Abstract
Objective
To evaluate and compare patient and neurologist preferences for relapsing–remitting multiple sclerosis (RRMS) treatments with respect to benefits and risks associated with common and novel disease-modifying therapies, including brain volume loss (BVL).
Methods
Patients with non-highly-active RRMS and neurologists in the United Kingdom completed an online cross-sectional survey. Patients completed one discrete choice experiment (DCE) exercise and providers completed two, one focusing on treatment for non-highly-active RRMS and another focused on highly active RRMS. Respondents chose between two treatment profiles that varied on seven attributes identified in qualitative research: 2 year disability progression; 1 year relapse rate; rate of BVL; and risks of gastrointestinal symptoms, flu-like symptoms, infection and life-threatening event. Bayesian modeling was used to estimate attribute-level weighted preferences.
Results
Patients (n = 144) prioritized slowing the rate of BVL, followed by reducing risk of infection, rate of 2 year disability progression and 1 year relapse rate. For non-highly-active patients, neurologists (n = 101) prioritized slowing the rate of BVL, followed by reducing 2 year disability progression, risk of infection and 1 year relapse rate. For highly active patients, neurologists prioritized lowering the 1 year relapse rate, followed by slowing the rate of BVL and 2 year disability progression. In all three DCEs, rate of BVL was approximately twice as important as reducing the risks of flu-like symptoms, gastrointestinal symptoms and life-threatening event.
Conclusions
This study highlights similarities in treatment preferences for non-highly-active RRMS among patients and neurologists and differences in neurologists’ preferences for treating non-highly-active vs. highly active RRMS. This research identifies BVL as a treatment outcome that should be discussed when physicians engage in shared decision-making with patients.
Introduction
Multiple sclerosis (MS) is a chronic inflammatory disease that impairs the functioning of the central nervous system and causes neurologic disabilitiesCitation1. MS is estimated to affect more than 2 million people worldwide, including 130,000 people in the United KingdomCitation2. The most common symptoms of MS include fatigue, chronic pain, mobility problems and cognitive impairmentCitation3, and the disease is most often identified during early adulthood between the ages of 20 and 50 yearsCitation4. Relapsing–remitting MS (RRMS) is the most frequently encountered form of the disease and is characterized by temporary periods of worsening neurologic function (i.e. relapses, flare-ups or exacerbations), followed by partial or complete recovery periods (i.e. remissions)Citation1,Citation5. Each patient’s disease course is unique and can vary with regard to their symptoms, rate of progression and response to treatmentCitation6.
There remains no cure for MS, and thus current treatments seek to slow disease progression and provide symptomatic relief. Disease-modifying therapies (DMTs) compose the mainstay of RRMS management. These agents vary according to treatment efficacy (e.g. relapse rates) and safety profiles (e.g. risk of developing progressive multifocal leukoencephalopathy)Citation7. Brain volume loss (BVL) is a well documented characteristic of MS disease progressionCitation8. Whereas all individuals lose brain volume as they age, several reports have indicated that this loss occurs more rapidly among those with MS, with BVL correlated with lesion burden on magnetic resonance imaging, age, duration of disease and disability levelsCitation9–14. BVL is observed in the earliest stages of MS and is associated with long-term disease progressionCitation15,Citation16. Evidence suggests that BVL is also associated with deficits in cognitive function, with studies showing that decreases in grey matter, most notably in the cortex and thalamus, can lead to long-term impairmentCitation17–19. Once lost, brain volume cannot be recovered and, as neurologic damage accumulates, the symptoms of MS can become permanent, resulting in progressive disability and declining quality of life (QoL)Citation20–22. Treatment with DMTs has been shown to slow the rate of BVL to varying degreesCitation23–26.
Given that currently available agents target symptomatic management rather than disease careCitation27, understanding patient and physician preferences for the varying benefit–risk profiles of DMTs in RRMS may lead to more informed treatment decision-making and improved patient outcomes. Despite the large body of research regarding patient preferences for MS treatmentsCitation28–31, studies have not explored the importance of BVL among patients and few investigations have explored physician preferences or compared patient and physician preferences in this domainCitation32. Therefore, the current study sought to assess and compare preferences with respect to the benefits and risks associated with common and novel DMTs in RRMS among patients with RRMS and neurologists who treat RRMS in the United Kingdom.
Methods
This study involved three phases: (1) a targeted literature review and concept elicitation interviews with patients with RRMS and neurologists who treat RRMS to inform survey content by determining the features (attributes) that most influence treatment choice; (2) cognitive interviews to refine the survey content wording; and (3) online, cross-sectional stated preference surveys completed in the United Kingdom by neurologists and patients with RRMS. The surveys were administered between 16 March and 22 May 2020.
Patients included in this study were ≥18 years of age at the time of the survey and self-reported a health care provider diagnosis of RRMS. Patients were excluded if they were taking a medication typically prescribed in highly active RRMS (e.g. alemtuzumab, natalizumab, cladribine, rituximab) or had never undergone treatment for RRMS with a DMT and were not planning to in the 6 months following the time of survey. Physicians included in this study were board-certified or -eligible neurologists, had been in practice for 3 years or more, spent at least 75% of their time in direct patient care, and managed at least 20 patients with MS and at least 10 patients with RRMS in the 3 months prior to the time of survey.
Patients with RRMS were recruited from panels, including the Kantar Profiles International Patient Panel, Dynata, Borderless Access and Empanel tomorrow. Survey invitations were sent to panel members previously identified as having MS. In addition, when needed, panel members with autoimmune conditions and general panel members were sent prequalification questionnaires. Survey invitations were sent to those who qualified. Neurologists were recruited from health care panels, including the Kantar Profiles International Physician Panel, Sermo, SurveyHealthcareGlobus, M3 Global Panel and GLocalMind. Health care providers identified as neurologists were sent survey invitations. All participants provided informed consent, and the study was granted exemption from ethics review by the Pearl IRB (Indianapolis, IN; IRB Study Number: 19-KANT-189).
Survey content
Treatment preferences were evaluated using a discrete choice experiment (DCE). A DCE survey is designed to assess participants’ willingness to accept trade-offs among hypothetical treatment profiles, which are described using treatment attributes of varying levels.
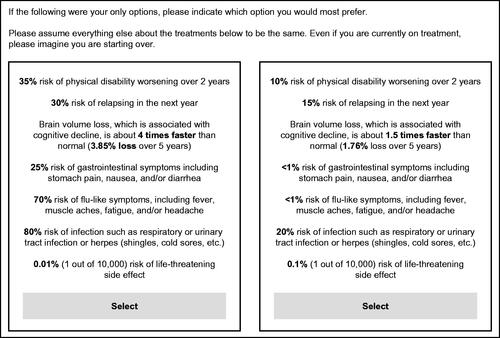
In a series of presented choices, respondents viewed two hypothetical treatment profiles side-by-side, consisting of combinations of seven treatment features (“attributes”) that varied with respect to their “attribute levels.” Patients completed one DCE exercise and neurologists completed two DCEs, one considering treatment for non-highly-active RRMS and another considering treatment for highly active RRMS. The same attributes and levels were used across all DCEs ().
Table 1. Attributes and levels included in the DCE.
The combinations of levels shown across choice tasks in the DCE were based on a balanced design with overlapCitation33. shows an example choice task.
Figure 1. Example of a DCE choice task seen by respondents. Abbreviation. DCE, Discrete choice experiment.
The attributes were selected through qualitative interviews with 10 patients and 10 neurologists using open-ended questions to identify key treatment attributes influencing treatment choiceCitation34. Cognitive interviews with five adult patients with RRMS and five neurologists were performed to ensure that the attributes and levels were being interpreted as intended. The levels used for each attribute were based on the range of performance observed in select DMTs approved for the treatment of RRMSCitation23–26,Citation35–40. More information on the selection of levels for BVL can be found in Supplemental Appendix A.
Prior to the DCE exercises, the surveys included an introductory section describing the meaning of BVL, as well as a series of items where respondents rated each of the attribute levels. This section helped familiarize respondents with the treatment attributes in the DCE choice tasks. These rating items were also used as an indicator of inattentiveness in responses.
The survey also included patient sociodemographic, clinical and treatment characteristics, and neurologist sociodemographic, professional history and practice characteristics. The Multiple Sclerosis Self-Efficacy Scale (MSSE) assessed patients’ self-efficacy, which measures self-efficacy in function maintenance and control over MS symptoms from patients’ perspectives. Patients rated the certainty to which they could perform specific behaviors in increments of 10 on a scale from 10 to 100 (10 = very uncertain, 50 = moderately certain and 100 = very certain)Citation41. The MSSE consists of two 9-item subscales: Function (MSSE-F) and Control (MSSE-C), which measure confidence with functional abilities and managing symptoms, respectively. Items were averaged to compute the two subscale scores (range: 10–100), and subscales were summed to compute a total score (range: 20–200)Citation42. Higher scores indicate greater self-efficacy.
Patients’ cognitive function was assessed using the Multiple Sclerosis Quality of Life (MSQOL)-54 Cognitive Function subscaleCitation43. The measure includes four items rating the frequency of problems with cognitive function over the past month on a scale from 1 (all of the time) to 6 (none of the time). Ratings are converted to increments of 20 on a 0–100 scale (0 = “all of the time,” 20 = “most of the time,” 40 = “a good bit of the time,” 60 = “some of the time,” 80 = “a little of the time” and 100 = “none of the time”) and are averaged to form the Cognitive Function score. Higher scores indicate higher cognitive function.
Statistical analysis
Descriptive statistics characterized the patient and neurologist samples and included means and standard deviations, or medians and ranges, for continuous and count variables, and frequency and percentage for categorical variables. A hierarchical Bayesian (HB) model was fitted to the choice data from the DCE to estimate preference weights for each attribute and attribute level. Mean preference weights were used as the point estimates for the HB model coefficients. To evaluate willingness to make trade-offs, the magnitude of change between levels of one attribute was compared with the magnitude of change between levels of a different attribute. The conditional relative importance of each attribute was calculated at the respondent level by dividing the range of each attribute (utility of most favorable level minus utility of least favorable level) by the sum of the ranges of all attributes and multiplying by 100.
One-way analysis of variance (ANOVA) tests were used to examine whether an attribute’s relative importance differed between the two DCEs among neurologists (non-highly-active RRMS vs. highly active RRMS) and between patients and neurologists. As the study was designed to exclude patients on a medication typically used in highly active RRMS, comparisons between patients and neurologists were conducted only for the non-highly-active RRMS DCE. One-way ANOVA tests were also used to examine whether an attribute’s relative importance differed between select subgroups (patients: age, line of therapy, disability [measured by the MSSE total score] and cognitive function [measured by the MSQOL-54 Cognitive Function scale]; neurologists: years in practice [median split], most frequently prescribed type of MS medication for non-highly-active RRMS [oral vs. both equally], practice type [university/teaching vs. community hospital practice] and RRMS patient volume in the preceding 3 months [median split]).
Analysis was conducted using SPSS Statistics 23 (IBM, Armonk, NY) for descriptive statistics and subgroup analyses and Lighthouse Studio 2018 Version 9.8.0 (Sawtooth Software, Provo, UT) for the DCE analysis.
Results
Of a total of 2254 patients and 177 neurologists who completed an online screener, 184 and 105, respectively, qualified for and completed the survey. As 40 patients and 4 neurologists were flagged for potential inattention in their responses, preference weights were calculated with and without these respondents and, given the results, they were removed from analyses. The reasons respondents were flagged for potential inattention included two or more illogical responses to the attribute rating items, survey and/or choice task completion in less than half of the median completion time, and/or patient self-reporting being diagnosed with every comorbidity (patients only). The final analysis sample included 144 patients and 101 neurologists.
Patients, on average, were 42.8 years; 68.8% were female. The majority were in a committed relationship or married (68.1%) and had children in their household (61.8%) (). Almost half were college educated (45.8%), a little over half were employed (55.6%), and about one fifth were on long- or short-term disability (19.4%) at the time of the study. The most frequently reported comorbidities included depression (18.1%) and anxiety (15.3%). About three quarters of patients (72.9%) were on a prescription medication for RRMS. Patients reported a moderate to high level of certainty in their ability to perform functional abilities and manage symptoms, with a median MSSE-F score of 71.1 (range: 10–100) and a median MSSE-C score of 56.7 (range: 10–100). Overall, the median MSQOL-54 Cognitive Function score of 60 (range: 0–100) indicates that the patient sample had problems with their cognitive function “some of the time” in the past month ().
Table 2. Patient characteristics.
The majority of neurologists practiced in an academic setting (80.2%), with an average of 12.8 years in practice. Neurologists spent an average of 85.5% of their time in direct patient care and treated a median of 40 patients with RRMS in the past 3 months ().
Table 3. Neurologist characteristics.
Treatment preferences
Among patients, the most important treatment attribute was the rate of BVL, followed by the risk of infection, rate of 2 year disability progression, 1 year relapse rate, risk of flu-like symptoms, risk of gastrointestinal symptoms and risk of a life-threatening event ().
Figure 2. Relative importance of treatment attributes; 95% confidence intervals are depicted. Abbreviations. BVL, Brain volume loss; GI, Gastrointestinal; RRMS, Relapsing–remitting multiple sclerosis.
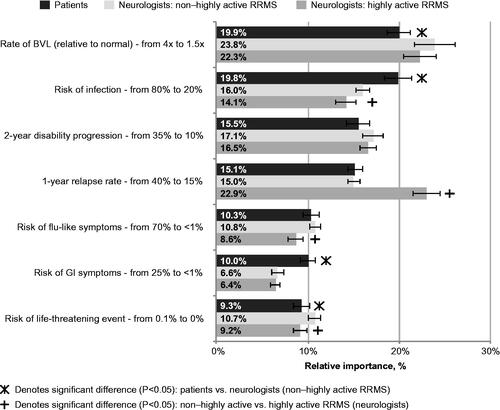
The most important treatment attribute among neurologists when considering non-highly-active RRMS was the rate of BVL, followed by the rate of 2 year disability progression, risk of infection, 1 year relapse rate, risk of flu-like symptoms, risk of a life-threatening event and risk of gastrointestinal symptoms. Reducing the rate of BVL was approximately twice as important as reductions in the risk of flu-like symptoms, gastrointestinal symptoms and a life-threatening event for non-highly-active RRMS patients and neurologists when considering treatment for non-highly-active RRMS ().
When neurologists considered treatment for highly active RRMS, the most important treatment attribute was 1 year relapse rate, followed by the rate of BVL, the rate of 2 year disability progression, risk of infection, risk of life-threatening event, risk of flu-like symptoms and risk of gastrointestinal symptoms (). Lowering the 1 year relapse rate and slowing the rate of BVL were at least twice as important as reducing the risk of flu-like symptoms, gastrointestinal symptoms and a life-threatening event.
Reducing the 1 year relapse rate was rated as more important in the highly active RRMS than the non-highly-active RRMS treatment setting (22.9% vs. 15.0%; p < .001), whereas reducing the risk of infection (16.0% vs. 14.1%; p = .012), the risk of flu-like symptoms (10.8% vs. 8.6%; p < .001) and the risk of a life-threatening event (10.7% vs. 9.2%; p = .002) was more important in the non-highly-active RRMS than the highly active RRMS treatment setting.
Reducing the risk of infection (19.8% vs. 16.0%; p < .001) and the risk of gastrointestinal symptoms (10.0% vs. 6.6%; p < .001) was more important to non-highly-active RRMS patients than neurologists when considering treatment for non-highly-active RRMS, whereas slowing the rate of BVL (23.8% vs. 19.9%; p = .011) and reducing the risk of a life-threatening event (10.7% vs. 9.3%; p = .025) were more important to neurologists when considering treatment for non-highly-active RRMS than non-highly-active RRMS patients ().
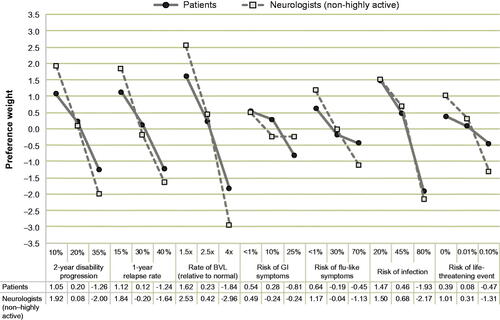
The attribute-level preference weights further illustrate the trade-offs that non-highly-active RRMS patients and neurologists considering treatment for non-highly-active RRMS are willing to make (). For example, patients would be willing to accept an increase in risk of infection from 20% to 45% (change in preference weight: 1.47–0.46 = 1.01) in exchange for reducing the rate of BVL from 2.5 times to 1.5 times relative to normal (1.62–0.23 = 1.39). In another example, when considering treatment for non-highly-active RRMS, neurologists would be willing to accept an increase in risk of flu-like symptoms from <1% to 30% in exchange for reducing the rate of BVL from 4 times to 2.5 times relative to normal (1.21 vs. 3.38).
Figure 3. Attribute-level preference weights. Preference weights should not be interpreted by themselves. Instead, the magnitude of change within one attribute should be compared with change within another attribute. Abbreviations. BVL, Brain volume loss; GI, Gastrointestinal.
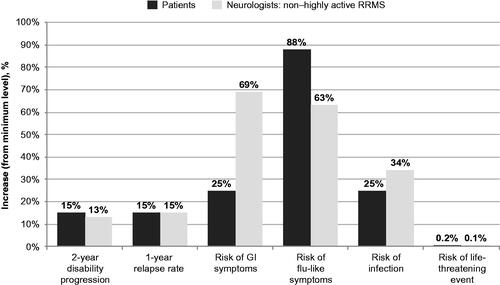
illustrates the increases in each attribute from the minimum level that patients and neurologists are willing to accept in exchange for slowing the rate of BVL from 2.5 times to 1.5 times relative to normal. Specifically, to slow BVL from 2.5 times to 1.5 times relative to normal, patients are willing to accept larger increases in risk of flu-like symptoms than neurologists considering non-highly-active RRMS (88% vs. 63%), whereas neurologists considering non-highly-active RRMS are willing to accept larger increases in risk of gastrointestinal symptoms (69% vs. 25%) and risk of infection (34% vs. 25%) than patients. However, patients and neurologists considering treatment for non-highly-active RRMS were comparable in the increases that they would be willing to accept in the rate of 2 year disability progression, the 1 year relapse rate and the risk of a life-threatening event to slow the rate of BVL from 2.5 times to 1.5 times relative to normal.
Subgroup analyses
No meaningful differences in relative importance were found by age, line of therapy, disability and cognitive function among patients; nor by years in practice, most frequently prescribed type of MS medication, practice type and patient volume among neurologists when considering non-highly-active RRMS. Preferences also did not differ by years in practice, practice type or patient volume among neurologists when considering highly active RRMS.
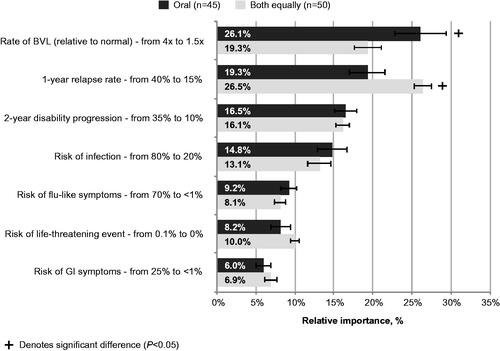
When considering highly active RRMS patients, significant differences were identified in the relative importance estimates for decreasing the rate of BVL and the 1 year relapse rate by most frequently prescribed type of MS medication for non-highly-active RRMS (). Specifically, decreasing the rate of BVL from 4 times to 1.5 times was the most important treatment attribute among neurologists who most frequently prescribe oral medications for non-highly-active RRMS and was significantly more important to these neurologists than those who equally prescribe both oral and injectable medications (26.1% vs. 19.3%; p = .006). Conversely, decreasing the 1 year relapse rate from 40% to 15% was most important to neurologists who equally prescribe both oral and injectable medications for non-highly-active RRMS and was significantly more important to these neurologists than those who most frequently prescribe oral medications (26.5% vs. 19.3%; p = .001).
Figure 5. Relative importance of treatment attributes in highly active RRMS DCE: neurologists by most frequently prescribed medication for non-highly-active RRMS. Note: 95% confidence intervals are depicted. Abbreviations. BVL, Brain volume loss; DCE, Discrete choice experiment; GI, Gastrointestinal, RRMS, Relapsing–remitting multiple sclerosis.
Discussion
This study showed that treatment preferences of non-highly-active RRMS patients and neurologists treating non-highly-active RRMS patients were generally aligned, with importance placed on reducing the rate of BVL, reducing the risk of infection and slowing the rate of 2 year disability progression. The priority placed on slowing the rate of BVL in both of these study groups emphasizes the shared importance of this aspect of MS progression. Previous preference-based studies in MSCitation28–31 have not included BVL, and thus the current results make an important contribution to the literature. Greater understanding of patient and provider preferences can help emphasize the importance of effective communication and shared decision-making, and ensure that research and clinical priorities align with patient priorities.
As the consistency of this pattern of preferences became apparent among neurologists in the treatment of non-highly-active RRMS, notable differences emerged in the context of treating highly active disease. In this scenario, neurologists prioritized lowering the 1 year relapse rate, followed by reducing the rates of BVL and 2 year disability progression. Whereas this may be expected, with the reduction of relapse rates in the short term remaining a priority among those experiencing high symptom burden, the consistent importance placed on reducing BVL even among these patients is noteworthy. As noted, BVL has been consistently associated with disease progression, cognitive deficits and long-term disabilityCitation9–14,Citation17–19. It is thus understandable why this factor holds such importance to patients, with cognitive functioning intimately tied to psychosocial outcomes, social engagement and QoLCitation44. Moreover, impacts on cognitive functioning were one of the most frequently cited areas of importance among patients and neurologists in the prior qualitative researchCitation34, reinforcing that the associated BVL should be considered in tandem with disability progression and relapse rates in treatment decision-making.
Because of time and resource constraints, BVL is not routinely measured, nor is it currently a therapeutic target in clinical practice. However, the importance placed on reducing the rate of BVL among the patients and neurologists surveyed suggests that this is a priority to both stakeholders and should thus be part of the discussion when exploring treatment options. In addition, more research in this area is needed to develop treatment guidance related to BVLCitation45. Further, the importance of reducing the rate of BVL emphasizes the need for novel therapeutic agents that can target this component of disease progression, thus limiting cognitive impairments and promoting long-term functioning among patients.
This study has limitations. The DCE methodology relies on self-reported data and hypothetical scenarios, and thus may not reflect the true preferences of patients and neurologists should they face such decisions with real clinical, financial and emotional consequences. Additionally, the number of attributes that can be included in a DCE is limited and therefore may not reflect all the aspects of a treatment that can influence preferences, such as treatment costs, and may not reflect other clinical outcomes used to measure treatment response, such as quality of lifeCitation46 or spinal cord atrophyCitation47. Additionally, the online survey format may have represented a barrier to individuals without easy access to the internet or comfort with online activities and thus could have underrepresented such individuals, as well as those with more severe disease or physicians with heavier workloads. This study overrepresented younger patients; while the median age of a person with MS in the current study was 42.5 years, the median age of a person with MS in the United Kingdom is 55–59 yearsCitation48. Therefore, results may not generalize to the entire MS population in the United Kingdom. However, preferences did not differ by patient age in the current study. In addition, in the current study, 80% of the neurologists practiced in an academic setting, which may have underrepresented neurologists from a community setting; thus, the results may not be generalizable to the entire neurologist population in the United Kingdom. Nevertheless, the preferences of neurologists did not differ between neurologists practicing in a university/teaching practice and neurologists practicing in a community hospital practice. Whereas such limitations must be acknowledged, efforts were made to recruit a representative population and minimize such bias, with targeted recruitment of respondents, the listing of several diagnoses (patients) and medical specialties (neurologists) to mask the target population, mapping attribute levels to clinical evidence, and constructing choice questions that mimicked realistic clinical choices.
Conclusions
For non-highly-active RRMS patient groups, UK patients and neurologists possess similar priorities in treatment preferences, with both valuing reducing the rate of BVL, 2 year disability progression and infection among their top three priorities. However, when considering highly active RRMS patients, UK neurologists placed highest importance on reducing the annual relapse rate, followed by reducing the rate of BVL. Both UK patients and neurologists placed lower importance on reducing risk of a life-threatening event effect, potentially owing to the very low risk of the event occurring. This research highlights differences in perspectives between patients and neurologists in the United Kingdom and identifies BVL as a treatment outcome that should be considered as a topic when physicians engage in shared decision-making with their patients.
Transparency
Declaration of funding
The study was sponsored by Bristol Myers Squibb, Princeton, NJ. The authors received medical writing support from Errol J. Philip PhD, who is a paid consultant of Kantar Health, and Jennifer Ken-Opurum PhD of Kantar Health, New York, NY, and editorial support from Samantha Rivera MS of Peloton Advantage LLC, an OPEN Health company, Parsippany, NJ, sponsored by Bristol Myers Squibb.
Author contributions
T.T., J.K. and K.B. contributed to the conception of the study. O.W., M.J.C.M., d.S.M. and K.B. were involved in data analysis. M.J.C.M. drafted the manuscript. All authors contributed to the design of the study, interpretation of the data and the critical review of the manuscript. All authors provided the final approval of the manuscript and agreed to be accountable for all aspects of the work.
Declaration of financial/other relationships
O.W., M.J.C.M., d.S.M. and K.B. have disclosed that they are employees of Kantar Health, who received funding from Bristol Myers Squibb to conduct this study. T.T. and J.K. have disclosed that they are employees and shareholders of Bristol Myers Squibb. CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Supplemental Material
Download MS Word (20.5 KB)Acknowledgements
The authors acknowledge Errol J. Philip PhD and Jennifer Ken-Opurum PhD for their assistance with medical writing and Peloton Advantage LLC (Parsippany, NJ), an OPEN Health company, for editorial support.
Data availability statement
BMS policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
References
- Lee J, Dunn J. Mobility concerns in multiple sclerosis—studies and surveys on US patient populations of relevance to nurses. US Neurol. 2013;9(1):17.
- MSTrust. Prevalence and incidence of multiple sclerosis 2020 [Internet] [cited 2020 Nov 11]. Available from: https://www.mstrust.org.uk/a-z/prevalence-and-incidence-multiple-sclerosis
- Antao L, Shaw L, Ollson K, et al. Chronic pain in episodic illness and its influence on work occupations: a scoping review. Work. 2013;44(1):11–36.
- Schapiro R. Managing the symptoms of multiple sclerosis. 5th ed. New York: Demos Medical Publishing; 2007.
- Hooper K. Managing progressive MS [Internet]. National MS Society; 2018 [cited 2020 Nov 11]. Available from: https://www.nationalmssociety.org/NationalMSSociety/media/MSNationalFiles/Brochures/Brochure_Managing_Progressive_MS.pdf
- Rae-Grant A, Day GS, Marrie RA, et al. Practice guideline recommendations summary: disease-modifying therapies for adults with multiple sclerosis: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90(17):777–788.
- Tran JQ, Hartung JP, Peach RJ, et al. Results from the first-in-human study with ozanimod, a novel, selective sphingosine-1-phosphate receptor modulator. J Clin Pharmacol. 2017;57(8):988–996.
- Bermel RA, Bakshi R. The measurement and clinical relevance of brain atrophy in multiple sclerosis. Lancet Neurol. 2006;5(2):158–170.
- Chard DT, Griffin CM, Parker GJ, et al. Brain atrophy in clinically early relapsing–remitting multiple sclerosis. Brain. 2002;125(Pt 2):327–337.
- De Stefano N, Matthews PM, Filippi M, et al. Evidence of early cortical atrophy in MS: relevance to white matter changes and disability. Neurology. 2003;60(7):1157–1162.
- Eshaghi A, Prados F, Brownlee WJ, et al. Deep gray matter volume loss drives disability worsening in multiple sclerosis. Ann Neurol. 2018;83(2):210–222.
- Radue EW, Barkhof F, Kappos L, et al. Correlation between brain volume loss and clinical and MRI outcomes in multiple sclerosis. Neurology. 2015;84(8):784–793.
- Roosendaal SD, Bendfeldt K, Vrenken H, et al. Grey matter volume in a large cohort of MS patients: relation to MRI parameters and disability. Mult Scler. 2011;17(9):1098–1106.
- Sanfilipo MP, Benedict RH, Sharma J, et al. The relationship between whole brain volume and disability in multiple sclerosis: a comparison of normalized gray vs. white matter with misclassification correction. Neuroimage. 2005;26(4):1068–1077.
- Moccia M, Quarantelli M, Lanzillo R, et al. Grey:white matter ratio at diagnosis and the risk of 10-year multiple sclerosis progression. Eur J Neurol. 2017;24(1):195–204.
- Siffrin V, Vogt J, Radbruch H, et al. Multiple sclerosis – candidate mechanisms underlying CNS atrophy. Trends Neurosci. 2010;33(4):202–210.
- Horakova D, Kalincik T, Dusankova JB, et al. Clinical correlates of grey matter pathology in multiple sclerosis. BMC Neurol. 2012;12:10.
- Minagar A, Barnett MH, Benedict RH, et al. The thalamus and multiple sclerosis: modern views on pathologic, imaging, and clinical aspects. Neurology. 2013;80(2):210–219.
- Popescu BFG, Lucchinetti CF. Meningeal and cortical grey matter pathology in multiple sclerosis. BMC Neurol. 2012;12:11.
- Gold R, Wolinsky JS, Amato MP, et al. Evolving expectations around early management of multiple sclerosis. Ther Adv Neurol Disord. 2010;3(6):351–367.
- Jacques FH. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2015;84(9):963.
- Tedeholm H, Lycke J, Skoog B, et al. Time to secondary progression in patients with multiple sclerosis who were treated with first generation immunomodulating drugs. Mult Scler. 2013;19(6):765–774.
- Cohen JA, Comi G, Selmaj KW, et al. Ozanimod vs interferon β-1a: clinical and MRI results of RADIANCE part B – a 2-year phase 3 trial in relapsing multiple sclerosis [abstract 280]. Mult Scler J. 2017;23(3 Suppl):981–982.
- De Stefano N, Silva DG, Barnett MH. Effect of fingolimod on brain volume loss in patients with multiple sclerosis. CNS Drugs. 2017;31(4):289–305.
- Khan O, Bao F, Shah M, et al. Effect of disease-modifying therapies on brain volume in relapsing–remitting multiple sclerosis: results of a five-year brain MRI study. J Neurol Sci. 2012;312(1–2):7–12.
- Zivadinov R, Dwyer MG, Carl E, et al. Evaluating the effect of teriflunomide on whole brain atrophy in the phase 3 TOPIC study [abstract p870]. Presented at 34th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS); 2018 Oct 10–12; Berlin, Germany.
- Tullman MJ. A review of current and emerging therapeutic strategies in multiple sclerosis. Am J Manag Care. 2013;19(2 Suppl):S21–S27.
- Jonker MF, Donkers B, Goossens LMA, et al. Summarizing patient preferences for the competitive landscape of multiple sclerosis treatment options. Med Decis Making. 2020;40(2):198–211.
- Visser LA, Louapre C, Uyl-de Groot CA, et al. Patient needs and preferences in relapsing–remitting multiple sclerosis: a systematic review. Mult Scler Relat Disord. 2020;39:101929.
- Webb EJD, Meads D, Eskyte I, et al. A systematic review of discrete-choice experiments and conjoint analysis studies in people with multiple sclerosis. Patient. 2018;11(4):391–402.
- Mansfield C, Thomas N, Gebben D, et al. Preferences for multiple sclerosis treatments: using a discrete-choice experiment to examine differences across subgroups of US patients. Int J MS Care. 2017;19(4):172–183.
- Poulos C, Wakeford C, Kinter E, et al. Patient and physician preferences for multiple sclerosis treatments in Germany: a discrete-choice experiment study. Mult Scler J Exp Transl Clin. 2020;6(1):2055217320910778.
- Johnson FR, Lancsar E, Marshall D, et al. Constructing experimental designs for discrete-choice experiments: report of the ISPOR Conjoint Analysis Experimental Design Good Research Practices Task Force. Value Health. 2013;16(1):3–13.
- Tencer T, Will O, Nguyen J, et al. Neurologist and patient preferences in multiple sclerosis: UK and US qualitative research findings [abstract PND107]. Value Health. 2019;22(3):S757.
- Biogen. Avonex (interferon beta-1a) injection [package insert]. Cambridge (MA): Biogen; 2019.
- Genentech. Ocrevus (ocrelizumab) injection [package insert]. South San Francisco (CA): Genentech; 2017.
- Novartis. Efficacy and safety of fingolimod in patients with relapsing–remitting multiple sclerosis (FREEDOMS). NLM identifier: NCT00289978; 2012.
- Serono EMD. Rebif (interferon beta-1a) [package insert]. Rockland (MA): Serono EMD; 2019.
- Teva. Copaxone (glatiramer acetate injection) [package insert]. Parsippany (NJ): Teva; 2019.
- Vermersch P, Martinelli V, Pfleger C, et al. Benefit–risk assessment of cladribine using multi-criteria decision analysis (MCDA) for patients with relapsing–remitting multiple sclerosis. Clin Ther. 2019;41(2):249–260.e18.
- Schwartz CE, Coulthard-Morris L, Zeng Q, et al. Measuring self-efficacy in people with multiple sclerosis: a validation study. Arch Phys Med Rehabil. 1996;77(4):398–398.
- Chiu CY, Motl RW. Further validation of the Multiple Sclerosis Self-Efficacy Scale. Disabil Rehabil. 2015;37(26):2429–2438.
- Vickrey BG, Hays RD, Harooni R, et al. A health-related quality of life measure for multiple sclerosis. Qual Life Res. 1995;4(3):187–206.
- Silveira C, Guedes R, Maia D, et al. Neuropsychiatric symptoms of multiple sclerosis: state of the art. Psychiatry Investig. 2019;16(12):877–888.
- Azevedo CJ, Pelletier D. Whole-brain atrophy: ready for implementation into clinical decision-making in multiple sclerosis? Curr Opin Neurol. 2016;29(3):237–242.
- Nowinski CJ, Miller DM, Cella D. Evolution of patient-reported outcomes and their role in multiple sclerosis clinical trials. Neurotherapeutics. 2017;14(4):934–944.
- Moccia M, Valsecchi N, Ciccarelli O, et al. Spinal cord atrophy in a primary progressive multiple sclerosis trial: improved sample size using GBSI. Neuroimage Clin. 2020;28:102418.
- Kantar Health. Epi Database, United Kingdom 2020 [Internet]. New York: Kantar Health; 2010 [updated 2020 Jun 23; cited 2021 Jun 2]. Available from: www.epidb.com