Abstract
Objective
National Comprehensive Cancer Network (NCCN) guidelines for cutaneous melanoma (CM) recommend physicians consider increased surveillance for patients who typically have lower melanoma survival rates (stages IIB-IV as determined by the American Joint Committee on Cancer (AJCC), 8th edition). However, up to 15% of patients identified as having a low recurrence risk (stages I-IIA) experience disease recurrence, and some patients identified as having a high recurrence risk will not experience any recurrence. The 31-gene expression profile test (31-GEP) stratifies patient recurrence risk into low (Class 1) and high (Class 2) and has demonstrated risk-appropriate impact on disease management and clinical decisions.
Methods
Five-year plans for lab work, frequency of clinical visits, and imaging pre- and post-31-GEP test results were assessed for a cohort of 509 stage I-III patients following an interim subset analysis of 247 patients.
Results
After receiving 31-GEP results, 50.6% of patients had a change in management plans in at least one of the following categories—clinical visits, lab work, or surveillance imaging. The changes aligned with the risk predicted by the 31-GEP for 76.1% of patients with a Class 1 result and 78.7% of patients with a Class 2 result. A Class 1 31-GEP result was associated with changes toward low-intensity management recommendations, while a Class 2 result was associated with changes toward high-intensity management recommendations.
Conclusion
The 31-GEP can stratify patient recurrence risk in patients with CM, and clinicians understand and apply the prognostic ability of the 31-GEP test to alter patient management in risk-appropriate directions.
PLAIN LANGUAGE SUMMARY
When caught early, cancer of the skin can usually be removed, and patients have excellent chances of survival. However, some patients will have their cancer come back or spread to a new location in their body.
The 31-gene expression profile (GEP) test measures the expression levels of 31 genes from an individual patient’s tumor. A proprietary formula uses this information to identify the risk of recurrence or spread as low risk (Class 1) or high risk (Class 2). Cancers with low-risk 31-GEP scores have a lower chance of cancer recurrence or spread than patients with a high-risk score.
In this study, we wanted to determine if doctors treated patients with low-risk scores differently from patients with high-risk scores. We found that doctors changed approximately half of patient treatment plans (doctor visits, lab work, or imaging to see if the cancer has come back) after learning the 31-GEP test results. Doctors usually planned less frequent follow-up visits for Class 1 results and more frequent follow up for Class 2 results.
This study found doctors understand and make changes to their treatment plans based on the patient’s 31-GEP test result.
Introduction
Rising cutaneous melanoma (CM) incidence and declining mortality ratesCitation1 suggest that recent advances in surveillance imaging and adjuvant therapy are efficaciousCitation2–4. However, therapies are not without side effects and, along with disease surveillance, are costly; therefore, there is an increased need to allocate resources to CM patients likely to experience recurrence or other poor outcomesCitation5. Therefore, the National Comprehensive Cancer Network (NCCN) has developed guidelines to improve allocation of resources and has developed guidance on follow-up visits, bloodwork, and imaging for patients with CM based on American Joint Committee on Cancer (AJCC 8th edition) staging, which provides a population-based prognosis for melanoma-specific survival (MSS) based on primary tumor characteristics (e.g. Breslow thickness and ulceration status), sentinel lymph node (SLN) status, and distant metastasesCitation6. The NCCN recommends that patients with a low risk of death from their disease (stage I-IIA) have follow-up visits every 6–12 months for 5 years and no routine imaging or blood workCitation7. In contrast, for patients with an increased risk of death from melanoma (stage IIB-IV), guidelines recommend increased follow-up frequency (every 3–6 months) and regular lab work and imaging to detect metastasesCitation7.
Most new melanoma patients are diagnosed with early-stage disease, classified as low risk, and are not eligible for more intensive management or adjuvant therapy by NCCN guidelinesCitation8,Citation9. However, variability in 5-year survival rates of CM patients within each AJCC stageCitation10–12 suggests that certain patients classified as low risk by current guidelines may have an unknown high risk of metastasis unaccounted for by current clinicopathologic staging factorsCitation13,Citation14. Advanced imaging can detect metastases while tumor burden is lowCitation3,Citation15–17, and is associated with improved survival outcomesCitation18,Citation19; therefore, identifying misclassified patients may increase recurrence-free (RFS), distant metastasis-free (DMFS), and MSS.
Gene expression profiling (GEP) uses the genomic signature of tumors to predict each patient’s recurrence or metastasis risk, and the NCCN guidelines for uveal melanoma and breast cancer incorporate the use of validated GEP tests into recommendations for patient managementCitation20–24. Additional GEP tests have been developed for prostateCitation25,Citation26 and thyroid cancersCitation27. A 31-GEP test was developed to measure the expression of 28 discriminant gene targets and three control genes to evaluate the risk of CM metastasis based on the primary tumor molecular profileCitation22,Citation23. This previously validated 31-GEP test stratifies CM as Class 1 (low risk) or Class 2 (high risk) and is an independent predictor of regional or distant metastatic recurrence and deathCitation22,Citation23. Previous reports have shown that clinicians used the 31-GEP test to alter management intensity in risk-appropriate directionsCitation28–30. Importantly, Schuitevoerder et al.Citation31 reported that 31-GEP class accounted for 52% of the changes in patient management decisions. The clinical utility of the 31-GEP was further confirmed by Berger et al. and Dillon et al., who demonstrated risk-appropriate changes in management using the 31-GEPCitation28,Citation29.
The current study was designed as a follow-up to Dillon et al.’s clinical utility interim analysis, which assessed clinician’s management changes after receiving 31-GEP results for 247 patients with stage I-II CM. In the current study, 262 patients with stage I-III melanoma and a 31-GEP result were combined with the cohort from the previously reported interim analysis by Dillon et al. to validate 31-GEP-mediated melanoma management strategies in a large cohort of patients (n = 509). We tested the hypothesis that clinicians use the 31-GEP test to alter patient management intensity in risk-appropriate directionsCitation29.
Methods
Data collection and patient population
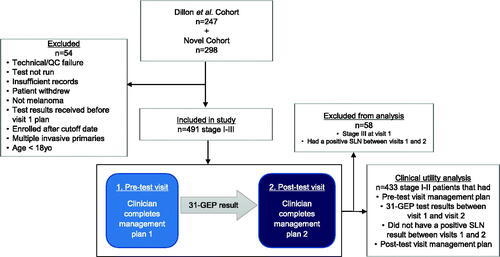
Patients were enrolled consecutively from March 2016 to March 2019 at 17 participating centers with 39 different providers () under an IRB-approved protocol, with an interim analysis of a subset of stage I-II patients previously reportedCitation29. Eligibility criteria included patients ≥18 years old, classified as stage I-III, and able to give informed consent. Exclusion criteria included patients for which use of the 31-GEP is not intended (i.e. melanoma in situ or stage IV melanoma at the time of diagnosis). Patients were enrolled primarily from surgical oncology centers (74.4%), followed by dermatology (20.8%), and medical oncology centers (3.9%) ().
Table 1. Provider demographics.
Table 2. Patient demographics.
This study enrolled 298 additional patients with stage I-III CM to combine with the interim analysis of a previously reported cohort (). Due to the low number of patients with stage III CM and to avoid the potentially confounding factor of increased management due to receiving a positive SLN, only patients with stage I-II CM that did not receive a positive SLN result between visits 1 and 2 were included in clinical utility analysis.
Of 545 patients initially enrolled in total, 54 were excluded due one of the following reasons as detailed in —technical or quality control failure, 31-GEP results obtained before the first visit, a known concurrent primary tumor, detection of lymphocytic leukemia, downgrading of the tumor to non-malignant melanoma by expert opinion, or unconfirmed date of birth. Further, 31 patients were diagnosed with stage III CM and 27 patients were upstaged from stage I-II to stage III between visit one and visit two and were excluded from clinical utility analysis, leaving 433 patients with stage I-II melanoma in the final analysis for clinical utility ().
Management
At the initial patient visit before 31-GEP testing, clinicians recorded recommendations for patient management for imaging frequency and modality, frequency of clinical visits, and lab work. After receiving 31-GEP results for each patient, clinicians again recorded recommendations for patient management. Clinician-recommended management changes were classified as increased, decreased, or no change based on overall change in frequency of management as well as change within each category of frequency for lab work and physical exams, as well as both frequency and type of imaging, pre- and post-31-GEP test recommendations.
NCCN management guidelines
Based on NCCN guidelines for patient management in stage I-IIA and stage IIB-IIC melanomaCitation7, we classified clinician-recommended patient follow-up according to the following rules: high risk: >4 clinical visits within the first two years, and any imaging indicated; low risk: ≤4 clinical visits in the first two years and no imaging; or intermediate risk: either ≤4 clinical visits within the first two years AND imaging OR >4 clinical visits in the first two years and no imaging.
Statistical analysis
The sample size was calculated to reduce the 95% confidence interval while expecting a 20% change in management based on 31-GEP test results. A patient sample size of 250 was found to meet these requirements. Pre- and post-test management decisions were compared by Fisher’s exact test or Chi-squared. Kaplan-Meier and log-rank tests compared survival outcomes between patients with Class 1 and Class 2 results. Analyses were performed in R version 3.3.2 (University of Auckland, NZ) according to prespecified analysis plans.
Results
Provider and patient characteristics
Provider characteristics are provided in . We did not see any significant differences in 31-GEP utility based on provider variables. Patient characteristics from the 433 combined patient cohort are shown in . Most patients were classified as stages I-IIA (384/433; 88.7%) and did not have ulceration (346/433; 79.9%). Stage I-IIA accounted for 97.3% (319/328) of Class 1 patients compared with 61.9% (65/105) of Class 2 patients. Class 1 patients had lower Breslow thickness (median 0.9 vs. 2.2 mm, p<.001) and mitotic rate (median 1.0 vs. 3.0, p<.001) than Class 2 patients.
Impact of the 31-GEP test on the management of stage I-II melanoma
We quantified the frequency of planned 5-year follow-up from pre- and post-31-GEP test management plans for patients with stage I-II melanoma. Overall, clinicians changed their management strategies for 50.6% (219/433) of patients (). For patients with a Class 1 result with a change in management, 76% of the changes resulted in decreased management intensity. Conversely, proposed changes in management for patients with a Class 2 result included increased frequency or intensity of imaging, bloodwork, or clinical visits 79% of the time (). Overall, post-31-GEP test management plans changed for the frequency of clinical visits, blood work, and imaging in 19.9% (86/433), 35.8% (155/433), and 37.0% (160/433) of patients, respectively (). Physicians most commonly decreased planned management intensity for lab work (65.1%, 28/43), frequency of clinical visits (66.3%, 61/92), and imaging intensity (74.2%, 49/66) after receiving a Class 1 result for their patient. Conversely, physicians typically increased planned management intensity for lab work (74.4%, 32/43), frequency of clinical visits (81.0%, 51/63), and imaging intensity (72.3%, 68/94) after receiving a Class 2 result for their patient (, ).
Figure 2. Percent of 5-year overall management that changed for patients with stage I-II melanoma after receiving the 31-GEP test result.
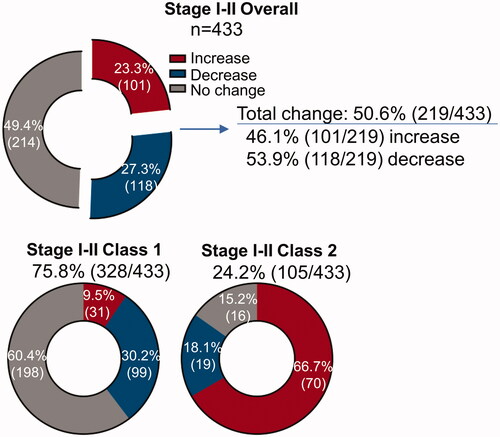
Figure 3. Percent proposed 5-year lab work, clinic visit, and imaging frequency that changed after receiving 31-GEP result for low- and high-risk populations.
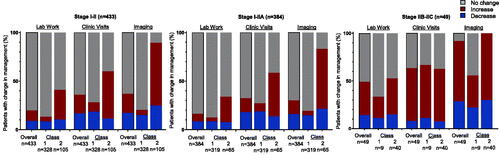
Table 3. Change in planned melanoma management in alignment with 31-GEP test results.
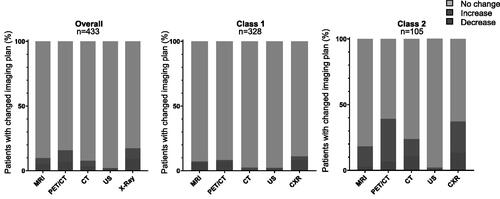
Next, we looked at which imaging modalities were associated with increased or decreased patient management based on the 31-GEP Class result (). Little change was seen in recommendations for ultrasound. On the other hand, physicians recommended increased X-ray, PET/CT, and Brain MRI for patients with a Class 2 result compared to reduced imaging recommendations by these modalities for patients with a Class 1 result.
Figure 4. Percent 5-year surveillance imaging plans that changed for patients with stage I-II melanoma after receiving 31-GEP result. The percent of patients with stage I-II melanoma for whom clinicians increased, decreased or did not change management plans after receiving either a Class 1 or Class 2 31-gene expression profile (31-GEP) result. MRI, magnetic resonance imaging; PET/CT, positron emission tomography/computed tomography; CT, computed tomography; US, ultrasound; CXR, chest x-ray.
Next, we categorized management plans as high-risk (similar to recommendations for stage IIB-IIC) or low-risk (similar to recommendations for stage I-IIA) as defined by NCCN guidelines. For stage I-II patients that had a change in management that moved them from one NCCN risk category to another following a Class 1 result, physicians tended (79.9%, 55/69) to change plans in concordance with a low-risk NCCN plan (; blue), with the majority going from a combination management plan (management plan comprised elements of NCCN high- and low-risk management) to a low-risk management plan (69.1%, 38/55) or from a high-risk to intermediate-risk plan (29.1%, 16/55). On the other hand, when clinicians changed management after receiving a Class 2 result for their patient, they tended to choose plans in concordance with a high-risk NCCN management plan (82.1%, 32/39) (; red), with the majority going from an intermediate-risk plan to a high-risk management plan (53.1%, 17/32) or from a low-risk to intermediate-risk management plan (34.45%, 11/32).
Table 4. Number of patients with planned management at each NCCN-based risk level at visit 1 and visit 2.
Discussion
NCCN bases management recommendations for CM patients on AJCC staging. NCCN recommends less intensive management for stage I-IIA patients than stage IIB-IV patients over the first five years after CM diagnosisCitation7. These recommendations include fewer clinical visits and no imaging recommendations for stage I-IIA patients. Further, subjective measurements of tumor characteristics or SLN tumor burden can result in discrepancies in diagnosis and under- or overestimation of metastatic risk leading to under- or over-treatment of the patientCitation32,Citation33. Here we report the final analysis to a previously reported initial analysisCitation29, showing that clinicians familiar with the 31-GEP test understand and utilize the test to alter plans for patient management intensity in a risk-appropriate direction. The results of this study demonstrate that the 31-GEP test led to management plan changes for 51% of patients based on 31-GEP results (). Most changes in patients with a Class 1 result led to reduced intensity in the management plan for the stage I-II population overall.
A Class 1 result for patients with stage I-II CM led to reduced management intensity within NCCN-based management strategies. A Class 2 result led to increased management intensity within NCCN-based management strategies (). Clinicians changed plans to more definitive risk categories after receiving the 31-GEP test results. For patients with a Class 1 result, clinicians changed 28% of intermediate-risk plans (a mix of high-risk and low-risk NCCN management strategies) to fully low-risk plans, and 41% of intermediate-risk plans were changed to fully high-risk plans after receiving a Class 2 result. These results suggest that the 31-GEP test increased clinician confidence in low-risk or high-risk management strategies for patients with stage I-II melanoma for whom there was initial uncertainty concerning the optimal management strategy. Further, clinicians are using the 31-GEP in a thoughtful manner in conjunction with clinicopathologic features to ensure patients are receiving the most appropriate care. With the considerable medical costs associated with surveillance of patients who do not have a recurrenceCitation17, using a 31-GEP Class 1 result in conjunction with AJCC staging criteria may reduce the economic burden. Similarly, a Class 2 result for patients with stage IIB-IIC CM reduced discordant management plans. Thus, the 31-GEP may add confidence that a patient’s molecular biology and AJCC staging are aligned, thereby increasing concordance with NCCN guidelines.
The study’s primary aim was to provide expanded evidence of the clinical utility of the 31-GEP test to alter patient management in a risk-appropriate direction. We report future patient management plans but did not capture if the clinicians or patients followed the proposed plans, which could impact the findings, nor did we assess patient survival or disease relapse, as these were beyond the scope of this study. However, previous publications have shown that the 31-GEP test is able to predict patient outcomesCitation22,Citation23,Citation34. Additionally, the data do not incorporate patient psychological needs or desires that may impact compliance with management intensity changesCitation35. It is also expected that physicians do not rely solely on the 31-GEP test results for patient management and are likely influenced by other factors, which may explain why some patients with Class 1 results were monitored more frequently (and that some with Class 2 results had plans that included less monitoring). Therefore, studies are planned to determine the effect of GEP results on patient psychology related to their diagnosis and determine the degree to which clinicians adhere to proposed management plans. However, the primary outcomes of the study confirm previous reports that clinicians use the test in a risk-appropriate manner to guide patient management strategies within the framework of NCCN-based guidelinesCitation28,Citation29,Citation31.
Conclusion
The 31-GEP test is a molecular prognostic measure of metastatic risk that can help clinicians develop appropriate management strategies. Patients at high molecular risk of metastasis may benefit from more intensive surveillance or treatment. In contrast, patients at low molecular risk of metastasis may benefit from less intensive surveillance or treatment. This study demonstrates that clinicians use the 31-GEP in conjunction with AJCC staging to modulate disease management strategies for patients with stage I-II disease.
Transparency
Declaration of funding
This study was funded by Castle Biosciences, Inc.
Declaration of financial/other relationships
AQ, BM, KC, OZ, and RC are employees and options holders at Castle Biosciences, Inc. LD is a consultant for Castle Biosciences, Inc. JV and AJ are on the speaker’s bureau at Castle Biosciences, Inc. MM, RD, and MF have no conflicts to disclose. Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Author contributions
LD, MM, RD, JV, AJ, and MF contributed patients. LD, MM, RD, JV, AJ, MF, AQ, BM, KC, OZ, and RC contributed to analyzing the data and/or writing or critically editing the manuscript.
Ethics statement
The study was approved by the WCG Institutional Review Board. Patients were included in the study only after giving informed consent.
Acknowledgements
None.
Data availability statement
Patient data will not be made publicly available.
References
- American Cancer Society. Cancer Facts & Figures 2019. 2019. 76. https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html.
- Freeman M, Laks S. Surveillance imaging for metastasis in high-risk melanoma: importance in individualized patient care and survivorship. Melanoma Manag. 2019;6:55. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6582455/.
- Ibrahim AM, Le May M, Bossé D, et al. Imaging intensity and survival outcomes in high-risk resected melanoma treated by systemic therapy at recurrence. Ann Surg Oncol. 2020;27(10):3683–3691.
- Leiter U, Buettner PG, Eigentler TK, et al. Is detection of melanoma metastasis during surveillance in an early phase of development associated with a survival benefit? Melanoma Res. 2010;20(3):240–246.
- Lee AY, Droppelmann N, Panageas KS, et al. Patterns and timing of initial relapse in pathologic stage II melanoma patients. Ann Surg Oncol. 2017;24(4):939–946.
- Gershenwald JE, Scolyer RA, Hess KR, et al. Melanoma staging: evidence-based changes in the American joint committee on cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–492.
- Swetter SM, Thompson JA, Coit DG, et al. NCCN clinical practice guidelines in oncology. Cutaneous melanoma. Version 3.2020. USA: National Comprehensive Cancer Network (NCCN); 2020.
- Whiteman DC, Baade PD, Olsen CM. More people die from thin melanomas (1 mm) than from thick melanomas (>4 mm) in Queensland, Australia. J Invest Dermatol. 2015;135(4):1190–1193.
- Howlader N;N. SEER cancer statistics review, 1975–2012. Bethesda, MD: National Cancer Institute; 2014. Available from: https://seer.cancer.gov/archive/csr/1975_2012/.
- Lo SN, Scolyer RA, Thompson JF. Long-term survival of patients with thin (T1) cutaneous melanomas: a breslow thickness cut point of 0.8 mm separates higher-risk and lower-risk tumors. Ann Surg Oncol. 2018;25(4):894–902.
- Maurichi A, Miceli R, Camerini T, et al. Prediction of survival in patients with thin melanoma: results from a multi-institution study. J Clin Oncol. 2014;32(23):2479–2485.
- Schuitevoerder D, Heath M, Cook RW, et al. Impact of gene expression profiling on decision-making in clinically node negative melanoma patients after surgical staging. J Drugs Dermatol. 2018;17(2):196–199.
- Niebling MG, Haydu LE, Karim RZ, et al. Pathology review significantly affects diagnosis and treatment of melanoma patients: an analysis of 5011 patients treated at a melanoma treatment center. Ann Surg Oncol. 2014;21(7):2245–2251.
- Patrawala S, Maley A, Greskovich C, et al. Discordance of histopathologic parameters in cutaneous melanoma: clinical implications. J Am Acad Dermatol. 2016;74(1):75–80.
- Podlipnik S, Carrera C, Sánchez M, et al. Performance of diagnostic tests in an intensive follow-up protocol for patients with American joint committee on cancer (AJCC) stage IIB, IIC, and III localized primary melanoma: a prospective cohort study. J Am Acad Dermatol. 2016;75(3):516–524.
- Park TS, Phan GQ, Yang JC, et al. Routine computer tomography imaging for the detection of recurrences in high-risk melanoma patients. Ann Surg Oncol. 2017;24(4):947–951.
- Livingstone E, Krajewski C, Eigentler TK, et al. Prospective evaluation of follow-up in melanoma patients in Germany – results of a multicentre and longitudinal study. Eur J Cancer. 2015;51(5):653–667.
- Poklepovic A, Carvajal R. Prognostic value of low tumor burden in patients with melanoma. Oncology. 2018;32(9):e90–e96.
- Joseph RW, Elassaiss-Schaap J, Kefford R, et al. Baseline tumor size is an independent prognostic factor for overall survival in patients with melanoma treated with pembrolizumab. Clin Cancer Res. 2018;24(20):4960–4967.
- Giuliano AE, Connolly JL, Edge SB, et al. Breast Cancer-Major changes in the American joint committee on cancer eighth edition cancer staging manual: updates to the AJCC breast TNM staging system: the 8th edition. CA Cancer J Clin. 2017;67(4):290–303.
- Wang M, Wu K, Zhang P, et al. The prognostic significance of the oncotype DX recurrence score in T1-2N1M0 estrogen receptor-positive HER2-Negative breast cancer based on the prognostic stage in the updated AJCC 8th edition. Ann Surg Oncol. 2019;26(5):1227–1235.
- Gerami P, Cook RW, Russell MC, et al. Gene expression profiling for molecular staging of cutaneous melanoma in patients undergoing sentinel lymph node biopsy. J Am Acad Dermatol. 2015;72(5):780–785.e3.
- Gerami P, Cook RW, Wilkinson J, et al. Development of a prognostic genetic signature to predict the metastatic risk associated with cutaneous melanoma. Clin Cancer Res. 2015;21(1):175–183.
- Grossman D, Kim CC, Hartman RI, et al. Prognostic gene expression profiling in melanoma: necessary steps to incorporate into clinical practice. Melanoma Manag. 2019;6(4):MMT32.
- Cooperberg MR, Simko JP, Cowan JE, et al. Validation of a cell-cycle progression gene panel to improve risk stratification in a contemporary prostatectomy cohort. J Clin Oncol. 2013;31(11):1428–1434.
- Cuzick J, Swanson GP, Fisher G, et al. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: a retrospective study. Lancet Oncol. 2011;12(3):245–255.
- Chudova D, Wilde JI, Wang ET, et al. Molecular classification of thyroid nodules using high-dimensionality genomic data. J Clin Endocrinol Metab. 2010;95(12):5296–5304.
- Berger AC, Davidson RS, Poitras JK, et al. Clinical impact of a 31-gene expression profile test for cutaneous melanoma in 156 prospectively and consecutively tested patients. Curr Med Res Opin. 2016;32(9):1599–1604.
- Dillon LD, Gadzia JE, Davidson RS, et al. Prospective, multicenter clinical impact evaluation of a 31-Gene expression profile test for management of melanoma patients. J of Skin. 2018;2(2):111–121.
- Scott AM, Dale PS, Conforti A, et al. Integration of a 31-Gene expression profile into clinical Decision-Making in the treatment of cutaneous melanoma. Am Surg. 2020;86(11):1561–1564.
- Schuitevoerder D, Heath M, Massimino K, et al. Impact of genetic expression profile on decision-making in clinically node negative melanoma patients after surgical staging. Seattle: WA; 2017.
- Gaudi S, Zarandona JM, Raab SS, et al. Discrepancies in dermatopathology diagnoses: the role of second review policies and dermatopathology fellowship training. J Am Acad Dermatol. 2013;68(1):119–128.
- Fayne RA, Macedo FI, Rodgers SE, et al. Evolving management of positive regional lymph nodes in melanoma: past, present and future directions. Oncol Rev. 2019;13(2):433. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6902307/.
- Gastman BR, Gerami P, Kurley SJ, et al. Identification of patients at risk of metastasis using a prognostic 31-gene expression profile in subpopulations of melanoma patients with favorable outcomes by standard criteria. J Am Acad Dermatol. 2019;80(1):149–157.e4.
- Trotter SC, Sroa N, Winkelmann RR, et al. A global review of melanoma follow-up guidelines. J Clin Aesthet Dermatol. 2013;6(9):18–26.