Abstract
Objective
Efficacy and safety of an oral thyme/ivy syrup for the treatment of acute cough was previously demonstrated in a randomized clinical trial. Here, we present real-life data from a pharmacy-based, observational study on the effectiveness and tolerability of another thyme/ivy combination (BNO 1200, Bronchipret drops).
Methods
This observational, prospective, uncontrolled study was conducted in 305 German pharmacies. Cough and associated symptoms, cough severity and health related quality of life (HRQoL) were assessed at baseline, after 4 days and at individual end of treatment (EoT) in patients with acute cough due to upper respiratory tract infections who bought BNO 1200. Patients took BNO 1200 until resolution of cough and completed three validated questionnaires: patient-adapted Bronchitis Severity Score (BSS), cough severity visual analogue scale (VAS) and the Leicester Cough Questionnaire (LCQ-acute). They also rated speed of onset of treatment action on a VAS and tolerability.
Results
A total of 749 patients participated in the study; 730 were included in the analysis. Mean treatment duration was 7.0 ± 3.6 days. Symptom severity assessed by BSS improved from 8.7 ± 3.8 score points (baseline) to 2.4 ± 2.6 at EoT (p < .0001). Clinically relevant improvements (MCID = 17 mm) in cough severity were reported by 87.2% of patients at EoT. HRQoL improved significantly (12.2 ± 3.3 points at baseline vs. 18.5 ± 2.7 at EoT; p < .0001), exceeding the MCID (=2 points) in 90.0% of patients. Tolerability was rated “good” or “very good” by 98.0% of patients. No adverse drug reactions were reported.
Conclusion
Patients with acute cough taking BNO 1200 had a significant reduction in BSS, cough severity and improvement in HRQoL confirming RCT data with the syrup formulation. BNO 1200 was well tolerated.
Introduction
Acute cough is one of the most common symptoms of acute bronchitis and common coldCitation1. Although the majority of cases are self-limiting without any long-term sequelae, acute cough has a considerable impact on patients’ quality of life (QoL) and is often associated with absenteeism from work and loss of productivityCitation2,Citation3. Due to its predominantly viral nature, treatment of acute cough with antibiotics is not appropriateCitation4–6 and fosters the occurrence of side effects and increasing bacterial resistanceCitation7. Symptomatic treatment is indicated to relieve the considerable discomfort of the patientsCitation1,Citation4,Citation8. Over-the-counter (OTC) products from the pharmacy are widely used for treatment of acute cough, e.g. in GermanyCitation9, amongst them herbal remedies.
Efficacy and tolerability of both a thyme/ivy and a thyme/primrose combination (Bronchipret syrup and film-coated tablets, respectively) for treating cough in acute bronchitis have been shown in randomized, placebo-controlled clinical trials (RCTs)Citation4,Citation10. The combinations are approved medicinal products in several countries and are recommended by the German guidelines for the treatment of acute coughCitation1. In preclinical in vitro and in vivo studies anti-inflammatory characteristics and normalizing effects regarding mucus viscosity have also been shownCitation11–14. A systematic review and meta-analysis confirmed that ivy-, primrose- or thyme-based remedies reduce both frequency and severity of acute coughCitation15. Here we present real-world data for another thyme/ivy preparation (BNO 1200, Bronchipret dropsFootnotei, oral liquid) which is qualitatively comparable to Bronchipret syrup but rather used in adults due to a higher daily dose of fluid extracts. Most patients with acute cough do not seek a physician’s attention and instead seek advice and over the counter treatments from pharmacies. Hence, we investigated the effectiveness and tolerability of BNO 1200 in patients with acute cough due to upper respiratory tract infection in a large, real-world, pharmacy-based, observational study.
Methods
Study participants
Eligibility criteria were the purchase and current intake of the thyme/ivy combination BNO 1200 for acute cough due to upper respiratory tract infection, a minimum age of 18 years as well as the willingness and ability to credibly and timely complete the questionnaire. Non-eligibility criteria were any contraindications against the medication, i.e. known hypersensitivity to ivy, thyme or other labiates, birch, mugwort, celery, or any other ingredient.
BNO 1200
BNO 1200 (Bronchipret drops, oral liquid) are approved as medicinal product in Germany, where the study took place, for improving the symptoms of acute inflammatory bronchial diseases and acute inflammation of the respiratory tract with the accompanying symptom “cough with viscous mucus”. Patients were asked to take the medication orally 3 times per day in fixed doses of 2.6 ml each (≙ 1.387 g herbal fluid extracts), amounting to 7.8 ml (≙ 4.134 g herbal fluid extracts) per day, as approved and recommended in the package insert. BNO 1200 had to be self-administered outside of the pharmacy. The duration of treatment was at discretion until resolution of symptoms. There was no restriction to treatment duration or use of other OTC preparations.
Study design
The study was a prospective, open-label, uncontrolled, multicenter, pharmacy-based, observational study. A total of 305 pharmacies throughout Germany took part in the study, which started in October 2019 and ended in April 2020. Suitable participants were asked by the pharmacist after purchase of BNO 1200 whether they were willing to participate in an observational study and to complete a questionnaire. The questionnaire contained sections to be filled in before first intake of BNO 1200, after 4 days of use and at the individual end of the intake. Both consultation by pharmacists and purchase decision of customers were independent from the participation in the study. In case a participant took BNO 1200 for less than 4 days, the questionnaire prompted the participants to skip the Day 4 documentation and to fill in the end of treatment section immediately, in which, amongst others, the total duration of BNO 1200 intake needed to be specified.
Outcome measures
The patient questionnaire comprised general patient characteristics (age, gender, smoking status) and information on the treatment (reason for purchasing BNO 1200, duration of symptoms, therapeutic status, duration of intake). In addition, the questionnaire contained three different validated tools, namely the Bronchitis Severity Score (BSS), a visual analogue scale (VAS) for cough severity and the Leicester Cough Questionnaire for acute cough (LCQ-acute). All three tools had to be completed at three timepoints: before first intake of the study medication, after 4 days of use (if applicable, see above) and at the individual end of the treatment. Time to onset of action as well as the patients’ subjective assessment of effectiveness, tolerability and their satisfaction with the treatment were collected at the last timepoint.
Bronchitis Severity Score
The BSS is a validated, acute bronchitis-symptom specific questionnaireCitation16. Here, a patient-adapted version of the BSS was used to measure the severity of cough and associated symptomsCitation17. In total, five items were assessed: cough, sputum (sputum production/expectoration), rattles, chest pain during coughing and dyspnea. Each item was rated on a 5-point Likert scale from 0 (absent) to 4 (very severe) contributing to the overall score (sum of all items) ranging from 0 to 20 with 20 describing the most severe cough. The response to the medication was calculated as the absolute and relative change from baseline in the BSS. In addition, a responder analysis was carried out, defined as number of patients with a change of ≥50% from baseline.
Cough severity visual analogue scale
Cough severity was assessed on a VAS from 0 mm (no cough) to 100 mm (strongest cough imaginable). A change of ≥17 mm in VAS was defined as clinically relevant. This threshold was based on previous findings of a minimal clinically important difference (MCID) of 17 mm VASCitation2. In addition, a responder analysis was performed.
Leicester Cough Questionnaire
The cough-specific quality of life was assessed with the validated LCQ-acuteCitation18,Citation19 that evaluates the health status over a 24 h period. The questionnaire contains 19 items in three domains: physical, psychological and social. Each item is ranked on a 7-point Likert scale from 1 to 7 (higher values representing a better quality of life). Mean scores are subsequently calculated for each domain, and their sum is the overall score ranging from 3 to 21. With an MCID of 2 in the questionnaire for acute coughCitation2, the LCQ response was defined as an improvement ≥2 points. In addition, the LCQ items “sleep disturbance due to coughing” and “frequency of coughing bouts per day” were analyzed separately.
Onset of action
Patients reported the subjective time to onset of action using a 100 mm VAS ranging from 0 (very slow) to 100 (very fast).
Tolerability
Tolerability was rated by patients as “very good”, “good”, “moderate”, “poor” or “insufficient”.
Overall effectiveness and satisfaction
Furthermore, patients were asked to provide an assessment of treatment effectiveness and their overall satisfaction on a scale “very good”, “good”, “moderate”, “poor” or “insufficient”. For effectiveness rating, verbal category descriptives were provided in addition (“very good” = complete recovery or marked improvement of symptoms, “good” = improvement of symptoms, “moderate” = symptoms unchanged, “poor” = symptoms deteriorated, “insufficient” = symptoms markedly deteriorated).
Ethics and data protection
The study was reported to the German Federal Institute for Drugs and Medical Devices (BfArM) before commencement according to the national regulatory requirements for observational studies/non-interventional studies (NIS). This was a non-interventional study as patients had already purchased BNO 1200 for their cough and used it according to the package insert. Thus, the study was exempted from ethics committee review and informed consent. Participants returned the completed questionnaire anonymously and free of charge to the Clinical Research Organization involved using the provided return envelopes. The survey was conducted entirely anonymously and no patient identifying data (e.g. name, initials or date of birth) was collected, excluding the possibility for any follow-ups. Pharmacists involved in the study could not access any of the completed questionnaires.
Data analysis and statistics
Predefined subgroups
To account for the dynamic nature of cough, subgroup analyses were performed based on the duration of symptoms at baseline (≤2 days, 3–6 days, 7–14 days, >14 days). Additional subgroup analyses were performed according to the total BSS score at baseline (<8 vs. ≥8), age of patients (18–64 vs. ≥65), gender (female, male) and smoking status (regular smoker, occasional smoker, former smoker, non-smoker).
Data set
All information collected from the returned questionnaires was entered into an SAS database. During data collection, all responses for items evaluated on a VAS were converted to numerical data and entered into the database.
Data analysis
Statistical analyses of treatment effects on cough symptoms and quality of life in a real-life setting were purely descriptive, as being standard for observational studies, using frequency tables for categorical data with usually adjusted relative frequencies. Numerical data were presented as mean together with the standard deviation, median, first and third quartile, as well as minimum and maximum. Wilcoxon signed-rank tests were performed with a two-sided alpha of .05 to assess significant differences between measurements.
Handling of missing data
Missing values were generally not replaced due to the descriptive character of the observational study and analyses were performed accordingly. Missing values could for example be the case for Day 4 assessments when participants stopped taking BNO 1200 before Day 4 and filled in the EoT assessment immediately, as instructed.
Relative changes in the BSS were considered missing if either the baseline or the post-treatment score were not available or if the baseline score was 0. Relevant changes in cough severity as measured by VAS were omitted if changes in cough severity could not be given due to missing values. For the LCQ-acute, the respective domain was set as missing if more than two items were lacking for the physical domain or more than one item was lacking for the psychological or social domain. The overall score was set as missing as soon as one domain score was missing in accordance with the instructions from the questionnaire developers.
Results
Participant demographics and baseline characteristics
In total, 749 patients returned questionnaires, which was about a third of total questionnaires distributed to the pharmacies (N = 2.600). The exact number of questionnaires distributed by pharmacies to patients is not known. Nineteen patient questionnaires were excluded from the analysis because participants either failed to disclose their age (N = 7) or were under the age of 18 (N = 12). The mean age of the remaining 730 participants was 45.1 ± 15.0 years with the vast majority being between 18 and 64 years of age (N = 654, 89.6%). Two thirds of patients were non-smokers (N = 483, 66.6%) and there were more females (67.2%) than males (32.8%). Of the 730 patients analyzed, the majority reported acute cough symptoms at baseline for either 3–6 days (N = 299, 41.1%) or 1–2 days (N = 268, 36.8%). A total of 110 patients (15.1%) had had cough symptoms for 1–2 weeks and only a few patients had had cough for more than 2 weeks before their first intake of medication (N = 51, 7.0%).
Mean baseline BSS, cough severity and LCQ-acute are shown in . Participants suffered from severe cough and impaired HRQoL at baseline. The strongest BSS symptoms were cough and sputum. Smokers reported more expectoration and stronger chest pain during coughing compared to non-smokers. Also, men scored higher on the BSS than women, who were non-smokers more frequently. Cough severity VAS at baseline was rated higher in men, regular or occasional smokers and patients with a BSS ≥8.
Table 1. Participant demographics and baseline characteristics.
Sleep disturbances most of the time due to cough within 24 h before the first intake of the medication were reported by 56.7% of patients. Similarly, 69.1% experienced coughing bouts at least several times during the day at baseline.
Patients suffering from acute cough for longer periods (1–2 weeks or longer) generally fared worse in total BSS, cough severity VAS and LCQ at baseline.
Mean treatment duration
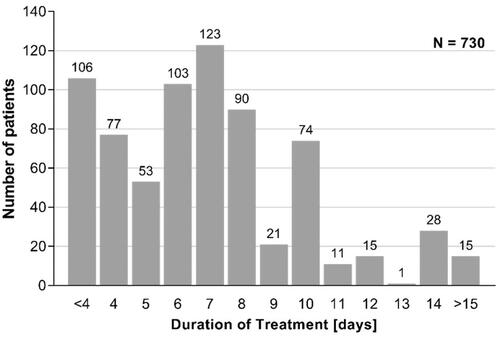
Mean treatment duration was 7 ± 3.6 days (median: 7.0 days) with a minimum of 2 days and a maximum of 45 days. For patients with symptom duration of 1 to 2 weeks prior to treatment start, with a baseline BSS ≥8 and women, mean duration of treatment was slightly increased. Frequency distribution of treatment duration is presented in .
Effectiveness
Bronchitis Severity Score
After 4 days of treatment, a significant reduction of symptom severity on the BSS from 8.7 ± 3.8 to 5.8 ± 3.3 was observed for patients who took BNO 1200 for ≥4 days and who provided data. This is a difference of 2.9 ± 3.3 points (p < .0001), equaling a relative change of 31.0%. At the end of treatment, the mean overall BSS was 2.4 ± 2.6, a significant reduction of 6.3 points compared to baseline and equivalent to a relative improvement of 70.0% (p < .0001) ( and ). Patients with longer duration of symptoms before treatment or more severe symptoms (BSS ≥8) showed larger absolute and relative changes in BSS during and after treatment. The proportion of responders, predefined by a relative change in BSS ≥50%, was 36.7% of patients at Day 4 and 85.7% at the end of treatment.
Figure 2. BSS at baseline and during treatment (mean ± SD). *Wilcoxon p < .0001, two-sided alpha = .05. The number in the bars denotes the respective mean value. Lower N number at Day 4 is due to patients with treatment duration <4 days (N = 106) or due to data not provided.
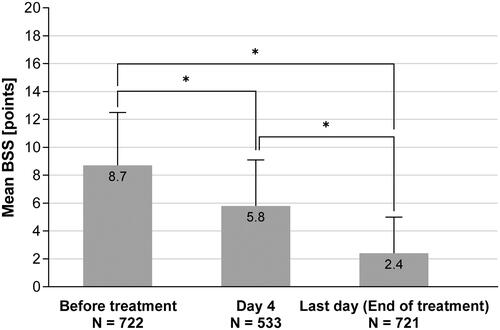
Table 2. Key effectiveness assessments.
At baseline, cough and sputum were the strongest symptoms. By the end of the treatment, cough and sputum had diminished to mild symptoms on average, while the other observed symptoms (rattles, chest pain during coughing and dyspnea) were rated with BSS values just above 0, indicating hardly any remaining symptoms.
Cough severity
Cough severity was measured on a 100 mm VAS and decreased from 58.2 ± 20.4 mm at baseline to 36.0 ± 18.9 mm (p < .0001) at Day 4 for patients who took BNO 1200 for ≥4 days and who provided data, and to 14.9 ± 17.0 mm (p < .0001) at the individual end of treatment ( and ).
Figure 3. Course of cough severity measured on 100 mm VAS (Mean ± SD). *Wilcoxon p < .0001, two-sided alpha = .05. The number in the bars denotes the respective mean value. Lower N number at Day 4 is due to patients with treatment duration <4 days (N = 106) or due to data not provided.
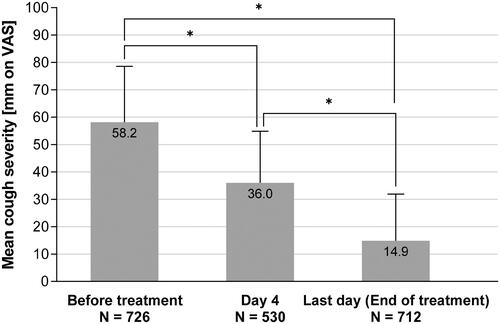
Clinically relevant improvements, defined by changes of at least 17 mm or more on the VAS, were reported by 87.2% of patients at the end of treatment. The proportion of patients achieving a clinically relevant improvement at the end of treatment was similar for patients with BSS <8 and ≥8 (81.5% vs. 90.9%), while at Day 4 more patients with stronger symptoms reported clinically relevant improvements (74.5% vs. 57.1%).
Leicester Cough Questionnaire
Improvements in quality of life were observed for the overall score of the LCQ-acute questionnaire as well as for each individual domain. On average, patients scored 12.2 ± 3.3 points on a scale from 3 (lowest QoL) to 21 (highest QoL) at baseline and improved to 18.5 ± 2.7 points (p < .0001) by the end of the treatment, indicating only very little or no remaining impact of the symptoms on the patients’ quality of life ( and ).
Figure 4. Improvement of quality of life during treatment according to LCQ (mean ± SD). *Wilcoxon p < .0001, two-sided alpha = .05. The number in the bars denotes the respective mean value. Lower N number at Day 4 is due to patients with treatment duration <4 days (N = 106) or due to data not provided.
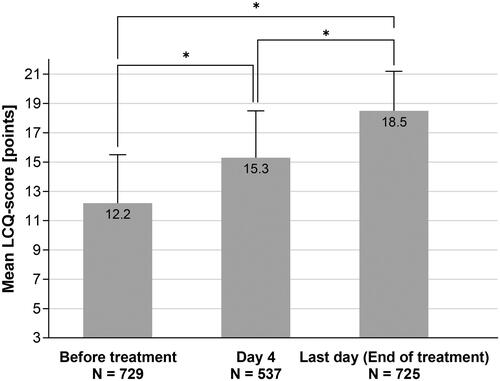
The minimal clinically important difference of at least 2 points in the LCQ questionnaire was achieved by 90.9% of patients at the end of treatment. This LCQ response rate was higher than average (93.9%) in patients with a BSS ≥8 at baseline.
By the end of treatment, the majority of patients were no longer affected by cough at night (LCQ question number 10): 79.4% reported that their sleep was disturbed either “hardly any of the time” or “none of the time”. Similarly, the frequency of coughing bouts during the day (LCQ question number 11) also clearly decreased until end of treatment. The proportion of patients with coughing bouts “all of the time”, “most of the time” or “several times during the day” was 69.1% at baseline, and 4.6% at the end of treatment. Correspondingly, the proportion of patients without any or only very few coughing bouts during daytime at the end of treatment was high with 69.7%.
Onset of action
The average score for the onset of treatment effects on VAS was 65.2 ± 23.5 mm, with 100 mm indicating the fastest possible onset. Patients with the shortest duration of symptoms (1–2 days) reported the fastest onset of action with 68.9 ± 23.9 mm, whereas patients with a longer duration of symptoms (1–2 weeks) reported the slowest onset of action with 61.4 ± 22.1 mm among all patients.
Overall effectiveness and satisfaction
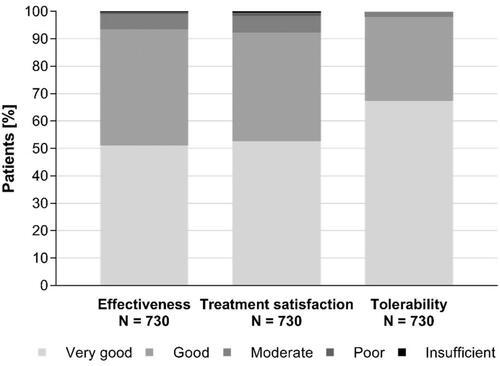
Overall effectiveness was assessed by 93.5% of patients as “good” or “very good” with more than half of all patients (51.1%) reporting a “very good” effectiveness, equivalent to a complete recovery or at least markedly improved symptoms. A “good” effectiveness, equivalent to improved symptoms, was reported by 42.4% of patients. This positive perception of the effectiveness was also reflected by the overall satisfaction with the treatment which was rated as “very good” or “good” by 92.3% of patients ().
Tolerability
Patients could report any adverse drug reactions (ADRs) to the pharmacists throughout the study. No ADRs were reported. Furthermore, tolerability was rated as “good” or “very good” by the vast majority of patients (98.0%) and no ratings for “poor” or “insufficient” tolerability were recorded ().
Discussion
The treatment with BNO 1200 was generally well received by the 730 evaluable out of 749 study participants with acute cough: both effectiveness and tolerability were rated positively. Patients reported a clear reduction of cough and associated symptoms already after 4 days of treatment (if patients took BNO 1200 for ≥4 days and provided data) and further improvements by the end of the treatment. In addition, the patients’ quality of life improved during treatment with fewer cough-related disturbances during night and day. No ADRs were reported and patient ratings showed a high satisfaction with the treatment.
Cough and sputum are considered to be the major symptoms of acute bronchitisCitation16. For these, a clear improvement on the BSS was observed in this study. Patients whose symptoms had been present for only a few days before starting the treatment had lower BSS – indicating less severe symptoms – and also showed a better quality of life at baseline in comparison to patients with a persistence of symptoms for longer periods (1–2 weeks or longer) before beginning the treatment. Patients with shorter durations of symptoms reported a faster onset of positive treatment effects than patients that had waited longer before starting the treatment.
This study reflects a real-life setting where cold medications such as the investigated BNO 1200 are obtained as OTC products in pharmacies. As the study design aimed at generating real-world data all results are based on patient-reported outcomes without a control group.
Previously, however, a similar thyme/ivy combination (Bronchipret syrup) was investigated in a placebo-controlled study in patients with acute bronchitis/cough in a clinical trial settingCitation4. The placebo-controlled study reported a statistically significant change from baseline in the BSS of −3.1 score points for the thyme/ivy combination on Day 4 vs. −2.3 score points for placebo (p < .0001) (baseline: 8.2 score points for verum vs. 8.3 for placebo; Day 4: 5.1 score points for verum vs. 6.0 for placebo). On Day 10, at the predefined end of this clinical trial, the change from baseline in the BSS was −6.6 score points for the thyme/ivy combination vs. −5.0 score points for placebo (p < .0001) (day 10: 1.61 score points for verum vs. 3.3 for placebo). Similarly, in the observational study reported here, the change from baseline in the BSS was −2.9 score points for BNO 1200 on Day 4 (if applicable) and −6.3 score points on the last day of treatment, which was on average on Day 7. Moreover, the RCT showed a significantly greater reduction of coughing bouts after 7–9 days of treatment for the syrup (68.7% of patients) compared to placebo (47.6%; p < .0001). We also saw a pronounced reduction in coughing bouts, which were here only assessed qualitatively, i.e. at the end of the treatment. Only 4.6% of patients complained of persisting coughing bouts. The observational non-controlled pharmacy-based study presented here shows similar results in a real-life setting.
The baseline BSS and the course of the BSS are in the same range as those in different placebo-controlled studies with herbal remedies in acute bronchitis. Taken together, even when considering the different study designs, inclusion/eligibility/exclusion/non-eligibility criteria, patient populations, study schedules and assessments, but the same outcome (BSS on Day 4), BNO 1200 seems to show similar positive effects in this pharmacy-based study as observed in controlled RCTsCitation4,Citation10,Citation20–22. This also confirms and underlines the validity of the BSS in different study settings. Moreover, LCQ and VAS show superiority compared with the natural history of acute bronchitisCitation2.
The study presented here has some limitations that are directly related to its nature of a pharmacy-based, non-controlled, observational questionnaire study: First of all is the lack of a placebo control. In the self-limiting condition of acute cough a large placebo effect has been describedCitation23. It is possible that a placebo effect of similar magnitude as observed in previous trials may have occurred in the current study. However, the purpose of the current study was not an RCT, it was a real-world observational study. Notwithstanding, as a similar thyme/ivy combination had shown efficacy over placebo in a placebo-controlled clinical trial with similar effect magnitudes as observed in this studyCitation4, it can be concluded that the observed effect reflects not only a mere placebo effect.
In this study, the therapeutic results were evaluated only subjectively. Indeed, the aim of this study was to capture the patient’s perspective when using an OTC product by means of patient reported outcome (PRO) measures. Mainly well recognized and validated tools have been used for this purpose such as the BSS, cough severity VAS and the LCQ. As mentioned above, similar effect magnitudes were observed in an RCT in which the assessment of the BSS was done by physicians.
The tool used for assessing onset of action (VAS) has not been validated yet. It was used to get an impression of how fast effects of the medication on symptoms are perceived by the patients by means of a simple, easily understandable assessment in analogy to the cough severity VAS. It needs to be validated in future studies since in a self-limiting disease the onset of action is a valuable outcome for effectiveness and efficacy.
Only a third of questionnaires distributed to the pharmacies were returned to the clinical research organization (CRO), which might at first sight seem to be a poor response rate. However, this number needs to be regarded as an indirect response rate. The true response rate is not known as it is unknown how many questionnaires were indeed distributed to BNO 1200 customers. In fact, the response rate in this study is not unexpectedly low and confirms findings from other questionnaire-based studiesCitation24,Citation25.
Concerning the timepoints at which the questionnaire was to be completed, it needs to be pointed out that fewer patients filled in the questionnaire on Day 4 than at baseline and EoT. This is due to the fact that, by design, patients could report EoT earlier than on Day 4 due to the different disease course in different patients. For this case the questionnaire was designed to prompt the participants directly to the EoT documentation, which is common practice even in RCTs. A total of 106 patients reported EoT earlier than Day 4 (). In addition, approximately 80 participants did not provide data for Day 4 or single Day 4 assessments when taking BNO 1200 for ≥4 days. This reflects real life, which was the focus of this study, and can’t be compared to the artificial setting of clinical trials in which missing values can be easily queried. Missing values were not replaced in contrast to confirmatory clinical trials due to the purely descriptive analysis of the observational study.
In summary, the current study adds evidence obtained from a broad spectrum of patients in a large pharmacy-based real-world setting for effectiveness and a favorable benefit–risk ratio of the thyme/ivy combination BNO 1200.
Although common cold may resolve without treatment, the fast onset of action leading to faster relieve improves the patients’ well-being and may shorten the duration of the disease. Here, we demonstrate clear improvements of cough and associated symptoms, cough severity and quality of life after 4 days of treatment with BNO 1200. The benefits of starting treatment early in the course of the disease were illustrated by the faster onset of action in patients that only had symptoms for 1–2 days.
The positive effects described can be attributed to the pharmacological actions of the key components, thyme and ivy. While thyme is an antibacterial secretolytic, ivy is an antispasmodic expectorant. Both agents have anti-inflammatory and mucus-normalizing properties that may contribute to the effectiveness of the medication shown hereCitation12–14.
Conclusion
This large non-interventional, pharmacy-based, real-life study provides evidence for the effectiveness and tolerability of the thyme/ivy combination BNO 1200 in acute cough, supporting efficacy and a highly favorable risk–benefit ratio shown earlier in RCTs with similar herbal drug combinations. Starting the treatment early in the course of the disease is associated with faster symptom relief.
Transparency
Declaration of funding
This study and the open access of this paper were sponsored by Bionorica SE, Neumarkt, Germany.
Author contributions: All authors contributed to the conception of the study, analyses performed in this manuscript and interpretation of the data, provided critical review and revisions of the manuscript for intellectual content, and provided final approval.
Declaration of financial/other relationships
P.K. and S.S.B. have disclosed that they have received honoraria from Bionorica SE, Neumarkt, Germany for scientific services. C.B.B., J.S. and D.A.S. have disclosed that they are employees of Bionorica SE, Neumarkt, Germany. CMRO peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Trial number: NIS-No. 7348 in the database for observational studies/non-interventional studies (NIS) of the German Federal Institute for Drugs and Medical Devices (BfArM).
Acknowledgements
Winicker Norimed GmbH, Nuremberg, Germany is acknowledged for CRO services. Dr. Moritz Klinghardt and Dr. Alexander Boreham (co.medical, Berlin, Germany) are acknowledged for medical writing supported by Bionorica SE.
Notes
i Bionorica SE, Neumarkt i.d.OPf., Germany.
References
- Kardos P, Dinh QT, Fuchs K-H, et al. Guidelines of the German respiratory society for diagnosis and treatment of adults suffering from acute, subacute and chronic cough. Pneumologie. 2019;73(3):143–180.
- Lee KK, Matos S, Evans DH, et al. A longitudinal assessment of acute cough. Am J Respir Crit Care Med. 2013;187(9):991–997.
- Thielmann A, Gerasimovska-Kitanovska B, Koskela TH, et al. Self-care for common colds: a European multicenter survey on the role of subjective discomfort and knowledge about the self-limited course – the COCO study. PLoS One. 2018;13(4):e0195564.
- Kemmerich B, Eberhardt R, Stammer H. Efficacy and tolerability of a fluid extract combination of thyme herb and ivy leaves and matched placebo in adults suffering from acute bronchitis with productive cough. A prospective, double-blind, placebo-controlled clinical trial. Arzneimittelforschung. 2006;56(9):652–660.
- Albert RH. Diagnosis and treatment of acute bronchitis. Am Fam Physician. 2010;82(11):1345–1350.
- Smith MP, Lown M, Singh S, et al. Acute cough due to acute bronchitis in immunocompetent adult outpatients: CHEST Expert Panel Report. Chest. 2020;157(5):1256–1265.
- Martin D, Konrad M, Adarkwah CC, et al. Reduced antibiotic use after initial treatment of acute respiratory infections with phytopharmaceuticals – a retrospective cohort study. Postgrad Med. 2020;132(5):412–418.
- Tackett KL, Atkins A. Evidence-based acute bronchitis therapy. J Pharm Pract. 2012;25(6):586–590.
- Healthcare Marketing. Selbstmedikation mit OTC-Produkten beliebter als Arztbesuch [Self-medication with OTC products more popular than doctor’s visit] [Internet]. Healthcare Marketing; 2015 [cited 2020 Nov 10]. Available from: http://www.healthcaremarketing.eu/unternehmen/detail.php?nr=33531
- Kemmerich B. Evaluation of efficacy and tolerability of a fixed combination of dry extracts of thyme herb and primrose root in adults suffering from acute bronchitis with productive cough: a prospective, double-blind, placebo-controlled multicentre clinical trial. Arzneimittelforschung. 2007;57(9):607–615.
- Kardos P. Phytotherapy in acute bronchitis: what is the evidence? Clin Phytosci. 2015;1(1):2.
- Seibel J, Kryshen K, Pongrácz JE, et al. In vivo and in vitro investigation of anti-inflammatory and mucus-regulatory activities of a fixed combination of thyme and primula extracts. Pulm Pharmacol Ther. 2018;51:10–17.
- Seibel J, Pergola C, Werz O, et al. Bronchipret syrup containing thyme and ivy extracts suppresses bronchoalveolar inflammation and goblet cell hyperplasia in experimental bronchoalveolitis. Phytomedicine. 2015;22(13):1172–1177.
- Seibel J, Wonnemann M, Werz O, et al. A tiered approach to investigate the mechanism of anti-inflammatory activity of an herbal medicinal product containing a fixed combination of thyme herb and primula root extracts. Clin Phytosci. 2018;4(1):1–9.
- Wagner L, Cramer H, Klose P, et al. Herbal medicine for cough: a systematic review and meta-analysis. Complement Med Res. 2015;22(6):359–368.
- Kardos P, Lehrl S, Kamin W, et al. Assessment of the effect of pharmacotherapy in common cold/acute bronchitis – the bronchitis severity scale (BSS). Pneumologie. 2014;68(8):542–546.
- Kardos P, Beeh KM, Sent U, et al. Characterization of differential patient profiles and therapeutic responses of pharmacy customers for four ambroxol formulations. BMC Pharmacol Toxicol. 2018;19(1):40.
- Birring SS, Prudon B, Carr AJ, et al. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax. 2003;58(4):339–343.
- Yousaf N, Lee KK, Jayaraman B, et al. The assessment of quality of life in acute cough with the Leicester Cough Questionnaire (LCQ-acute). Cough. 2011;7(1):4.
- Gruenwald J, Graubaum H-J, Busch R. Efficacy and tolerability of a fixed combination of thyme and primrose root in patients with acute bronchitis. Arzneimittelforschung. 2005;55(11):669–676.
- Gillissen A, Wittig T, Ehmen M, et al. A multi-centre, randomised, double-blind, placebo-controlled clinical trial on the efficacy and tolerability of GeloMyrtol forte in acute bronchitis. Drug Res. 2013;63(01):19–27.
- Matthys H, Eisebitt R, Seith B, et al. Efficacy and safety of an extract of Pelargonium sidoides (EPs 7630) in adults with acute bronchitis: a randomised, double-blind, placebo-controlled trial. Phytomedicine. 2003;10:7–17.
- Eccles R. The powerful placebo effect in cough: relevance to treatment and clinical trials. Lung. 2020;198(1):13–21.
- Hindi A, Parkhurst C, Rashidi Y, et al. Development and utilization of the medicines use review patient satisfaction questionnaire. Patient Prefer Adherence. 2017;11:1797–1806.
- Otter SJ, Kumar S, Gow P, et al. Patterns of foot complaints in systemic lupus erythematosus: a cross sectional survey. J Foot Ankle Res. 2016;9:10.