Abstract
Objective
Head-to-head trials comparing siponimod with fingolimod or ofatumumab in patients with multiple sclerosis (MS) are lacking. Instead, the comparative efficacy of siponimod can be derived from indirect treatment comparisons (ITCs). We assessed the suitability of ITCs leveraging individual patient data from relevant phase III trials across different MS phenotypes.
Methods
One siponimod trial in patients with secondary progressive MS (SPMS), four fingolimod trials (three in relapsing-remitting MS [RRMS], and one in primary progressive MS [PPMS]), and two ofatumumab trials in relapsing MS (RMS) were considered. The suitability of ITCs was evaluated based on trial design, patient eligibility criteria, baseline patient characteristics, placebo response, and outcome definitions for each trial. Analyses deemed feasible were conducted using one-to-one propensity score matching (PSM).
Results
An ITC between siponimod in SPMS and either fingolimod in RRMS or ofatumumab in RMS was not feasible because of insufficient overlap in key patient characteristics (e.g. disability level and relapse history) and differences in placebo response. However, a comparison between siponimod in SPMS and fingolimod in PPMS was feasible because of sufficient overlap in eligibility criteria and baseline characteristics. One-to-one PSM demonstrated siponimod was favored relative to fingolimod for time to 6- and 3-month confirmed disability progression though not significantly different (hazard ratio 0.76 [95% confidence interval 0.48–1.20; p-value = .240] and hazard ratio 0.80 [95% confidence interval 0.52–1.22; p-value = .300], respectively).
Conclusions
For trials in MS, clinical phenotype is an important determinant of ITC feasibility. An ITC between siponimod in SPMS and either fingolimod in RRMS or ofatumumab in RMS was not feasible. The only feasible comparison was between siponimod in SPMS and fingolimod in PPMS.
Introduction
Multiple sclerosis (MS) is the leading cause of disability in young and middle-aged people and affects ∼2.8 million individuals worldwideCitation1. Several clinical phenotypes have been identified for MSCitation2. Relapsing-remitting MS (RRMS) is the most common MS phenotype, affecting 70% to 80% of MS patients, and is characterized by the occurrence, recurrence, or worsening of symptoms of neurologic dysfunction (relapses) followed by complete or partial remissionCitation3. Secondary progressive MS (SPMS) is characterized by a gradual neurological deterioration independent of relapses after an initial relapsing-remitting course with or without superimposed relapsesCitation3. The term relapsing MS (RMS) describes both RRMS and SPMS with superimposed relapses (i.e. relapsing forms of MS). Primary progressive MS (PPMS) affects 15–20% of MS patients and presents as a gradual deterioration from disease onset with few or no relapsesCitation3.
Disease-modifying therapies (DMTs) are the standard of care for people with MS. They reduce both relapse frequency and the underlying acute focal inflammation in the CNS visible on magnetic resonance imaging (MRI) scans, albeit with varying degrees of efficacyCitation4. They also reduce the risk of disability progression with limited efficacyCitation4. Beta interferons (IFN beta) and glatiramer acetate (GA) collectively comprise the BRACE therapies (BetaseronFootnotei, RebifFootnoteii, AvonexFootnoteiii, CopaxoneFootnoteiv, ExtaviaFootnotev), which were the first DMTs approved, and are still widely used. Although they have relatively benign safety profiles, the injectable BRACE therapies are less effective compared to more modern oral or injectable DMTsCitation5,Citation6.
Siponimod is an oral sphingosine 1-phosphate receptor modulator studied in SPMS and recently approved with diverse labels, e.g. in the United States (US) (patients with RMS)Citation7, the European Union (EU), and Canada (adult patients with SPMS with active disease)Citation8,Citation9, and Australia (adult patients with SPMS)Citation10. Approval of siponimod was based on a phase III, double-blind, randomized EXPAND trial that demonstrated oral siponimod significantly reduced the risk of disease progression in a broad SPMS population that included patients with a high level of disabilityCitation11.
Fingolimod was the first oral sphingosine 1-phosphate modulator approved for MS. Fingolimod is approved for adult and pediatric patients with RMS in the US, Canada (RRMS in adults), and AustraliaCitation12–14 and highly active RRMS in the EUCitation15. Fingolimod has been evaluated in patients with PPMSCitation16 and RRMSCitation5,Citation17,Citation18 but not in SPMS patientsCitation18. In the phase III trial in patients with PPMS, disability progression and brain volume change were not different between the fingolimod and placebo groups, although fingolimod was found to improve MRI outcomes relative to placeboCitation16.
Ofatumumab is a subcutaneous anti-CD20 monoclonal antibody treatment recently approved for adult patients with RMS in the US and AustraliaCitation19,Citation20, RMS with active disease in the EUCitation21, and RRMS with active disease in CanadaCitation22. In two phase III trials, ofatumumab was more effective in reducing focal inflammation (relapses and CNS inflammation as measured by MRI) and delayed disability worsening compared to teriflunomideCitation24.
There are no head-to-head randomized clinical trials (RCTs) directly comparing siponimod to either fingolimod or ofatumumab, so their relative efficacy is unknown. In the absence of direct evidence, indirect treatment comparisons (ITCs) can be used to explore their relative effect if it is feasible to perform valid ITCs based on the available clinical trial evidence, which needs to be assessed. Given their similar listing status in the US, there may be a tendency for researchers to want to include the three therapies in an ITC, such as a network meta-analysis (NMA), which simultaneously compares the relative efficacy of therapies in the absence of direct evidence. However, a recent review of NMAs in MS discussed the importance of the population being similar in terms of MS phenotype when undertaking such analysesCitation23. Indirectly comparing trials in different MS phenotypes may produce invalid results if there are substantial phenotype-driven differences in patient populations (e.g. age and stage of disease). The pivotal trial for siponimod was conducted in patients with a different MS phenotype compared to trials for fingolimod and ofatumumab, so systematically evaluating cross-trial differences to assess the validity of undertaking ITCs is particularly important to avoid misleading comparisons of treatment effects. We undertook this present work to establish whether there is merit in evaluating siponimod in an MS phenotype for which it had not been directly evaluated, thereby aiming to provide more clarity on caveats regarding the comparability of different MS phenotypes and the clinical implications for healthcare providers and patients.
Propensity score matching (PSM) is a common approach for comparing the efficacy of two or more treatments using observational dataCitation24–27. More recently, PSM has also been used to derive ITCs using individual patient data (IPD) from multiple RCTs evaluating therapies with similar therapeutic indicationsCitation25,Citation26. Propensity score methods leverage IPD for both patient populations being compared to correct for differences between populations that are expected to bias comparisons. We considered PSM and other propensity score-based ITC approaches using IPD from pivotal trials evaluating fingolimod, ofatumumab, and siponimod. We were in a unique position with this study because IPD was available for trials of each therapy. Consequently, we could adjust for cross-trial differences to allow for more valid indirect estimates of efficacy.
This study aimed to evaluate the suitability of performing PSM-based ITC analyses involving siponimod and trials in different MS phenotypes by systematically evaluating cross-trial heterogeneity between the EXPAND trial and the phase III trials evaluating fingolimod and ofatumumab. Where feasible, we conducted PSM analyses to evaluate the comparative efficacy of siponimod vs. fingolimod or ofatumumab for the treatment of MS using IPD from the respective pivotal trials for these therapies.
Methods
Data sources
The three pre-specified therapies of interest were siponimod, fingolimod, and ofatumumab. The double-blind, phase III, randomized EXPAND trial compared siponimod to placebo in patients with SPMSCitation11. Four double-blind, phase III, randomized trials compared fingolimod to placebo (FREEDOMSCitation18 and FREEDOMS IICitation17 in RRMS) or intramuscular IFN beta-1a (TRANSFORMSCitation5 in RRMS, while INFORMS evaluated patients with PPMSCitation16). The double-blind, phase III, randomized ASCLEPIOS I/II trials were identically designed trials that compared ofatumumab to teriflunomide in patients with RMSCitation28. The design characteristics of the trials are summarized in .
Table 1. Design of trials included in this study.
Ethics
Novartis Pharma AG was the sponsor of all trials of all three pre-specified therapies of interest used in this study. Concept and design for PSM analyses underwent Novartis Pharma AG approval and EVERSANAFootnotevi was provided access to anonymized data from all trials of interest as described previously, to run the analyses after approval. All trials were registered with the U.S. National Library of Medicine ClinicalTrials.gov. Trial protocols were institutional review board- or independent ethics committee-approved. Trials were conducted by the International Conference on Harmonization Guidelines for Good Clinical Practice and the principles of the Declaration of Helsinki.
Feasibility assessment
Systematic differences between trials may compromise the validity of ITCs, therefore the degree of heterogeneity in patient characteristics and trial design between trials was assessed. A qualitative assessment of between-trial heterogeneity based on the trial design, patient eligibility criteria, baseline patient characteristics, placebo arm outcome values (i.e. placebo response), and outcome definitions was performed to ensure similarity. The exchangeability assumption underlying each potential ITC was assessed by comparing outcomes for placebo arms between clinical trials, where applicable.
The following study characteristics were extracted for each trial if reported: general publication details (e.g. author, study location, year, journal, and NCT number), trial design (e.g. phase, blinding, allocation, and trial duration), patient eligibility criteria, baseline patient characteristics (e.g. age, sex, Expanded Disability Status Scale [EDSS] score, and duration of disease), intervention details (e.g. therapy, dose, route, and frequency), and trial-specific outcome definitions for the available outcomes of interest. We used IPD for each trial to evaluate standardized mean differences (SMDs) and the overlap of key baseline patient characteristics between trials.
Where feasible, ITCs were conducted using PSM to evaluate the comparative efficacy of siponimod vs. fingolimod or ofatumumab for the treatment of MS using IPD from pivotal trials for these therapies. Methodological details for the PSM analyses are provided in Supplemental Appendix A.
Outcomes of interest
Outcomes of interest (selected a priori) were time to 6-month confirmed disability progression (6mCDP), time to 3-month CDP (3mCDP), and annualized relapse rate (ARR), which were primary or secondary endpoints evaluated in the EXPAND, FREEDOMS, FREEDOMS II, INFORMS (CDP outcomes only), TRANSFORMS, and ASCLEPIOS I/II trials. These endpoints are clinically important and commonly assessed in MS trials. Notably, ARR was not reported in INFORMS, which was conducted in a PPMS population with no or very few relapses, and time to 6mCDP was not reported in TRANSFORMS, which was a one year study and so not suited to measuring this outcome.
Results
Feasibility assessment
Trial design
All included trials were double-blind, parallel-group, phase III RCTs (). Trials had either a placebo, IFN beta, or teriflunomide as comparators, as described previously. Trial durations ranged from one year (TRANSFORMS) up to five years (INFORMS). Trial populations included RRMS (fingolimod), RMS (ofatumumab), SPMS (siponimod), and PPMS (fingolimod). In the two ofatumumab trials, most patients had RRMS (∼94%), and the remainder presented with active SPMS (∼6%).
Patient eligibility criteria
The results of a qualitative comparison of patient eligibility criteria for the included trials are summarized in . All trials included adult patients; however, the trials differed in inclusion/exclusion criteria regarding MS phenotype evaluated, baseline EDSS score, history of relapse or RRMS, and history of disease progression. All trials in relapsing MS enrolled patients with an EDSS score between 0.0 and 5.5, but the two trials in progressive MS required patients to have an EDSS score between 3.0 and 6.5 (EXPAND) or between 3.5 and 6.0 (INFORMS). Notably, the majority (56%) of the patients in EXPAND (in SPMS) had a baseline EDSS of ≥6.0, and so would have been excluded from the RRMS/RMS trials. Although EXPAND and all the included RRMS/RMS trials required history or a current diagnosis of RRMS, INFORMS excluded patients with a previous history of relapse and RRMS. Finally, EXPAND and INFORMS required documented progression in the prior two years. The ASCLEPIOS I/II trials also included patients with SPMS with disease activity in addition to patients with RRMS.
Table 2. Comparison of patient eligibility criteria for included trials.
Baseline patient characteristics
Baseline patient characteristics for the included trials are summarized in . Differences in disease-related baseline characteristics (e.g. EDSS score, duration of MS symptoms and disease, and relapse history) reflected the MS phenotypes studied. Baseline characteristics were substantially different between the siponimod arm in SPMS and the fingolimod and ofatumumab arms in RRMS or RMS trials, respectively. Differences were present but less pronounced in SPMS and PPMS trials. Density plots from the siponimod, fingolimod, or ofatumumab arm were generated using IPD to visualize the degree of overlap between the trial patient populations ( and Supplemental Appendix B). Siponimod-treated SPMS patients more closely resembled PPMS patients treated with fingolimod than RRMS/RMS patients treated with fingolimod or ofatumumab. As expected, baseline EDSS score and relapse history characteristics demonstrated a high degree of heterogeneity between patients with SPMS and patients with either RRMS or RMS for the included trials. The two populations (SPMS and RRMS/RMS) also differed substantially in terms of age, duration of MS, and proportion with T1 gadolinium-enhancing (Gd+) lesions at baseline. In contrast to the differences noted between SPMS and RRMS/RMS populations, baseline characteristics including EDSS score, relapse history, the proportion with Gd + lesions, and age were more similar for SPMS and PPMS patients vs. patients with RRMS/RMS.
Figure 1. Distribution of baseline patient characteristics across trials in different MS phenotypes: EXPAND (SPMS), FREEDOMS (RRMS), and INFORMS (PPMS). Note: The INFORMS trial was not included in the number of relapses plots because the PPMS patients did not have relapses. For the time since first MS symptom and time since MS diagnosis, comparisons with INFORMS (PPMS) may be biased by the systematic underestimation of MS duration in PPMS patients (who typically have accumulated lesions but not acute neurological events). Additional comparisons between trials included in this study can be found in Supplemental Appendix B. Abbreviations. EDSS: Expanded Disability Status Scale; MS: multiple sclerosis; PPMS: primary progressive multiple sclerosis; RRMS: relapsing-remitting multiple sclerosis; SPMS: secondary progressive multiple sclerosis.
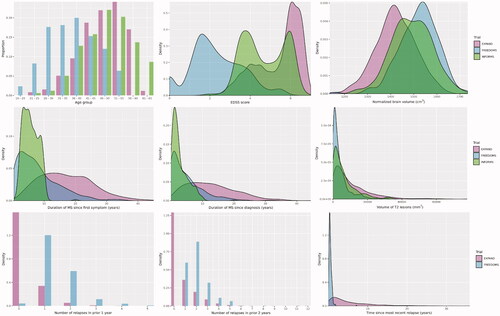
Table 3. Published patient baseline characteristics for the siponimod, fingolimod (0.5 mg), or ofatumumab treatment arm of included trials.
Outcome definitions
The outcome definitions for time to 6mCDP, time to 3mCDP, and ARR for each included trial are summarized in . For each of the reported outcomes, the definitions were described similarly across the trials.
Table 4. Summary of trial-specific outcome definitions.
Placebo response
All trials except TRANSFORMS and ASCLEPIOS I/II were placebo-controlled (), which allowed for assessment of placebo response for time to 6mCDP and 3mCDP endpoints. Placebo response was significantly different between EXPAND and the placebo-controlled trials in RRMS/RMS. Although more similar to one another than to the trials in RRMS/RMS, there were observable differences in placebo response (i.e. placebo arm time to CDP rates) between EXPAND and INFORMS, highlighting differences in the baseline characteristics of SPMS (EXPAND) and PPMS (INFORMS) populations.
There was considerable overlap between the placebo arm values of EXPAND and INFORMS for time to 6mCDP and 3mCDP (). However, there was a significantly higher rate of 6mCDP events and a trend toward a higher rate of 3mCDP in INFORMS compared to EXPAND. The HR (95% confidence interval [CI]; p-value) for EXPAND vs. INFORMS was 0.79 (0.63–0.99; p = .0383) and 0.85 (0.69–1.04; p = .103) for time to 6mCDP and 3mCDP, respectively.
Figure 2. Time to confirmed disability progression in placebo arms of the EXPAND, FREEDOMS, FREEDOMS II, and INFORMS trials. Note: The shaded areas of the plot that surround the curves represent the 95% confidence intervals around the point estimates at each time point. Curves were derived from IPD for the placebo arms of the indicated trials: EXPAND (SPMS), FREEDOMS (RRMS), FREEDOMS II (RRMS), and INFORMS (PPMS). Curves were generated before excluding relapsing patients from EXPAND and before conducting PSM on treatment arms (EXPAND and INFORMS). The TRANSFORMS (in RRMS) and ASCLEPIOS I/II (in RMS) trials were not included because they were not placebo-controlled. Abbreviations. 3mCDP: 3-month confirmed disability progression; 6mCDP: 6-month confirmed disability progression; IPD: individual patient data; PPMS: primary progressive multiple sclerosis; PSM: propensity score matching; RMS: relapsing multiple sclerosis; RRMS: relapsing-remitting multiple sclerosis; SPMS: secondary progressive multiple sclerosis.
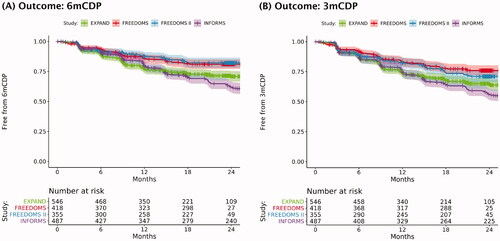
As expected, considerable overlap in placebo arm values was also noted between FREEDOMS and FREEDOMS II for time to 6mCDP and 3mCDP. However, between EXPAND and FREEDOMS/FREEDOMS II, a distinct difference was evident as shown by the separation of Kaplan-Meier curves for these pairs of trials (). The fingolimod trials in RRMS, where CDP often results from incomplete relapse recovery, each demonstrated a significantly lower rate of CDP events compared to the EXPAND trial in SPMS. The risk of 6mCDP events was significantly different in the placebo arms of EXPAND vs. FREEDOMS (HR 1.59 [95% CI 1.20–2.11; p = .00133]) and EXPAND vs. FREEDOMS II (HR 1.73 [95% CI 1.26–2.35; p < 0.001]). Similarly, the risk of 3mCDP events was significantly different in the placebo arms of EXPAND vs. FREEDOMS (HR 1.57 [95% CI 1.22–2.02; p < 0.001]) and EXPAND vs. FREEDOMS II (HR 1.32 [95% CI 1.02–1.70; p = .0357]).
Comparison of placebo response was not possible for TRANSFORMS and ASCLEPIOS I/II because of the lack of placebo arms for these trials.
Propensity score matching
An ITC between siponimod (EXPAND, in SPMS patients) and fingolimod (INFORMS, in PPMS patients) was feasible. Sufficient overlap in trial design, patient eligibility criteria, baseline patient characteristics, outcome definitions, and placebo response, as described previously, permitted the use of IPD to estimate the relative efficacy of the interventions within matched cohorts.
Patient selection and population alignment
Of the 1044 siponimod-treated SPMS patients in EXPAND, 366 patients with relapses in the two years before study entry were removed before the calculation of propensity scores to align more closely with the eligibility criteria in INFORMS. In total, 678 patients treated with siponimod 2 mg in EXPAND and 332 patients treated with fingolimod 0.5 mg in INFORMS were considered for the PSM analysis. Not all possible covariates could be matched (Supplemental Appendix C). After matching on seven covariates (age, baseline EDSS score, time since first MS symptom, timed 25-foot walk test, normalized brain volume, sex, and volume of T2 lesions), a total of 117 patients from each treatment group were included in the time to 6mCDP and time to 3mCDP analyses.
Before matching (and after excluding patients with prior relapses from EXPAND), six of the seven ranked covariates were imbalanced between treatment groups, with SMDs ≥0.20. In particular, matched SPMS patients who received siponimod had on average a long time since first MS symptom (SMD = 2.039), a lower normalized brain volume (SMD = 0.812), and a higher EDSS score (SMD = 0.673) at baseline than PPMS patients who received fingolimod 0.5 mg. Matching improved the balance across treatment groups, with SMDs <0.20. Descriptive statistics for all available patient characteristics before and after matching are presented in Supplemental Appendix C.
Before matching, the estimated probabilities of receiving siponimod, as predicted from baseline patient characteristics, were systematically higher among patients who received siponimod than those who received fingolimod 0.5 mg. After matching, the distribution of predicted probabilities of receiving siponimod was similar across treatment groups. Density plots of the propensity scores estimates for each treatment group before and after matching are presented in Supplemental Appendix C.
Time to CDP
Results of the one-to-one PSM analysis between siponimod and fingolimod are presented in . Although there was no statistically significant difference, a trend toward a greater delay in time to 6mCDP and 3mCDP favored siponimod compared to fingolimod (HR 0.76 [95% CI 0.48–1.20; p = .240] and HR 0.80 [95% CI 0.52–1.22; p = .300], respectively).
Table 5. Propensity score matching analysis results.
Scenario analyses wherein the lowest-ranked covariates were dropped from the one-to-one PSM analysis in a stepwise fashion (i.e. reducing the number of characteristics adjusted for) produced similar results as the primary analysis (Supplemental Appendix C). Similarly, additional analyses using other matching and weighting methods (i.e. three-way nearest neighbor matching and multinomial inverse probability of treatment weighting) produced results that were consistent with the primary one-to-one PSM analysis (Supplemental Appendix C).
Discussion
Before conducting ITCs, it is important to assess their feasibility based on the available clinical trial evidence. The validity of ITCs is dependent on whether there are systematic differences between trials, especially for patient characteristics that are treatment effect modifiersCitation29–32. We identified major differences in patient eligibility criteria, baseline patient characteristics, and placebo response that limited the feasibility of using PSM to conduct valid ITCs between siponimod in SPMS patients and either fingolimod in RRMS or ofatumumab in RMS. There was insufficient overlap in key patient characteristics, such as level of disability and relapse history. However, these characteristics were more similar in SPMS and PPMS patients as compared to RRMS/RMS patients, reflecting the relatively higher level of disability, lower rate of relapse (or absence of relapses), older age, and differing MRI inflammatory disease activity that characterize the progressive MS phenotypes. Relative to patients with progressive MS, RRMS/RMS patients are typically younger and have less advanced diseaseCitation33,Citation34. It was therefore feasible to use PSM to conduct valid ITCs between siponimod and fingolimod in patients with progressive MS. The results of our feasibility assessment support the separation of data for trials in different MS phenotypes when conducting ITCs, except in the case of comparing SPMS (EXPAND) and PPMS (INFORMS) using population-based methods to adjust for imbalances. As expected, even though there was some overlap between RRMS/RMS patients and SPMS patients in their baseline level of disability, patients with the same baseline EDSS score but a different MS phenotype (i.e. RRMS/RMS vs. SPMS) were still likely to differ in their relapse history, disease duration, and age.
In addition to patient characteristics, placebo response provides an indication of the degree of comparability in the trial populations. To meet the assumption of exchangeability (i.e. the patients could have been included in either trial in a pairwise ITC), the placebo response of the comparator trial populations must be similar. Although more similar to one another than to the trials in RRMS/RMS, there were observable differences in placebo response between EXPAND and INFORMS that highlight the differences in the SPMS (EXPAND) and PPMS (INFORMS) populations before conducting adjusted analyses.
The importance of conducting a detailed assessment of cross-trial heterogeneity when undertaking ITCs was recently demonstrated by Samjoo et al. for trials in SPMS patientsCitation35. In this case, the feasibility assessment highlighted the appreciable cross-trial heterogeneity that would have caused biased results if traditional ITCs, such as Bucher ITCs or NMA were conducted. Instead, population-based ITC methods that accounted for disparities in clinical covariates by leveraging IPD were found to be a more valid approach to examining comparative efficacy. Previous studies in MS and several other therapeutic areas have provided insights into the impact of adjustments on the validity of ITCsCitation35–38. In these examples, the clinical interpretation of the comparative efficacy of interventions was altered when accounting for differences in clinically relevant covariates.
The results of our ITC feasibility assessment underscore the importance of systematically evaluating cross-trial differences and identifying whether there is a need for covariate adjustment via PSM or comparable methodologies. Given the magnitude of the between-trial differences we observed, it was not methodologically appropriate to include SPMS (EXPAND) in a network of RRMS/RMS trials (FREEDOMS, FREEDOMS II, TRANSFORMS, and ASCLEPIOS I/II). However, it was feasible to indirectly compare trials in SPMS (EXPAND) and PPMS (INFORMS) using population-based methods to adjust for imbalances. These findings support the separation of data for trials in relapsing MS and progressive MS when conducting ITCs. From a clinical standpoint, it is important to exercise caution when interpreting the results of ITCs of trials in different MS phenotypes. People with relapsing MS cannot be readily compared to those with progressive MS. Notably, MS phenotype definitions can differ in clinical practice and between RCTs. For example, different SPMS definitions based on measures of progression and relapse history have been used by cliniciansCitation2,Citation39. Our findings regarding the feasibility of ITCs of trials in different MS phenotypes may not be generalizable to other indirect comparisons if the MS phenotype definitions differ for the included trials.
For MS trials, eligibility criteria related to disease phenotype can substantially influence how similar patient populations are and thus whether ITCs are feasible. Supporting the findings of this study, the importance of MS phenotype as a determinant of ITC feasibility has been noted in other recently published ITCs and feasibility assessments for ITCs. Samjoo et al.Citation35 concluded that ITCs were not feasible between the EXPAND siponimod trial in SPMS patients and the OPERA I/II ocrelizumab trials in RMS patientsCitation6 in part because the patient populations were too dissimilar. Substantial differences in baseline patient characteristics between EXPAND and OPERA I/II were noted for age, EDSS score, time since onset of MS symptoms, exposure to prior DMTs, prior relapse history, and MRI lesion measures. We observed a similar level of heterogeneity between EXPAND and ASCLEPIOS I/II, which is unsurprising given that the ASCLEPIOS I/II trials, like the OPERA I/II trials, were conducted in RMS patients with a low mean EDSS score (i.e. <3.0) at baseline. Unlike our study, Samjoo et al. did not have access to IPD for the comparator trials, which prevented a specific investigation of patients with progressive diseaseCitation35. In NMAs by the Institute for Clinical and Economic ReviewCitation40 and Melendez-Torres et al.Citation41, the authors conducted separate analyses for trials in patients with RRMS and progressive disease phenotypes (PPMS or SPMS). Other recent ITCs in MS have focused on a single MS phenotypeCitation42–46. In agreement with the findings of our study, Filippini et al.Citation47 considered RRMS separately in an NMA from progressive disease phenotypes, but combined trial data for patients with SPMS, progressive-relapsing MS, and PPMS, reasoning that these MS phenotypes were sufficiently similar because the course of MS is similar after the onset of progression regardless of prior history.
In the absence of randomized head-to-head comparative clinical trial data, and provided it is appropriate given the evidence base, PSM is a valuable method that enables a pairwise comparison of two treatments from separate trials using IPD. In addition to more broadly assessing the feasibility of ITCs for trials in patients with different MS phenotypes, this study used PSM to generate matched comparisons of siponimod (EXPAND) in SPMS and fingolimod (INFORMS) in PPMS, permitting an assessment of their relative efficacy in terms of time to CDP in patients with these MS phenotypes. After matching, treatment with siponimod was associated with a greater delay in disability progression, though this did not reach statistical significance. Findings were also robust across additional analyses using alternative propensity score methods for both CDP outcomes. Notably, the PSM analysis was a post-hoc analysis not included in statistical analysis plans for RCTs considered in this study and so was not planned to be powered to detect statistical significance.
A notable strength of the present study is that we were able to leverage IPD from multiple RCTs evaluating several DMTs in various MS phenotypes. The availability of IPD allowed us to conduct a rigorous assessment of the comparative efficacy between siponimod and fingolimod and between siponimod and ofatumumab in the absence of a head-to-head trial. Although ITCs involving methods, such as NMA can be performed using aggregate data, the results of these analyses may be biased by trial-level heterogeneity. In contrast, IPD provides more flexibility in adjusting for heterogeneity using standard covariate adjustment, matching, or reweighting techniques.
Our study had several limitations. First, we were unable to examine placebo responses for three of the trials because they did not include placebo arms, and there may be additional trial and patient characteristics that were not captured by the trials but influenced patient outcomes. Second, although we used PSM to adjust for imbalances in demographics and clinical characteristics between trials, ITCs using PSM may be confounded by unobserved but relevant differences between the two groups. To account for this, covariates available in both trials were identified and ranked based on prognostic importance a priori, which enabled adjustment of seven (out of nine) factors between the treatment groups. The two outstanding covariates (duration of SPMS and years since most recent relapse) did not apply to INFORMS because this study was in a PPMS population, which has no or very few relapses. Third, the relatively small number of patients informing the PSM comparison of siponimod with fingolimod reduced the statistical power and effect size for this analysis. Finally, we excluded SPMS patients with relapses (in the two years before study entry) in EXPAND from the PSM analysis. Although removing these patients reduced the number available for matching, it improved the alignment between EXPAND and INFORMS in terms of patient eligibility criteria (i.e. no relapsing MS).
Conclusion
For trials in MS, clinical phenotype is a key determinant of ITC feasibility. In this study, the SPMS phenotype (chronic progression of disability with or without overlaid relapses) as assessed in EXPAND sufficiently resembled the PPMS phenotype (progressive disease from onset) as assessed in INFORMS, permitting the estimation of comparative efficacy using PSM. For time to 6mCDP and 3mCDP, siponimod was favored relative to fingolimod though not significantly different (HR 0.76 [95% CI 0.48–1.20; p = .240] and HR 0.80 [95% CI 0.52–1.22; p = .300], respectively). However, trials evaluating other MS phenotypes (RRMS in FREEDOMS, FREEDOMS II, and TRANSFORMS; and RMS in ASCLEPIOS I/II) were too dissimilar to compare with SPMS, making comparative analyses infeasible. Our findings underscore the importance of assessing population differences across MS trials evaluating different MS phenotypes, as disparities may be too great for even population-based adjustment techniques to overcome and allow for valid comparisons of comparative efficacy. As such, comparative efficacy analyses incorporating different MS phenotypes, without adjustment, should be interpreted with caution.
Transparency
Declaration of funding
This research was supported by Novartis Pharma AG.
Declaration of financial/other relationships
IAS, EW, AH, PS, CD, and CC are paid employees of EVERSANA, which was contracted by Novartis Pharma AG to work on this project. CC has disclosed is also a shareholder of EVERSANA. RB and NA are salaried employees of Novartis Pharma AG, which is the manufacturer of fingolimod, ofatumumab, and siponimod. FD was a salaried employee of Novartis Pharma AG at the time of writing.
Author contributions
IAS, EW, AH, PS, CD, and CC were involved with study concept and design, analysis/interpretation of data, and drafting/revising of the manuscript. IAS, EW, and CC provided study supervision. IAS and CC critically reviewed the importance of intellectual content for the work. RB, FD, and NA assisted with the interpretation of the data and critically reviewed the importance of intellectual content for the work. All authors approved the final version of the manuscript.
Supplemental Material
Download PDF (1.2 MB)Acknowledgements
The authors thank Melody Zhao (employee of EVERSANA) for her contributions to the feasibility assessment and editorial assistance with this manuscript.
Notes
i Betaseron, Bayer, Leverkusen, Germany.
ii Rebif, Merck Serono, Darmstadt, Germany.
iii Avonex, Biogen, Cambridge, MA, USA.
iv Copaxone, Teva Neuroscience, Kansas City, MO, USA.
v Extavia, Novartis, Basel, Switzerland.
vi EVERSANA, Milwaukee, WI, USA.
References
- Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: insights from the atlas of MS, third edition. Mult Scler. 2020;26(14):1816–1821.
- Lublin FD, Reingold SC, Cohen JA, et al. Defining the clinical course of multiple sclerosis: the 2013 revisions. Neurology. 2014;83(3):278–286.
- Tafti D, Ehsan M, Xixis K. Multiple sclerosis. Treasure Island (FL): StatPearls Publishing; 2020.
- Vargas DL, Tyor WR. Update on disease-modifying therapies for multiple sclerosis. J Investig Med. 2017;65(5):883–891.
- Cohen JA, Barkhof F, Comi G, et al. Oral fingolimod or intramuscular interferon for relapsing multiple sclerosis. N Engl J Med. 2010;362(5):402–415.
- Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med. 2017;376(3):221–234.
- Novartis Pharmaceuticals. Mayzent (siponimod) highlights of prescribing information; 2020 [updated 2020 Jul; cited 2021 May 12]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/209884s002lbl.pdf
- Novartis Europharm Limited. Mayzent (siponimod) summary of product characteristics; 2021 [updated 2021 Jan 14; cited 2021 May 12]. Available from: https://www.ema.europa.eu/en/documents/product-information/mayzent-epar-product-information_en.pdf
- Novartis Pharmaceuticals Canada Inc. Mayzent (siponimod) product monograph; 2020 [updated 2020 Feb 20; cited 2021 May 12]. Available from: https://pdf.hres.ca/dpd_pm/00055111.PDF
- Novartis Pharmaceuticals Australia Pty Limited. Mayzent (siponimod) Australian product information; 2021 [updated 2021 Mar 10; cited 2021 May 12]. Available from: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/PICMI?OpenForm&t=&q=siponimod
- Kappos L, Bar-Or A, Cree BAC, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391(10127):1263–1273.
- Novartis Pharmaceuticals. Gilenya (fingolimod) highlights of prescribing information; 2019 [updated 2019 Dec; cited 2021 May 12]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/022527s031lbl.pdf
- Novartis Pharmaceuticals Australia Pty Limited. Gilenya (fingolimod) Australian product information; 2020 [updated 2021 Feb 9; cited 2021 May 12]. Available from: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/PICMI?OpenForm&t=&q=fingolimod
- Novartis Pharmaceuticals Canada Inc. Gilenya (fingolimod) product monograph; 2020 [updated 2020 Dec 15; cited 2021 May 12]. Available from: https://pdf.hres.ca/dpd_pm/00059239.PDF
- Novartis Europharm Limited. Gilenya (fingolimod) summary of product characteristics; 2021 [updated 2021 Feb 4; cited 2021 May 12]. Available from: https://www.ema.europa.eu/en/documents/product-information/gilenya-epar-product-information_en.pdf
- Lublin F, Miller DH, Freedman MS, et al. Oral fingolimod in primary progressive multiple sclerosis (INFORMS): a phase 3, randomised, double-blind, placebo-controlled trial. Lancet. 2016;387(10023):1075–1084.
- Calabresi PA, Radue E-W, Goodin D, et al. Safety and efficacy of fingolimod in patients with relapsing-remitting multiple sclerosis (FREEDOMS II): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Neurol. 2014;13(6):545–556.
- Kappos L, Radue EW, O'Connor P, et al. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362(5):387–401.
- Novartis Pharmaceuticals. Kesimpta (ofatumumab) highlights of prescribing information; 2020 [updated 2020 Aug; cited 2021 May 12]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/125326s070lbl.pdf
- Novartis Pharmaceuticals Australia Pty Limited. Kesimpta (ofatumumab) Australian product information; 2021 [updated 2021 Mar 4; cited 2021 May 12]. Available from: https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/PICMI?OpenForm&t=&q=ofatumumab
- Novartis Ireland Limited. Kesimpta (ofatumumab) summary of product characteristics; 2021 [updated 2021 Apr 16; cited 2021 May 12]. Available from: https://www.ema.europa.eu/en/documents/product-information/kesimpta-epar-product-information_en.pdf
- Novartis Pharmaceuticals Canada Inc. Kesimpta (ofatumumab) product monograph; 2021. [updated 2021 Jan 22; cited 2021 May 12]. Available from: https://pdf.hres.ca/dpd_pm/00059817.PDF
- Sormani MP, Wolff R, Lang S, et al. Overview of differences and similarities of published mixed treatment comparisons on pharmaceutical interventions for multiple sclerosis. Neurol Ther. 2020;9(2):335–358.
- Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res. 2011;46(3):399–424.
- Gensler LS, Chakravarty SD, Cameron C, et al. Propensity score matching/reweighting analysis comparing intravenous golimumab to infliximab for ankylosing spondylitis using data from the GO-ALIVE and ASSERT trials. Clin Rheumatol. 2020;39(10):2907–2917.
- Mann H, Andersohn F, Bodnar C, et al. Adjusted indirect comparison using propensity score matching of osimertinib to platinum-based doublet chemotherapy in patients with EGFRm T790M NSCLC who have progressed after EGFR-TKI. Clin Drug Investig. 2018;38(4):319–331.
- Stuart EA. Matching methods for causal inference: a review and a look forward. Stat Sci. 2010;25(1):1–21.
- Hauser SL, Bar-Or A, Cohen JA, et al. Ofatumumab versus teriflunomide in multiple sclerosis. N Engl J Med. 2020;383(6):546–557.
- Dias S, Sutton AJ, Ades A, et al. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network Meta-analysis of randomized controlled trials. Med Decis Making. 2013;33(5):607–617.
- Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR task force on indirect treatment comparisons good research practices: part 1. Value Health. 2011;14(4):417–428.
- Jansen JP, Naci H. Is network meta-analysis as valid as standard pairwise meta-analysis? It all depends on the distribution of effect modifiers. BMC Med. 2013;11(1):159.
- Salanti G. Indirect and mixed-treatment comparison, network, or multiple-treatments meta-analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Res Synth Methods. 2012;3(2):80–97.
- Dahlke F, Arnold DL, Aarden P, et al. Characterisation of MS phenotypes across the age span using a novel data set integrating 34 clinical trials (NO.MS cohort): age is a key contributor to presentation. Mult Scler J. 2021:1352458520988637.
- Weideman AM, Tapia-Maltos MA, Johnson K, et al. Meta-analysis of the age-dependent efficacy of multiple sclerosis treatments. Front Neurol. 2017;8:577.
- Samjoo IA, Worthington E, Haltner A, et al. The importance of considering differences in study and patient characteristics before undertaking indirect treatment comparisons: a case study of siponimod for secondary progressive multiple sclerosis. Curr Med Res Opin. 2020;36(7):1145–1156.
- Cameron C, Ewara E, Wilson FR, et al. The importance of considering differences in study design in network meta-analysis: an application using anti-tumor necrosis factor drugs for ulcerative colitis. Med Decis Making. 2017;37(8):894–904.
- Cameron C, Hutton B, Druchok C, et al. Importance of assessing and adjusting for cross-study heterogeneity in network meta-analysis: a case study of psoriasis. J Comp Eff Res. 2018;7(11):1037–1051.
- Thorlund K, Druyts E, Aviña-Zubieta JA, et al. Why the findings of published multiple treatment comparison meta-analyses of biologic treatments for rheumatoid arthritis are different: an overview of recurrent methodological shortcomings. Ann Rheum Dis. 2013;72(9):1524–1535.
- Lorscheider J, Buzzard K, Jokubaitis V, et al. Defining secondary progressive multiple sclerosis. Brain. 2016;139(Pt 9):2395–2405.
- Institute for Clinical and Economic Review. Disease-modifying therapies for relapsing-remitting and primary-progressive multiple sclerosis: effectiveness and value; 2017.
- Melendez-Torres G, Auguste P, Armoiry X, et al. Clinical effectiveness and cost-effectiveness of beta-interferon and glatiramer acetate for treating multiple sclerosis: systematic review and economic evaluation. Health Technol Assess. 2017;21(52):1–352.
- Li H, Hu F, Zhang Y, et al. Comparative efficacy and acceptability of disease-modifying therapies in patients with relapsing-remitting multiple sclerosis: a systematic review and network meta-analysis. J Neurol. 2020;267(12):3489–3498.
- Lucchetta RC, Tonin FS, Borba HHL, et al. Disease-modifying therapies for relapsing-remitting multiple sclerosis: a network meta-analysis. CNS Drugs. 2018;32(9):813–826.
- McCool R, Wilson K, Arber M, et al. Systematic review and network meta-analysis comparing ocrelizumab with other treatments for relapsing multiple sclerosis. Mult Scler Relat Disord. 2019;29:55–61.
- Samjoo IA, Worthington E, Drudge C, et al. Comparison of ofatumumab and other disease-modifying therapies for relapsing multiple sclerosis: a network meta-analysis. J Comp Eff Res. 2020;9(18):1255–1274.
- Siddiqui MK, Khurana IS, Budhia S, et al. Systematic literature review and network meta-analysis of cladribine tablets versus alternative disease-modifying treatments for relapsing-remitting multiple sclerosis. Curr Med Res Opin. 2018;34(8):1361–1371.
- Filippini G, Del Giovane C, Vacchi L, et al. Immunomodulators and immunosuppressants for multiple sclerosis: a network meta‐analysis. Cochrane Database of Systematic Reviews. 2013;(6):CD008933.
