Article title: Patient-reported treatment satisfaction with rivaroxaban in Japanese non-valvular atrial fibrillation patients: an observational study
Authors: Yukihiro Koretsune, Koichiro Kumagai, Shinichiro Uchiyama, Takeshi Yamashita, Masahiro Yasaka, Emi Watanabe-Fujinuma, Benjamin F. Banderas, Sayako Akiyama, Yutaka Okayama, Jean-Baptiste Briere, Gavin Dickie and Stefan J. Cano
Journal: Current Medical Research and Opinion
Bibliometrics: Volume 34, Number 12, pages 2157–2164
DOI: https://doi.org/10.1080/03007995.2018.1507315
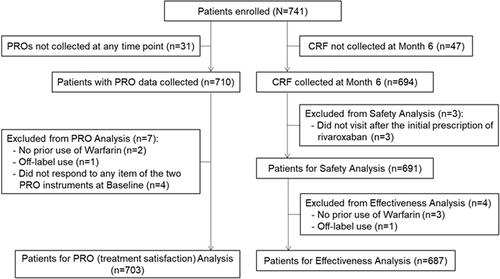
The authors have reported errors in the numbers of eligible patients for the PRO (treatment satisfaction) and effectiveness analyses, caused by misclassification of the prior treatment status (whether a patient had received prior treatment with Warfarin) and disease history in the original analyses. The study conclusions are not affected by these errors.
Pg. 2158
Study Population
Patients eligible for the PRO analysis were originally reported as n = 706. The correct number is n = 703. Additionally, the number of patients eligible for effectiveness analysis was previously reported as n = 665, however, the correct number is n = 687.
Pg. 2159
was revised as next to reflect the corrected study population.
Results
Study population
The first sentence in the section was revised to: The baseline characteristics of the 706 NVAF patients included in the PRO analysis are shown in .
Treatment satisfaction
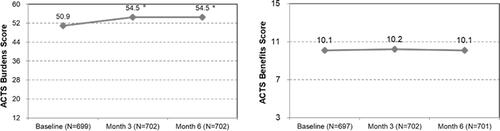
The 3rd sentence was revised to: The mean ACTS scores significantly improved at month 3 and month 6 compared with baseline in the Burden of Treatment scale (mean ± SD; 54.5 ± 6.4, 54.5 ± 6.5 vs. 50.9 ± 7.6, respectively; p < .001).
The 5th sentence was revised to: Quality of completion for the TSQM-II domains at baseline, month 3, and month 6 were ≥96% for all domains.
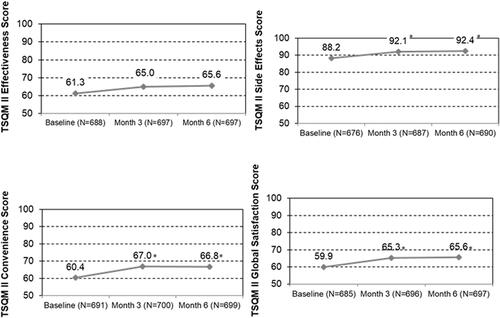
The last sentence was revised to: The mean TSQM-II sub-scale scores significantly improved at month 3 and month 6 compared to baseline for all four domains (mean ± SD; Effectiveness 65.0 ± 13.0, 65.6 ± 13.4 vs. 61.3 ± 13.4; Side Effects 92.1 ± 15.0, 92.4 ± 15.5 vs. 88.2 ± 17.8; Convenience 67.0 ± 13.0, 66.8 ± 13.1 vs. 60.4 ± 13.6; Global Satisfaction 65.3 ± 13.4, 65.6 ± 14.4 vs. 59.9 ± 13.9, respectively; all p < .001).
Pg. 2160
was revised as below. Since the number of eligible patients changed from n = 706 to n = 703, the frequencies and percentages of the demographic and clinical characteristics of the analysis population have changed.
Table 1. Patient characteristics at baseline (n = 703).
Exploratory sub-group analysis
The 2nd sentence was revised to: Significant differences were found in the Burdens of Treatment score, with older patients (p = .022), patients with high CHADS2 scores (p = .007), and patients with permanent AF (p = .008) demonstrating higher treatment satisfaction.
The 3rd sentence (“Patients who switched treatments due to difficulty in compliance also showed higher satisfaction after the switch with regard to reduced Burden of Treatment (p = .044).”) was deleted as it was no longer statistically significant after the re-analysis with the corrected study population.
The 8th sentence was revised to: Patients with low CHADS2 scores showed greater improvement in treatment satisfaction than patients with moderate or high CHADS2 scores in the ACTS Burdens of Treatment score (4.5 vs. 3.6, 3.7, respectively).
The 9th sentence was revised to: In addition, patients who were switched at least partly due to unstable INR consistently showed greater improvement in satisfaction than those who were switched not due to unstable INR in ACTS Burdens (4.3 vs. 3.3, respectively) and TSQM-II scores (Effectiveness: 5.6 vs. 3.7, Side Effects: 5.7 vs. 3.1, Convenience: 8.2 vs. 5.8, Global Satisfaction: 6.8 vs. 5.3).
The 10th sentence was revised to: Finally, those who were switched at least partly due to difficulty in patient compliance also showed greater improvement in satisfaction than those who were switched not due to difficulty in patient compliance in ACTS Burdens (4.2 vs. 3.7, respectively), and TSQM-II scores (Effectiveness: 6.0 vs. 4.3, Convenience: 7.9 vs. 6.7, Global Satisfaction: 6.7 vs. 5.9).
was revised as next. Since the number of eligible patients changed from n = 706 to n = 703, the ACTS Burdens and Benefits scores have changed.
Pg. 2162
was revised as below. Since the number of eligible patients changed from n = 706 to n = 703, the TSQM-II scores have changed.
was revised as below. Since the number of eligible patients changed from n = 706 to n = 703, the number of analysis samples (n), mean ACTS Burdens and Benefits scores and the standard deviations, and the p-values for each demographic/clinical characteristic have changed.
Table 2. The ACTS scores at month 6 by demographic/clinical characteristics and reasons for switching.
Pg. 2162
Safety/effectiveness analysis
The 1st sentence was revised to: Among 691 patients who were eligible for safety analysis, any bleeding occurred in 39 (5.6%) patients, of which four (0.6%) were major bleeding, and one (0.1%) was intracranial hemorrhage.
The 3rd sentence was revised to: Among 687 patients who were eligible for effectiveness analysis, no effectiveness events occurred except for ischemic stroke in four patients (0.6%). The incidence of these events was similar to that in a larger-scale, rivaroxaban PMS study in Japan24.