74 patients (58.7%). As shown in , many patients who did not receive L-AMB during the index stay went on to receive L-AMB as a subsequent treatment. Only one patient received AMPH-B (as a subsequent treatment, not during the index hospitalization). Time to first antifungal treatment, and doses/duration of L-AMB and AMPH-B treatment are summarized in . Claims for mucormycosis-related surgical procedures were not captured in this database analysis.
Figure 3. Treatment patterns for the main group. Abbreviations. AMPH-B, amphotericin B; L-AMB, liposomal amphotericin B. In the main group, a total of 126 patients were examined for treatment patterns, and each treatment percentage was calculated based on the main group total patient number. For the 1st treatment, 34 patients (27.0%) were prescribed L-AMB; 5 patients (4.0%) were prescribed L-AMB + other antifungals; and 74 patients (58.7%) were prescribed other antifungals, exclusive of L-AMB and AMPH-B. For the 2nd treatment, 1 patient (0.8%) was prescribed AMPH-B; 38 patients were prescribed L-AMB (L-AMB only: 14 patients, 11.1%; L-AMB + other antifungals: 24 patients, 19.1%); and other antifungal medications were given to 47 patients (37.3%), exclusive of L-AMB and AMPH-B. Details for 3rd and 4th treatments are shown in the figure.
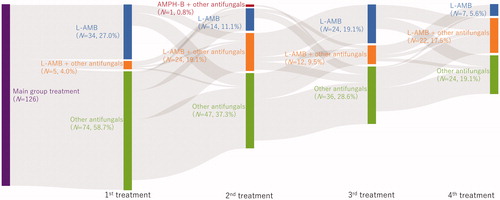
Table 4. Antifungal treatments: time to first antifungal treatment and doses/duration of L-AMB and AMPH-B treatment from the database study.
Database study: inpatient costs
Total inpatient costs for the index hospitalization are summarized in . For the main group, median total costs were equivalent to US$60,945 including US$29,283 in drug costs. The median cost of L-AMB treatment was US$10,243. Similar cost trends were observed in both the main group and the sub-group, although the total costs were US$9940 higher in the sub-group.
Table 5. Inpatient costs for the index hospitalization from the database study.
Discussion
The incidence of invasive fungal infections such as mucormycosis has risen in recent decades. This is likely due in part to medical advances that have improved the survival of immunocompromised patients, including transplant recipients and those who receive intensive chemotherapy for hematologic malignanciesCitation4,Citation17–23.
In Japan, however, there is a lack of knowledge regarding the clinical and economic burden of mucormycosis, including the HCRU associated with the disease. Our current study comprised two approaches—systematic literature review and retrospective database analysis—to evaluate comprehensively the real-world burden of mucormycosis in Japan. This systematic literature review is the first to include patient data from Japanese-language publications to understand this burden. Similarly, this retrospective database analysis in Japan is the first report to use a large-scale database of real-world data containing patients with mucormycosis to report clinical outcomes, HCRU, and inpatient costs (including treatment costs). This combined approach provides key information for understanding the true burden of the disease on healthcare systems. In addition, this study offers a unique perspective on treatment patterns by reporting on the initial and subsequent mucormycosis treatments received and by tracking patients who switched treatments over time.
We found that the sex distribution was similar between the findings of the literature review and the database study. Our finding that mucormycosis affects men more frequently than women is consistent with previous reportsCitation24–26. From the database analysis, we found that approximately half the patients hospitalized for mucormycosis in Japan were aged 65 years or older, but the systematic literature review showed that fewer than 30% of patients were aged 65 years or older. The reason for this difference might be a publication bias favoring the reporting of uncommon clinical cases in younger patients. From the literature review, overall mortality appeared to be higher than was found in our database study. This difference could also be explained by a publication bias favoring reports of fatal cases. Hematologic malignancies were identified as the most common risk factor in the literature review, and the most common comorbidity in the database study. These findings suggest that better management is needed to prevent patients with hematologic malignancies from contracting and developing mucormycosis.
Our database analysis found that median total inpatient costs for mucormycosis hospitalizations were US$60,945 with drug costs being the largest component (US$29,283). These median costs for mucormycosis hospitalizations appear broadly similar to those reported for some patients in Germany (mean overall direct treatment costs of €53,261)Citation8 and the USA (mean costs for patients with invasive mucormycosis infections of US$48,673)Citation13, but much lower than those reported for other patients in US studies (average total costs of US$90,272Citation7 to US$112,419Citation12) however, we acknowledge it is difficult to make a direct comparison of medical costs with those in other countries. These considerable variations in total cost estimates for mucormycosis hospitalizations likely reflect the considerable differences among the Japanese, German, and US health systems. The increased costs in the sub-group compared with the main group likely reflect not only the cost of L-AMB or AMPH-B but also that of antifungal treatments received prior to L-AMB or AMPH-B; some patients in the main group may not have received any of these treatments.
From the database analysis of treatment patterns during the index stay, L-AMB was received by 31.0% of patients in our study, and other antifungals were received by 58.7%. Many patients who did not receive L-AMB during the index stay went on to receive L-AMB as a subsequent treatment, which suggests patients may experience delays before amphotericin-based mucormycosis treatment is initiated. However, we could not assess the impact of early treatment at the patient level because the timing of administration of the antifungal therapy in relation to the timing of the first diagnosis of mucormycosis cannot be identified in the hospital claims database as the data which is captured only relates to prescriptions which are supplied, and full clinical records with specific details of administration are not available.
Our database analysis findings are largely consistent with those of a recent analysis of patients hospitalized for mucormycosis in GermanyCitation8. In that study, mortality during the index hospitalization was 41.3%, compared with 35.7% in the main group of our database analysisCitation8. Both studies identified hematologic malignancies as the most common risk factor or baseline comorbidity (91.3% of patients described by Heimann et al. had hematologic disease, including 45.7% with acute myeloid leukemia and 13.0% with acute lymphatic leukemia)Citation8. Similarly, both studies identified antifungal drug costs as the primary cost-driver in mucormycosis management. Heimann et al. reported a mean overall direct treatment cost of €53,261 for patients hospitalized with invasive mucormycosis, with mean antifungal treatment costs of €22,819 per patientCitation8.
However, the German patients described by Heimann et al. (mean hospital stay durations of 36.9 days in general hospital wards, 1.5 days in an intermediate care unit, 9.7 days in a bone marrow transplant ward, and 8.6 days in an ICU wardCitation8) experienced considerably shorter hospital stays than those in our database analysis (mean duration of index stay, 94 days; mean duration of ICU stay, 9 days). Furthermore, the US patients described by Zilberberg et al. (mean length of hospital stay, 24.5 daysCitation7) Kontoyiannis et al. (median length of hospital stay, 17 daysCitation12) and Menzin et al. (mean length of hospital stay, 18.7 daysCitation13) experienced even shorter hospital stays. Reasons for these differences observed across studies are unclear but might be attributable to the different treatment options for mucormycosis available in each country. As mentioned above, oral antifungal drugs active against mucormycosis (such as posaconazole) were not available in Japan during the study period of our database analysis. Thus, the necessity of intravenous treatment with AMPH-B or L-AMB might explain the extremely prolonged lengths of hospital stays in Japan compared with other countries.
In a recent systematic review and meta-analysis of 851 adult patients with mucormycosis published between 2000 and 2017, all-cause 90-day mortality was found to be 41%Citation27. Among 785 patients for whom treatment details were available in that analysis, initial treatment with combination antifungals did not reduce 90-day mortality compared with AMPH-B or L-AMB monotherapy (p = .541), but concomitant surgical and antifungal therapy was associated with reduced 90-day mortality compared with antifungals alone (p < .001).
These findings suggest that the availability of first-line antifungal treatments with good efficacy remains an urgent unmet need in mucormycosis managementCitation27. In our database analysis, fewer than half the patients hospitalized for mucormycosis received AMPH-B or L-AMB as first-line or later treatments, which suggests a possible delay in initiating appropriate drug therapies.
The limitations of our systematic literature review include the fact that many data, such as length of hospital stay, treatment duration, and costs, were not reported in the primary articles. Furthermore, publication bias may exist, such that uncommon or unique cases are over-represented in the clinical literature. In addition, the literature data up to the end of October 2018 (the most comprehensive data available at the time of the database analysis) were used in the systematic literature review with the intention of providing context informing the design of the retrospective database. Thus, the data in 2019 were not included for the analysis. The limitations of our retrospective, descriptive database analysis include the fact that data were captured from diagnosis-procedure-combination (DPC) hospitals only; claims from non-DPC hospitals were not included. It was not possible to calculate the per hospital discharge rate of mucormycosis as it was not possible to associate patients to specific hospitals in the database in order to calculate the rate. Additionally, these data were not specifically recorded for research purposes. As such, data recording errors and biases may exist, and some data may be missing; therefore, we defined a mucormycosis-related hospitalization as a patient’s first hospitalization with the ICD-10 B46.x diagnosis code. Of note, mortality data in the MDV database is subject to missing information and is only recorded for patients who died during their hospital stay. Also, we only found 45 patients having record of death in the 2010–2019 study period, and hence did not investigate the trends over time.
When assessing treatment patterns, the reasons for switching to subsequent therapies (which could include lack of response to treatment, patient dissatisfaction with treatment, or the occurrence of adverse events) were not captured in this analysis. Also, combination therapy of antifungal drugs is considered as off-label use in Japan, thus the treatment patterns and outcomes of combination therapy were not explored. Furthermore, novel triazole treatment options, such as posaconazole and isavuconazole, have not yet been approved in Japan, so the costs and outcomes of those treatments are not reflected in this analysis.
An additional limitation of our database study was the fact that mucormycosis-related surgical procedures were not captured in the database analysis; from our systematic literature review, 38.4% of the identified patients underwent surgery during the index hospitalization. Although surgery is an important component of mucormycosis treatment, the claims data did not indicate which surgical procedures were mucormycosis-related, and our study, therefore, focused on drug treatments for mucormycosis.
Because of these limitations, our findings and cost estimates may not be completely generalizable to regions outside of Japan. Nevertheless, the results of this database study demonstrate that, in Japan, mucormycosis is associated with substantial mortality, HCRU, and costs. Future studies to evaluate mucormycosis-associated HCRU in Japan in the context of new treatment options are warranted.
Conclusions
From our combined analysis that involved a systematic literature review and a retrospective database analysis, the high mortality associated with mucormycosis suggests a substantial clinical burden. Patients with hematologic malignancies were particularly likely to be diagnosed with mucormycosis, suggesting the need for better management of these patients at high risk for fungal infections.
From the database analysis, the substantial HCRU burden of mucormycosis in Japan includes hospitalizations and ICU stays as well as subsequent hospital readmissions for many patients. The substantial cost burden of mucormycosis includes hospitalization costs as well as drug and examination costs. Median total inpatient costs for mucormycosis hospitalizations in Japan were the equivalent of nearly US$56,000 with drug costs (mostly those for antifungal treatments) approximating US$29,283.
Transparency
Declaration of funding
This study and preparation of this manuscript were supported by funding from MSD KK.
Declaration of financial/other relationships
RU, SN, and GF are employees of MSD KK. DA is an employee of IQVIA and has a research/consulting agreement with MSD KK. Peer reviewers on this manuscript have received an honorarium from CMRO for their review work. One of these reviewers has disclosed that they have received research funding from Astellas to study isavuconazole. The remaining reviewers have no other relevant financial relationships or otherwise to disclose.
Author contributions
RU, SN, and GF contributed to the study design. DA contributed to the acquisition of study data. All authors contributed to the critical interpretation of data and drafting/revision of the manuscript content, have approved the final version of this manuscript, and take responsibility for the integrity of this research study.
Supplemental Material
Download MS Word (70.3 KB)Acknowledgements
The authors wish to thank Jeffrey Walter of IQVIA for assistance with preparation of the manuscript.
Data availability statement
The data that support this study’s findings are available from MSD KK upon request. Some restrictions apply to the availability of these data, which were used under license for this research study.
References
- Meis JF, Chakrabarti A. Changing epidemiology of an emerging infection: zygomycosis. Clin Microbiol Infect. 2009;15(Suppl 5):10–14.
- Asano-Mori Y. Diagnosis and treatment of mucormycosis in patients with hematological malignancies. Med Mycol J. 2016;57(4):J155–J162.
- Skiada A, Pagano L, Groll A, et al. European Confederation of Medical Mycology Working Group on Zygomycosis. Zygomycosis in Europe: analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) Working Group on Zygomycosis between 2005 and 2007. Clin Microbiol Infect. 2011;17(12):1859–1867.
- Shadrivova OV, Burygina EV, Klimko NN. Molecular diagnostics of mucormycosis in hematological patients: a literature review. JoF. 2019;5(4):112.
- Vučićević Boras V, Jurlina M, Brailo V, et al. Oral mucormycosis and aspergillosis in the patient with acute leukemia. Acta Stomatol Croat. 2019;53(3):274–277.
- Hartnett KP, Jackson BR, Perkins KM, et al. A guide to investigating suspected outbreaks of mucormycosis in healthcare. JoF. 2019;5(3):69.
- Zilberberg MD, Shorr AF, Huang H, et al. Hospital days, hospitalization costs, and inpatient mortality among patients with mucormycosis: a retrospective analysis of US hospital discharge data. BMC Infect Dis. 2014;14(1):310.
- Heimann SM, Vehreschild MJGT, Cornely OA, et al. Healthcare burden of probable and proven invasive mucormycosis: a multi-centre cost-of-illness analysis of patients treated in tertiary care hospitals between 2003 and 2016. J Hosp Infect. 2019;101(3):339–346.
- Skiada A, Lanternier F, Groll AH, European Conference on Infections in Leukemia, et al. Diagnosis and treatment of mucormycosis in patients with hematological malignancies: guidelines from the 3rd European Conference on Infections in Leukemia (ECIL 3). Haematologica. 2013;98(4):492–504.
- Cornely OA, Alastruey-Izquierdo A, Arenz D, et al. Mucormycosis ECMM MSG Global Guideline Writing Group. Global guideline for the diagnosis and management of mucormycosis: an initiative of the European Confederation of Medical Mycology in cooperation with the Mycoses Study Group Education and Research Consortium. Lancet Infect Dis. 2019;19(12):e405–e421.
- Katragkou A, Walsh TJ, Roilides E. Why is mucormycosis more difficult to cure than more common mycoses? Clin Microbiol Infect. 2014;20(suppl 6):74–81.
- Kontoyiannis DP, Yang H, Song J, et al. Prevalence, clinical and economic burden of mucormycosis-related hospitalizations in the United States: a retrospective study. BMC Infect Dis. 2016;16(1):730.
- Menzin J, Meyers JL, Friedman M, et al. Mortality, length of hospitalization, and costs associated with invasive fungal infections in high-risk patients. Am J Health Syst Pharm. 2009;66(19):1711–1717.
- Suzuki Y, Kume H, Togano T, et al. Epidemiology of zygomycosis: analysis of national data from pathological autopsy cases in Japan. Med Mycol J. 2017;58(3):E89–e95.
- Higo T, Kobayashi T, Yamazaki S, et al. Cerebral embolism through hematogenous dissemination of pulmonary mucormycosis complicating relapsed leukemia. Int J Clin Exp Pathol. 2015;8(10):13639–13642.
- Ino K, Nakase K, Nakamura A, et al. Management of pulmonary mucormycosis based on a polymerase chain reaction (PCR) diagnosis in patients with hematologic malignancies: a report of four cases. Intern Med. 2017;56(6):707–711.
- Dodds Ashley E, Drew R, Johnson M, et al. Cost of invasive fungal infections in the era of new diagnostics and expanded treatment options. Pharmacotherapy. 2012;32(10):890–901.
- Repetto EC, Giacomazzi CG, Castelli F. Hospital-related outbreaks due to rare fungal pathogens: a review of the literature from 1990 to June 2011. Eur J Clin Microbiol Infect Dis. 2012;31(11):2897–2904.
- Tsutsumi I, Kunisawa S, Yoshida C, et al. Impact of oral voriconazole during chemotherapy for acute myeloid leukemia and myelodysplastic syndrome: a Japanese nationwide retrospective cohort study. Int J Clin Oncol. 2019;24(11):1449–1458.
- Pegorie M, Denning DW, Welfare W. Estimating the burden of invasive and serious fungal disease in the United Kingdom. J Infect. 2017;74(1):60–71.
- Shekar M, Elumalai R, Elayaperumal I, et al. Prevalence and outcome of systemic fungal infections in renal transplant recipients – a tertiary care experience. Saudi J Kidney Dis Transpl. 2019;30(5):1137–1143.
- Lin CY, Wang IT, Chang CC, et al. Comparison of clinical manifestation, diagnosis, and outcomes of invasive pulmonary aspergillosis and pulmonary mucormycosis. Microorganisms. 2019;7(11):531.
- Stull K, Esterberg E, Ajmera M, et al. Use of antifungals and outcomes among inpatients at risk of invasive aspergillosis or mucormycosis in the USA: a retrospective cohort study. Infect Dis Ther. 2019;8(4):641–655.
- Saegeman V, Maertens J, Ectors N, et al. Epidemiology of mucormycosis: review of 18 cases in a tertiary care hospital. Med Mycol. 2010;48(2):245–254.
- Sarvestani AS, Pishdad G, Bolandparvaz S. Epidemiology and clinical characteristics of mucormycosis in patients with leukemia; a 21-year experience from southern Iran. Bull Emerg Trauma. 2014;2(1):38–43.
- Lee FY, Mossad SB, Adal KA. Pulmonary mucormycosis: the last 30 years. Arch Intern Med. 1999;159(12):1301–1309.
- Jeong W, Keighley C, Wolfe R, et al. Contemporary management and clinical outcomes of mucormycosis: a systematic review and meta-analysis of case reports. Int J Antimicrob Agents. 2019;53(5):589–597.