Abstract
Introduction
Repository corticotropin injection (RCI; Acthar Gel) is indicated to induce a diuresis or a remission of proteinuria in nephrotic syndrome (NS) without uremia of the idiopathic type or that due to lupus erythematosus. This study compares patient characteristics and measurable healthcare resource utilization (HCRU) between NS patients who received a prescription for RCI and then were either approved or denied treatment by their insurers.
Methods
A retrospective analysis of adults with NS from January 2015 to December 2018 was conducted using a de-identified open-source claims database. Patients were included in the study if they had ≥1diagnosis associated with NS, were age 18+, and had medical claims activity at some point in the year preceding (“baseline”) and year following (“follow up”) their first approved or denied RCI prescription. Baseline characteristics were reported with p-values indicating the significance of characteristics between cohorts. To assess outcomes, approved and denied patients were matched (1:1) using propensity-matching to account for underlying differences.
Results
Overall, 1,232 patients met inclusion criteria for the study. At baseline, approved patients were older than denied patients (mean age 53.9 vs. 48.4) and had higher rates of comorbidities. A greater proportion of approved patients required inpatient admissions (34.1 vs. 28.0%) and "high" doses of corticosteroids (CS) (26.2 vs. 20.7%) at baseline. Matched outcomes showed directionally more denied patients with inpatient admissions compared to approved (64 vs. 52) and a greater utilization of deep vein thrombosis ultrasound (12.2 vs. 6.6%) and dialysis (10.5 vs. 6.1%). Matched, denied patients had directionally greater CS use during follow-up both in the number of patients receiving CS (104 vs. 95) and the average annualized daily dose (4.1 vs. 3.4 mg).
Conclusion
Patients denied access to RCI treatment had directionally higher HCRU compared to matched, approved counterparts. Thus, the results of this study may aid providers and payers in evaluating scenarios where RCI may be beneficial and improve quality of care for NS patients.
Introduction
Nephrotic syndrome (NS) is a presentation of kidney disease characterized by elevated levels of protein in the urine (heavy proteinuria), low levels of albumin in the blood, (hypoalbuminemia), swelling of the lower extremities (peripheral edema), and abnormally high blood serum levels of fat and cholesterol (hyperlipidemia)Citation1–3. NS may develop as the result of a number of different primary, idiopathic conditions or secondary conditions that affect the kidneys. Primary causes of NS include focal segmental glomerulosclerosis (FSGS), minimal change disease (MCD), membranous nephropathy (MN), membranoproliferative glomerulonephritis (MPGN), and immunoglobulin-A nephropathy (IgAN). Common secondary diseases that may result in NS include lupus nephritis and diabetic nephropathy. Although NS is a rare condition - estimated to occur in 3 out of 100,000 adults every year - patients who present with NS have significant morbidity and mortality due to increased risk of complications such as infection, deep-vein or renal-vein thrombosis, bone disease and renal failureCitation1.
Current first-line treatments for NS attempt to target the underlying pathology while simultaneously mitigating the various complications associated with NS via background treatments (e.g. angiotensin-converting enzyme [ACE] inhibitors, angiotensin receptor blockers [ARBs], diuretics, beta blockers and calcium channel blockers)Citation3. In an attempt to achieve remission of NS, patients are often prescribed immunosuppressive agents such as corticosteroids (CS), calcineurin inhibitors (CNI), antimetabolytes or cytotoxic drugsCitation4,Citation5. Many of these patients, however, either experience relapse after remission or are resistant to these first-line immunosuppressive therapiesCitation4,Citation6.
In the 1950s, adrenocorticotropic hormone (ACTH) emerged as a safe and effective treatment for NS resulting in the reduction or remission of proteinuriaCitation5. Currently, ACTH is available in the U.S. as repository corticotropin injection (RCI: ActharFootnotei Gel), a naturally sourced complex mixture of adrenocorticotropic hormone analogues and other pituitary peptides, and is indicated to induce a diuresis or a remission of proteinuria in nephrotic syndrome without uremia of the idiopathic type or that due to lupus erythematosusCitation7. More recently, there is a growing body of evidence from clinical studies, retrospective case series, and case reports that show the effectiveness of RCI treatment in achieving complete or partial remission of proteinuria in NSCitation4–6,Citation8–20. Despite clinical evidence supporting the efficacy, safety, and economic benefits of RCI treatment for NS, third party payers deny many patients coverage for RCI through implementation of stringent utilization management. Coverage of RCI treatment for NS by US commercial plans is available only through a formulary exception (i.e. medical exception), request for a non-formulary specialty prescription. Although formulary restrictions are implemented to reduce drug utilization and associated drug costs, the resultant pharmacy cost savings may be offset by increased health care resource utilization and medical costsCitation21.
There is a relative paucity of research, however, regarding the impact of formulary restrictions on clinically relevant health care resource utilization (HCRU) measures among adult NS patients treated with RCI. This study aims to fill that gap, and build upon the growing body of literature in NS treatments, by comparing patient characteristics and outcomes among NS patients whose RCI prescriptions are approved by insurers (and therefore undergo RCI treatment) versus and those who are denied (and therefore are treated with other options). In doing so, the study evaluates measurable, clinically relevant (HCRU) across the approved and denied cohorts, controlling for underlying differences in demographic and clinical characteristics observed during the one-year period prior to patients' approval or denial ("baseline"). The analysis of demographic and clinical characteristics observed during the baseline period is itself informative as to the types of patients who may be more likely to have their prescription for RCI treatment either approved or denied by third party payers.
Patients and methods
Overview
To assess the resource use for NS patients ultimately receiving access to RCI treatment versus those patients who did not, and to understand the demographic and clinical characteristics of NS patients approved for RCI treatment compared to NS patients denied RCI treatment, this study implemented a retrospective cohort analysis of a de-identified open source claims database. Demographic and clinical characteristics and HCRU were assessed during a baseline period, defined as the 12 months preceding a patient's index date; the index date for each patient was defined as the date of the first approved RCI claim (for approved patients) or the date of the first denied claim (for denied patients). HCRU for approved and denied patients were compared during a follow-up period, defined as the 12 months following the index date. The difference in HCRU between NS patients approved for use of RCI versus those denied were assessed during the follow-up period using a matched cohort study design to account for baseline differences in patient demographic and clinical characteristics as well as prior HCRU and costs.
Data source
This study used a de-identified open source claims database (Symphony Health Integrated Dataverse [IDV]) that links data from pharmacy point-of-service systems, payer adjudication services (clearing houses), and direct prescription, medical, and hospital feeds. These data contain approximately 168 million longitudinally-tracked patients with prescription and medical claims in any recent year of the database. Patients in the database are representative of the U.S. population age and gender mix with claims from multiple payer types, including Medicaid (federal and state government health insurance program for individuals with low income) fee-for-service (FFS), Managed Medicaid, Medicare (federal health insurance program, predominantly for individuals aged 65+), and commercial payers. Within these payer types, a variety of payers are represented, each with varying plan, formulary and claim authorization criteria. While the specific details of individual plan structures are not contained in the data, the database captures prescription claims from two types of data streams: clearing-house claims that have been processed through a payer's adjudication system and direct-feed claims from pharmacy point-of-service systems. For claims processed through payers' adjudication systems, the data contain the full cycle of a patient's claim adjudication status making it possible to analyze a patient's history of denied and approved claims. For claims originating in pharmacy point-of-service systems, only the final approved status of the claim is available. All prescription claims in the data contain detailed information regarding final claim approval status (i.e. approved or denied), fill date, national drug code, days' supply and payment amounts, making it possible to track patients who received access to a particular drug versus those who were denied access by U.S. payers. The data also contain linked medical claims for 72% of individuals with prescription claims activity which allows for the assessment of HCRU outcomes among the patient population of interestCitation22.
Sample selection
In order to be eligible for the study, patients were required to have submitted at least one prescription claim for RCI, ultimately either approved or denied, during the four-year period of 1 January 2015 through 31 December 2018. Prescription claims for RCI were identified in the data using Generic Product Identification codes (GPI), National Drug Codes (NDC), and codes from the Healthcare Common Procedure Coding System (HCPCS). In addition to having at least one prescription claim for RCI in the data, patients were required to have at least one medical claim with an ICD-9-CM/ICD-10-CM diagnosis code indicating a condition associated with NS. Diagnoses associated with NS and their corresponding ICD-9-CM (580.xx-589.xx, 791.xx) and ICD-10-CM (N00-N08) were used to identify patients for the study sample.
Based on their prescription history in the data, patients meeting the above criteria were divided into two mutually exclusive cohorts: patients approved for RCI treatment (i.e. patients having at least one approved prescription claim in the data) and patients denied access to RCI (i.e. patients having no approved claims in the data). Patients in both cohorts were required to be age 18 or older on their index date. Because the data aggregate medical and prescription claim activity over time from a diverse set of sources and do not definitively report the timeframe of a patient's insurance eligibility, the study utilized the date-stamps of a patient's claims activity to approximate the length of a patient's insurance eligibility. To ensure that all relevant medical and prescription claims were captured during a patient's baseline and follow-up periods, patients were required to have at least one active claim in the data during the first three months of their baseline period (i.e. months 9–12 prior to their index date) and the last three months of their follow-up period (i.e. months 9–12 after their index date). To ensure that patient outcomes could be observed for the full 12-month follow-up period, the study required patients to have an index date occurring before 2018.
Statistical analyses
To compare outcomes between patients who were approved for RCI treatment and those who were denied, the study implemented a propensity score matching methodology that took into account the underlying differences between the two cohorts observed in the baseline periodCitation22. This approach allowed the study to create a scenario in which matched approved and denied patients were observably similar based on baseline measures that were available in the database, and to thereby carry out a more direct comparison of the difference in HCRU associated with RCI treatment for NS while reducing bias. Specifically, approved patients were matched to denied patients based on a “greedy” propensity score matching methodology using all characteristics assessed during the baseline periodCitation23 (See for a complete list of characteristics used in the matching algorithm). Denied patients were matched one-to-one to approved patients with the nearest propensity score (± ¼ of a standard deviation).
Table 1. Patient demographics and clinical characteristics during the baseline period.
Table 2. Approval process for nephrotic syndrome patients with full claim cycle.
For categorical variables, statistical significance was assessed using chi-squared tests for comparisons between pre-match outcomes, and McNemar tests for the matched cohorts. For continuous variables, statistical significance was assessed using Wilcoxon rank-sum tests (pre-match) and Wilcoxon signed-rank tests (post-match). Statistical analyses were performed separately for each cohort using SAS Enterprise Guide version 7.15 (SAS Institute Inc., Cary, NC, USA).
To better understand the impact of commercial payer coverage restrictions to access, sub-group analysis by payer type was conducted for the matched cohorts.
Study measures
Demographic, HCRU, and clinical characteristics relevant to the diagnosis and treatment of NS were evaluated for both the approved and denied cohorts. Patient demographics included patient age at the time of index date, gender, U.S. census region, the year in which their index date occurred, and insurance plan type (e.g. Medicare, Medicaid, Commercial, and Other/Unspecified). Clinical characteristics evaluated in the baseline period included the time elapsed since a patient's initial NS diagnosis in the data (including diagnoses that may date prior to the 12-month baseline period) in addition to the number of claims with a diagnosis for NS (as proxies for disease severity). Additionally, the number of patients with specific NS subtypes (FSGS, IgAN, MN, MCD, lupus nephritis, MPGN) was reported. During the baseline period, the study also reported patients' Charlson Comorbidity Index (a composite measure of the patient's health status) as well as the incidence of other comorbidities commonly observed in patients with NS including mood disorder diagnoses, mobility impairment indicators, cardiac comorbidities, pulmonary comorbidities, vascular comorbidities, bone disorders, anemia, and renal comorbidities.
In both the baseline and follow-up periods, the study assessed the use of selected treatments and procedures for NS such as NS background therapies, NS related treatments (e.g. CNI and mycophenolate mofetil [MMF]), dialysis, and renal transplant.
As an assessment of disease progression during the follow-up period, the study reported the number of patients with end stage renal disease and chronic kidney disease as well as the number of patients receiving targeted NS treatments (e.g. chlorambucil, cyclophosphamide, proteasome inhibitor, and rituximab). Additionally, patient utilization of ultrasounds, deep vein thrombosis (DVT) ultrasounds, dialysis, plasmapheresis, creatinine and proteinuria tests, renal transplant and kidney biopsies was reported.
Total all-cause HCRU was examined for approved and denied patients in both periods. Resource utilization was categorized by place of service to identify sources of differential utilization. Place of service categories included the following: inpatient, outpatient/physician office and other. To compare the utilization of prescription medication in both cohorts, the average number of prescriptions per patient was assessed. The study also analyzed patients' CS use (e.g. the average number of CS prescriptions per patient along with the average annualized daily dose), and the use of disease modifying anti-rheumatic drugs (DMARDs).
Where the descriptive and comparative observations do not cross conventional thresholds of statistical significance, the study highlights results with greater than 10% relative difference between approved and denied patients, to indicate directional differences of potential clinical interest.
Compliance with ethics guidelines
Because of the nature of the retrospective study design using previously collected, de-identified data, IRB approval was not necessary for this study.
Results
Sample selection
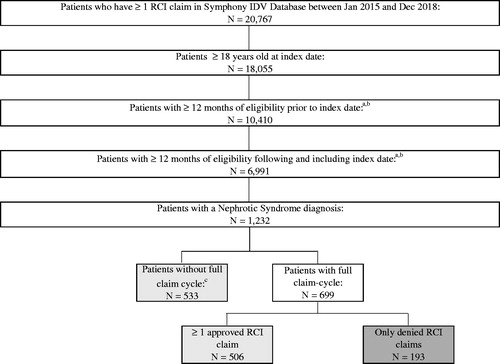
Of the 20,767 patients in the Symphony Health IDV database with at least one prescription claim for RCI during the study period, 1232 patients had a diagnosis of NS and met the inclusion criteria of being age 18 or older on their index date with at least 12 months of eligibility during both their baseline and follow-up periods (). Out of the 1232 NS patients eligible for the sample, 1039 patients had at least one approved claim for RCI while 193 patients had no approved RCI claims. Among all approved and denied patients in the sample, the full claim cycle (i.e. the history of approvals and/or denials of their RCI claims) was available for 699 (56.7%) patients; conversely, 533 (43.3%) patients had only direct feed claims. Because direct feed claims in the data originate from pharmacy point-of-sale feeds, they are necessarily approved claims (i.e. they are entered at the time of dispense and payment). Thus, the 533 patients with direct feed only claims are included in the 1039 patients that make up the approved cohort ().
Figure 1. Sample selection of patients with nephrotic syndrome and assignment to cohorts based on approval or denial of RCI claims. aFor patients that have at least 1 approved RCI claim, the index date is the date of the first approved claim. For patients that only have denied RCI claims, the index date is the date of the first denied claim. bA patient is assumed to have eligibility in the baseline period if they have a medical or prescription claim 9-12 months before the index date. A patient is assumed to have eligibility in the follow-up period if they have a medical or prescription claim 9-12 months after the index date. Symphony IDV does not contain eligibility information. cPatients without full-claim cycle are patients that have only direct feed claims, all of which are approved. Direct feed claims originate from point of sale data streams and therefore do not include denied claims. RCI repository corticotropin injection
Approval process
Among the 699 patients in the sample for whom the full claims cycle (i.e. full history of approvals and denials) was available, analysis of the approval process showed that 54.7% of patients with an approved prescription for RCI have no denied claims in the data, meaning that the remaining 45.3% of patients in the approved cohort had at least one denied claim prior to being approved. The mean number of approved claims per patient in the approved cohort was 7.9. For the 193 patients in the denied cohort, for whom the full claims cycle was available, the mean number of denied claims per patient was 2.9, indicating that these patients had their claims reviewed, on average, roughly 3 times before they were finally denied access to RCI treatment by their third-party payer ().
Table 3. Clinical outcomes and healthcare resource utilization during the follow-up period.
Baseline characteristics
During the 12-month baseline period, patients who had been approved for RCI treatment were observably different from the population denied access to RCI along a number of different dimensions (). Although most of the differences between the approved and denied cohorts during baseline did not reach conventional levels of statistical significance, the study reports measures that cross a threshold of >10% relative difference between approved and denied patients (unless otherwise noted). We interpret these differences to provide descriptive insights into the types of patients who may be more likely to have their prescription claims for RCI approved. Approved patients were older (mean age: 53.9) compared to denied patients (mean age: 48.4). Approved patients also had higher rates of comorbidities than denied patients in several of the categories assessed: bone disorders (16.8 vs. 13.0%); cardiac comorbidities (65.4 vs. 57.0%); pulmonary comorbidities (14.5 vs. 11.9%); vascular comorbidities (10.3 vs. 8.3%); and slightly higher rates (<10% difference) of renal comorbidities (80.3 vs. 78.2%). Previous treatment with diuretics and dialysis among approved patients was greater than among the denied cohort: (61.0 vs. 54.4%) and (3.7 vs. 2.6%), respectively ().
With regard to HCRU, a greater proportion of approved patients had at least one inpatient admission (34.1 vs. 28.0%); approved patients also had a higher mean number of outpatient visits than the denied cohort (14.9 vs. 12.8). A slightly greater utilization of prescription medication was observed among approved patients during the baseline with the average number of prescriptions being 53.7 compared to 49.9 for denied patients. Among prescription utilization, a slightly larger proportion (<10% difference) of approved patients received CS (62.0 vs. 59.6%) with more approved patients being prescribed a “high” dose of CS (i.e. >15 mg/day) than denied patients (26.2 vs. 20.7%). In total, this differential utilization translated to higher all-cause healthcare costs for approved patients compared to denied patients ($34,582 vs. $27,800) during the baseline period ().
Matching results
After matching, 94% of denied patients were matched to an approved patient with similar baseline characteristics for an overall sample of 181 matched pairs for whom outcomes were compared. In the matched cohort, none of the baseline characteristics were statistically different between approved and denied patients, indicating that the post-match sample of approved and denied patients were well balanced. While most of the outcomes observed among the matched sample did not reach conventional levels of statistical significance, we nonetheless report outcomes in categories that point to potentially clinically relevant distinctions between patients who were denied access to RCI treatment relative to those who were approved (). To identify potentially clinically relevant distinctions between the two cohorts the study reports outcomes among the matched sample that cross a threshold of >10% relative difference between approved and denied patients. The complete list of variables assessed during the follow-up is reported in .
Table 4. Clinical outcomes and healthcare resource utilization during the follow-up period (commercial plan type).
Healthcare resource use during the follow-up period
Among the matched cohort, NS patients denied access to RCI had greater HCRU than patients who had their RCI claims approved in a number of different areas. The denied cohort had more patients requiring an inpatient admission compared to the approved cohort during the follow-up period (64 denied vs. 52 approved). The denied cohort also had more patients requiring surgery visits than the approved cohort (21 vs. 18). Notably, denied patients underwent DVT ultrasound and dialysis procedures in higher proportions than approved patients during the follow-up period: (12.2 vs. 6.6%) and (10.5 vs. 6.1%), respectively ().
Prescription drug use and NS related treatments during the follow-up period
Although CS use was slightly greater among the approved cohort during the baseline period, more denied patients received CS prescriptions (104 vs. 95) and, on average, were prescribed a higher average annualized daily dose (4.1 vs. 3.4 mg) during the follow-up period. The use of NS background therapies such as ACE inhibitors, ARBs, anticoagulants, and calcium channel blockers during the follow-up period was also higher among denied patients than patients who were approved for RCI ().
Outcomes among commercially insured patients during the follow-up period
The data used in this study indicate the payer type associated with each prescription claim. Thus, it was possible to analyze outcomes for a subgroup of commercially insured patients in the sample (N = 114) to assess whether there were notably different results between the subset of commercially insured patients and patients in the overall sample. Among this subgroup, it was observed that the directional trends of worsening clinical outcomes for denied patients persisted. Specifically, commercially insured patients who are denied access to RCI had greater use of proteinuria tests (34.5 denied vs. 27.1% approved) and creatinine tests (41.8 denied vs. 32.2% approved) to assess renal function. Additionally, a higher proportion of denied patients had inpatient visits compared to approved patients (38.2 vs. 22.0%, respectively) with greater average lengths of inpatient stays (11.1 vs. 4.7 days, respectively). Denied patients also had greater utilization of dialysis (16.4 denied vs. 3.4% approved) and CS treatment (65.5 denied vs. 52.5% approved) at higher average annualized daily doses than approved patients (4.4 vs. 3.2 mg, respectively). Finally, a greater proportion of patients denied access to RCI had a diagnosis of end stage renal disease in the follow-up period compared to the approved cohort (21.8 vs. 15.3%, respectively) ().
Discussion
This study was the first of its kind to assess outcomes among NS patients in a real-world setting who were either approved or denied access to RCI treatment by their insurers. To implement this comparison, the study also assessed and controlled for numerous underlying differences in patient demographics, HCRU, and the incidence of comorbidities prior to patients' approval or denial of RCI treatment. While the results of the study did not reach conventional levels of statistical significance, the study reports measures that cross a threshold of >10% relative difference between approved and denied patients (unless otherwise noted).
At baseline, we found the approved cohort to be older and to have higher rates of cardiac, pulmonary, vascular, and bone comorbidities compared to the denied cohort. The approved cohort also exhibited greater prior use of diuretic medications and dialysis procedures than the denied cohort as well as a slight increase in the utilization of prescription medications, in general, and a higher use of CS, in particular. With regard to medical resource use, a higher proportion of approved patients had inpatient admissions and more outpatient visits than the denied cohort. Taken together, these observations imply that increased disease severity and rates of HCRU may play a role in the decision process of third-party payers when evaluating patients' prescription claims for RCI in the treatment of NS.
Matching approved and denied patients according to the complete set of characteristics measured in the baseline period allowed the study to reduce bias when comparing outcomes for patients who received access to RCI to those who did not. This comparison showed that denied patients had comparably worse indicators of clinical status and greater resource use in a number of categories during the 12-month follow-up period. Denied patients were more likely to require inpatient admissions than those in the approved cohort and underwent procedures for DVT ultrasound and dialysis at higher rates than patients who were approved for RCI treatment. The denied cohort's use of CS during the follow-up period also increased relative to the approved cohort where a greater number of denied patients received prescriptions for CS and, on average, at higher daily doses. Notably, the use of background therapies (e.g. ACEIs, ARBs, anticoagulants, and calcium channel blockers) to treat complications associated with NS was greater among patients in the denied cohort than that of approved patients. We interpret these results to indicate clinically relevant levels of continued disease activity and to highlight a potentially unmet need in patients who are denied access to RCI.
Because the data used in this study indicate the payer type for each prescription claim, it was possible to compare outcomes for a subset of the matched approved and denied cohorts with claims submitted to commercial payers. While the trends of generally worsening HCRU for denied patients hold after stratifying by payer type, the results showed a greater proportion of denied patients required continued assessment of renal function via proteinuria and creatinine tests, had higher utilization of dialysis and CS treatment, incurred more inpatient admissions and of greater lengths of stay, and had higher incidence of end stage renal disease in the follow-up period than their approved counterparts. These observations among the commercially insured subgroup further bolster the findings that there are important consequences for NS patients who are denied access to RCI treatment.
While this study was the first of its kind in that it compares HCRU in recent real-world settings among NS patients who received access to RCI treatment and those who did not, the findings are consistent with studies in other settings that show improved clinical outcomes in NS patients undergoing RCI treatment. For example, a combined prospective trial and retrospective review of patients with FSGS found that roughly one third of patients treated with RCI achieved remission; all patients achieving remission in that study were categorized as having steroid-resistant or steroid-dependent FSGS demonstrating the viability of RCI as a non-steroid treatment option for FSGSCitation20. Additionally, a prospective, open-label trial of RCI treatment in NS patients with various etiologies (e.g. MN, MCD, FSGS, and IgAN) showed that 7 out of 15 patients achieved a reduction in proteinuriaCitation6. Although the data used in our study did not contain lab results, precluding our study from assessing remission or reduction in proteinuria, we did observe directional improvements among the approved cohort relative to the denied in the reduced incidence of inpatient admissions, reduced use of dialysis and background therapies, as well as a comparative reduction in the use of CS. With regard to the use of CS in patients with NS of FSGS etiology, Tumlin et al. discuss the broad effort in clinical practice to find effective and safe alternatives to steroid therapy due to the increased risk of complications such as excessive weight gain, glucocorticoid-induced diabetes, and metabolic bone diseaseCitation10. Because the denied cohort in our study shows an increased use of CS treatment relative to approved patients, it may be the case that patients denied access to therapeutic alternatives such as RCI are at greater risk for steroid-induced complications. Another recent study has found that NS patients with MN type etiology are significantly burdened with high disease severity and incur substantial HCRU and costsCitation24. Further study on the benefits of RCI as an alternative first or second line treatment for NS may help clinicians and payers make decisions that reduce the burden of increased resource use and treatment induced adverse events commonly observed in NS patients.
Certain limitations of this study are inherent in the data used for the analysis. First, while the data are uniquely rich on a number of dimensions, given the rareness of NS overall, the sample size was limited after implementing the full set of analytic criteria and matching methodology. As a result, most outcomes did not reach conventional levels of statistical significance, and future research should explore, where possible, whether these observed trends hold with larger sample sizes. Second, because certain clinical information was unavailable in the data, measures of disease severity could be assessed by proxy only. Notably, the data do not contain lab values for clinical tests. Clinical measures of severity, therefore, were assessed based on treatment and resource use relying on ICD 9/10 diagnosis codes and CPT codes associated with a particular claim. Additionally, because the Symphony IDV is an open source claims database, aggregating claims for patients from a variety of different data streams, it is possible that not all patient claims are captured in the data. Third, while a proportion of patients in the approved cohort had their claims for RCI denied before ultimately being approved, the data do not indicate a reason for claim denials. Thus, the study could not explain the underlying dynamics that influenced denial and approval patterns for patients. We interpret these dynamics as the influence of different formulary structures and prior authorization requirements among payers as well as physician prescribing behaviors. A more detailed exploration of the factors that lead to approval (or denial) of RCI claims for NS patients could be the focus of future research. Finally, the data reflect results from a variety of payer types. While this adds to the richness of the data, the convenience sample from which the data are based may not be reflective of or generalizable to any particular payer, depending on the characteristics of their enrolled population.
Conclusions
This real-world study is the first to use rigorous methodologies to estimate the impact of using RCI in patients with NS using a uniquely-suited, claims database. As such, this study helps fill a gap in the literature addressing health economics outcomes and research for NS patients. The comparison of outcomes between approved and denied patients with similar baseline profiles showed that patients who were denied access to RCI treatment had directionally higher HCRU. These include higher rates of inpatient admissions, greater utilization of DVT ultrasound and dialysis treatments, as well as greater use of CS and NS background therapies. The findings of this study may therefore help providers and health plans review current situations in which RCI treatment may be beneficial to further improve quality of care and potentially improve outcomes in this important patient population.
Transparency
Declaration of funding
Sponsorship of this study and article processing charges were funded by Mallinckrodt Pharmaceuticals (Bedminster, NJ).
Declaration of financial/other relationships
JN and GJW are employees of Mallinckrodt Pharmaceuticals, which provided research funding to Analysis Group (employer of JBR, AW, MS, EB, and ND). MPP is a paid consultant for Mallinckrodt Pharmaceuticals.
Compliance with ethics guidelines
This article is based on previously collected, de-identified data and did not explicitly precipitate human or animal subjects.
Acknowledgements
No assistance in the preparation of this article is to be declared.
Data availability statement
The datasets generated during and/or analyzed during the current study are not publicly available because they are proprietary administrative health claims data owned by Symphony Health.
Notes
i Acthar is a registered trademark of Mallinckrodt ARD LLC., Bedminster, NJ, USA.
References
- Hull RP, Goldsmith DJ. Nephrotic syndrome in adults. BMJ. 2008;336(7654):1185–1189.
- Orth SR, Ritz E. The nephrotic syndrome. N Engl J Med. 1998;338(17):1202–1211.
- National Institute of Diabetes and Digestive and Kidney Diseases: National Institutes of Health. Nephrotic syndrome in adults; 2014 [cited 2020 Aug 18]. Available from: https://www.niddk.nih.gov/health-information/kidney-disease/nephrotic-syndrome-adults.
- Bomback AS, Tumlin JA, Baranski J, et al. Treatment of nephrotic syndrome with adrenocorticotropic hormone (ACTH) gel. Drug Des Dev Ther. 2011;5:147–153.
- Madan A, Mijovic-Das S, Stankovic A, et al. Acthar gel in the treatment of nephrotic syndrome: a multicenter retrospective case series. BMC Nephrol. 2016;17(1):37.
- Bomback AS, Canetta PA, Beck LH, Jr, et al. Treatment of resistant glomerular diseases with adrenocorticotropic hormone gel: a prospective trial. Am J Nephrol. 2012;36(1):58–67.
- Pilon D, Sheehan JJ, Szukis H, et al. Medicaid spending burden among beneficiaries with treatment-resistant depression. J Comp Eff Res. 2019;8(6):381–392.
- Tumlin JA, Galphin CM, Rovin BH. Steroid resistant nephrotic syndrome: a prospective, open label study of the safety and efficacy of combination tacrolimus and ACTHar gel therapy [ASN abstract FR-PO434]. J Am Soc Nephrol. 2015;26(Suppl):455A.
- Tumlin JA, Rovin BH, Paxton WG, et al. A prospective, open label study of the safety and treatment efficacy of ACTHar gel for fibrillary glomerulonepritis [ASN abstract SA-PO659]. J Am Soc Nephrol. 2016;27(Suppl):781A.
- Tumlin J, Galphin C, Santos R, et al. Safety and efficacy of combination ACTHar gel and tacrolimus in treatment-resistant focal segmental glomerulosclerosis and membranous glomerulopathy. Kidney Int Rep. 2017;2(5):924–932.
- Madan A. Repository corticotropin injection in a patient presenting with focal segmental glomerulosclerosis, rheumatoid arthritis, and optic neuritis: a case report. Int J Gen Med. 2015;8:119–124.
- Madan A, Mijovic-Das S, Stankovic A, et al. Treatment response to Acthar Gel in patients with idiopathic focal segmental glomerulosclerosis and idiopathic membranous nephropathy: a retrospective case series [ASN abstract TH-PO437]. J Am Soc Nephrol. 2014;25(Suppl):206A.
- Reuben S, Maroz N. Treatment of Fibrillary Glomerulonephritis with Use of Repository Corticotropin Injection (Acthar) [ASN abstract TH-PO018]. J Am Soc Nephrol. 2016;27(Suppl):99A.
- Bonilla NA, Parada XF, Henriquez MA. A case series on the treatment of nephrotic syndrome with adrenocorticotropin hormone (ACTH) gel in an office setting [ASN abstract PUB219]. J Am Soc Nephrol. 2015;26(Suppl):937A.
- Filippone EJ, Dopson SJ, Rivers DM, et al. Adrenocorticotropic hormone analog use for podocytopathies. IMCRJ. 2016;9:125–133.
- Marayati F, Long-Term HP. Acthar gel treatment of relapsing idiopathic membranous glomerulopathy: a case study [ASN abstract PUB206]. J Am Soc Nephrol. 2015;26(Suppl):934A.
- McMahon BA, Berliner AR. Treatment of severe resistant idiopathic membranous glomerulopathy with adrenocorticotropic hormone gel: a case report [ASN abstract SA-PO032]. J Am Soc Nephrol. 2015;26(Suppl):631A.
- Hladunewich MA, Cattran D, Beck LH, et al. A pilot study to determine the dose and effectiveness of adrenocorticotrophic hormone (H.P. Acthar Gel) in nephrotic syndrome due to idiopathic membranous nephropathy. Nephrol Dial Transplant. 2014;29(8):1570–1577.
- Lieberman KV, Pavlova-Wolf A. Adrenocorticotropic hormone therapy for the treatment of idiopathic nephrotic syndrome in children and young adults: a systematic review of early clinical studies with contemporary relevance. J Nephrol. 2017;30(1):35–44.
- Hogan J, Bomback AS, Mehta K, et al. Treatment of idiopathic FSGS with adrenocorticotropic hormone gel. Clin J Am Soc Nephrol. 2013;8(12):2072–2081.
- Park Y, Raza S, George A, et al. The effect of formulary restrictions on patient and payer outcomes: a systematic literature review. J Manag Care Spec Pharm. 2017;23(8):893–901.
- Rice JB, Panaccio MP, White A, et al. Consequences of insurance denials among U.S. patients prescribed repository corticotropin injection for acute exacerbations of multiple sclerosis. Neurol Ther. 2020. DOI:10.1007/s40120-020-00219-y
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424.
- Nazareth TA, Kariburyo F, Kirkemo A, et al. Patients with idiopathic membranous nephropathy: a real-world clinical and economic analysis of U.S. claims data. J Manag Care Spec Pharm. 2019;25(9):1011–1020.
